Abstract
INTRODUCTION
Extended liver resection for hepatocellular carcinoma can be performed safely and results in long-term survival in select patients. Caudate lobe as the sole remnant liver following extended liver resection for hepatocellular carcinoma has traditionally been considered a relative contraindication to resection for advanced tumors of the liver. This study evaluated this surgical technique and the results of patients with tumors who had undergone liver resection with the caudate lobe as the sole remnant liver.
PRESENTATION OF CASE
A 68-year-old man with a tumor (9 cm × 11 cm) located in Couinaud's segment VI + VII + VIII and another tumor (7 cm × 8 cm) located in segment IV + V underwent liver tumor resection.
DISCUSSION
Pathological examination of the resected tumors revealed HCC and mixed nodular cirrhosis. With a follow-up, the patient survived 28 months.
CONCLUSION
Despite its small volume, the caudate lobe has integrated bilateral blood supply system and proliferates easily. Liver resection is a feasible procedure that can be performed with an acceptable operative risk leading to long-term outcome in selected patients.
Keywords: Caudate lobe, Hepatocellular carcinoma, Hepatectomy, The future liver remnant (FLR)
1. Introduction
Extended liver resection has generally been accepted as the treatment of choice for large HCC in patients with well-preserved liver function. Caudate lobe-sparing subtotal hepatectomy for primary hepatolithiasis has been reported.1 But, extended hepatectomy for hepatocellular carcinoma (HCC) with the caudate lobe representing the only remnant liver following resection has not been previously reported.
2. Presentation of case
A 68-year-old man presented with the complaint of upper abdominal pain and discomfort for 1-month duration. Additional symptoms were asthenia and anorexia with a weight loss of 5 kg in past 5 months. He denied fever and chills, and jaundice. He denied high blood pressure, heart disease, or diabetes. Physical examination was normal. Serum liver function tests revealed a total bilirubin (TBIL) of 17.5 μmol/L, albumin (ALB) 38 g/L, alanine aminotransferase (ALT) 36 U/L, and aspartate aminotransferase (AST) 29 U/L. Renal function was normal. White blood cells were 3.57 × 9/L, hemoglobin 70.2 g/L; platelet: 157 × 109/L, prothrombin time (PT): 13.3S, and activated partial thromboplastin time (APTT): 27.4S. Tumor markers (alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA199)) were all within the normal range and the serum hepatitis panel was negative. Ultrasound showed multiple hypoechoic areas in the right liver lobe. The maximum diameter of each lesion ranged from 7 to 11 cm. Imaging characteristics were consistent with that of cirrhosis and the presence of left intrahepatic bile duct stones. Computed tomography (CT) and magnetic resonance imaging (MRI) scanning confirmed following findings: multiple liver tumors with cirrhosis, splenomegaly, esophageal varices and cholangitis of the liver, and compensatory hypertrophy of the caudate lobe (Fig. 1) were found. Indocyanine green retention rate at 15 min (ICG-15) was 12.1%. The future liver remnant (FLR) was about 40%.
Fig. 1.
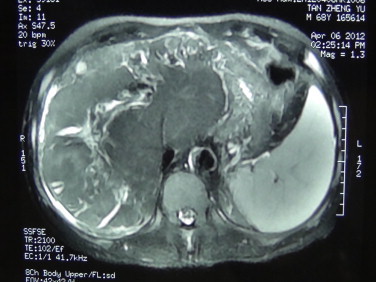
MRI: Multiple liver tumors with cirrhosis, splenomegaly, and esophageal varices. The cholangitis of the liver and caudate lobe compensatory hypertrophy were found.
At the time of the operation, the abdominal cavity was thoroughly explored to rule out extrahepatic disease. Intraoperative ultrasound was performed to assess the number and size of the lesions, and the relationship of the tumor to vascular structures. The left lobe was noted as atrophic with caudate lobe compensatory hypertrophy. A tumor (9 cm × 11 cm) was located in Couinaud's segments VI + VII + VIII and the other tumor (7 cm × 8 cm) was located in segments IV + V. The caudate lobe was found to protrude through the lesser omentum. After ruling out extrahepatic disease, the liver was fully mobilized and the suprahepatic cava was surrounded. The sheath of the porta hepatis was opened and a small portal branch that passes from the main portal vein to the caudate lobe was carefully preserved. The hepatic artery and the bile duct of the caudate lobe were also preserved. All vessels of the right lobe (segments V–VIII) and the left lobe (segments II–IV) were divided and ligated prior to the liver parenchymal transaction. The lesser omentum was opened and the caval left hepatic junction was exposed. The common venous trunk of the left hepatic vein and the middle hepatic vein was then divided and ligated. All short ducts draining the caudate were preserved. The right hepatic vein was then isolated, cross-clamped, and divided. During liver parenchymal transection, was performed under intermittent Pringle's maneuver (intermittent inflow occlusion of 15 min clamps time and 5 min unclamped interval) was employed, and liver resection was carried out by a clamp-crushing method. Incision was made between segments I and segments II + III. The liver capsule was incised and vessels of the left lobe encountered were ligated and divided. Great care was taken to protect the short hepatic vein and the left portal branch of the caudate lobe. A second incision was positioned to the right of the caudate lobe (between segments VI + VII + VIII and process portion + paracaval portion). Transection of the liver tissue was performed as close as feasible to the lines of segmental anatomy. It is essential to preserve the portal vein, hepatic artery, and bile duct of the caudate lobe during hepatectomy. One of the major issues is the preservation of inflow and outflow vasculature. The cut surfaces of the liver were managed with a combination of electrocautery and directly covered by greater omentum and hemostatic gauze. Only the hypertrophic caudate lobe was left as the remnant portion. The resected tumors located in the atrophic right and left livers are shown in Fig. 2.
Fig. 2.
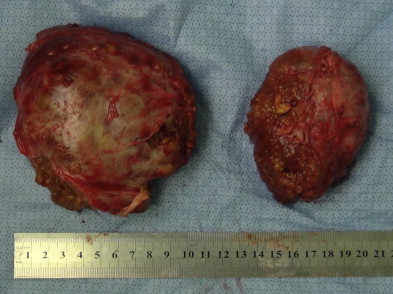
Samples of resection tumor.
The operation lasted for 3 h and the Warm ischemia time was 13 and 8 min. Intra-operative blood loss was 1800 ml and 4 U of RBC and 6 U of fresh plasma were transfused during the operation. A continuous abdominal double-cannula lavage with low negative pressure drainage was utilized for drainage of the abdominal cavity. The abdominal drain was removed on the fifth day after the operation. The patient recovered fully 15 days after the operation and was discharged from the hospital. Pathological examination of the resected tumors revealed HCC (II–III) and mixed nodular cirrhosis. The patient died of recurrence 28 months after the operation.
3. Discussion
The caudate lobe is generally divided into three regions: the left Spiegel lobe (Couinaud's segment I), the process portion (segment X), and the paracaval portion (segment IX).2,3 The caudate lobe extends to the hilum of the liver just posterior to the bifurcation of the portal vein. Cephalad portion of the caudate lobe lies posterior to the confluence of the left and middle veins as they enter the IVC on the left. The caudate lobe receives portal blood flow from both the left and, to a lesser extent, the right portal systems. Venous drainage occurs along its posterior aspect directly into the IVC through multiple small branches of variable size and location (short hepatic veins). Biliary drainage includes small tributaries to the right, but is predominantly through the left hepatic duct; hepatic arterial flow is mainly through a solitary branch from the main left hepatic artery and a second smaller branch from the right posterior sectorial artery.4
Despite the small volume, the caudate lobe has integrated bilateral blood supply system and proliferates easily. The atrophy caused by intrahepatic bile duct stones is always combined with compensatory hyperplasia. Therefore hyperplasia of the caudate lobe is always found in patients with intrahepatic bile duct stones, especially in end-stage patients to maintain basic liver functions.1
It is critical to assess the liver function of residual caudate lobe to avoid liver failure after hepatectomy. The residual liver function evaluation includes ICG5 and measurement of residual liver volume. A remnant liver volume-to-body weight ratio (RLV-BWR) ≥0.8% or the ratio of RLV-to-total liver volume (RLV-TLV) ≥30% is safe in predicting postoperative course after major hepatectomy in normal liver. Patients having RLV-BWR ≤0.5% or RLV-TLV ≤20% are at considerable risk for hepatic dysfunction and postoperative mortality.6–9 Our patient's ICG-15 was of normal range. The MRI scan also indicated the volume of the caudate lobe to be more than 30% of the whole liver (Fig. 3)6,8. In addition, the patient had intrahepatic bile duct stones for an unknown period of time; the hyperplasia present maintained the normal functioning of the caudate lobe. With adequate inflow, outflow and biliary drainage, a case such as this which is based on the caudate remnant alone is possible.
Fig. 3.
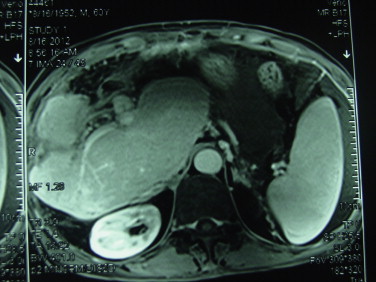
MRI: the caudate lobe showed compensatory hypertrophy after operation.
Resection of HCC with only a preserved caudate lobe is challenging for several reasons. First, from the anatomical standpoint, the caudate lobe was large and deep in the retroperitoneum, thus inhibiting visualization. Second, cirrhosis of the liver may necessitate the need for a larger liver remnant. Third, this anatomic resection requires a thorough knowledge of the anatomy of the liver and a high level of operative experience.
4. Conclusion
Hepatic resection may be performed for a large HCC within the caudate lobe, provided inflow, outflow and biliary drainage are preserved in addition to an adequate remnant. This unique case of the caudate being the sole remnant is the first such case reported to date.
Conflict of interest
None of the authors have any conflict of interest, neither in terms of funding nor of commercial associations.
Funding
None.
Ethical approval
Written consent form can be provided upon editor's request.
Author contributions
Conception and design was done by Wu Mengchao; Li Aijun and Tang Qinhe contributed in the acquisition of data; Li Aijun analysed and interpreted the data; Li Aijun and Yang Jiamei drafted the article; Wu Mengchao provided the final approval.
Key learning points
-
•
First, from the anatomical standpoint, the caudate lobe was large and deep in the retroperitoneum, thus inhibiting visualization.
-
•
Second, cirrhosis of the liver may necessitate a larger liver remnant.
-
•
Third, this anatomic resection requires a thorough knowledge of the anatomy of the liver and a high level of operative experience. Resection of HCC with only a preserved caudate lobe is challenging.
-
•
So, hepatic resection may be performed for a large HCC within the caudate lobe, provided inflow, outflow and biliary drainage are preserved in addition to an adequate remnant. This unique case of the caudate being the sole remnant is the first such case reported to date.
Acknowledgments
Special thanks to Professor Michael G. Sarrh and T. Clark Gamblin for their contribution to this article and for their help and guidance.
References
- 1.Dong J., Lau W.Y., Lu W., Zhang W., Wang J., Ji W. Caudate lobe-sparing subtotal hepatectomy for primary hepatolithiasis. Br J Surg. 2012;99(10):1423–1428. doi: 10.1002/bjs.8888. [DOI] [PubMed] [Google Scholar]
- 2.Kumon M. Anatomy of the caudate lobe with special reference to portal vein and bile duct. Acta Hepatol Jap. 1985;26:1193–1199. [Google Scholar]
- 3.Couinaud C. The paracaval segments of the liver. J Hep Bil Pancr Surg. 1994;2:45–151. [Google Scholar]
- 4.Chaib E., Ribeiro M.A.F., Jr., Collet Silva F.S., Saad W.A., Cecconello I. Surgical approach for hepatic caudate lobectomy: review of 401 cases. J Am Coll Surg. 2007;204:118–127. doi: 10.1016/j.jamcollsurg.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.G., Hwang S. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12(1):38–43. doi: 10.1007/s00534-004-0949-9. [DOI] [PubMed] [Google Scholar]
- 6.Shoup M., Gonen M., D’Angelica M. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7(3):325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 7.Truant S., Oberlin O., Sergent G., Lebuffe G., Gambiez L., Ernst O., Pruvot F.R. Remnant liver volume to body weight ratio > or = 0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204(1):22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmi A., Ruzzenente A., Conci S., Valdegamberi A., Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 9.Lin X.J., Yang J., Chen X.B., Zhang M., Xu M.Q. The critical value of remnant liver volume-to-body weight ratio to estimate posthepatectomy liver failure in cirrhotic patients. J Surg Res. 2014;188(2):489–495. doi: 10.1016/j.jss.2014.01.023. [DOI] [PubMed] [Google Scholar]


