Abstract
Mitochondrial dysfunction is increasingly recognized as an accomplice in most of the common human diseases including cancer, neurodegeneration, diabetes, ischemia/reperfusion injury as seen in myocardial infarction and stroke, and sepsis. Inflammatory conditions, both acute and chronic, have recently been shown to affect mitochondrial function. We here discuss the role of oxidative phosphorylation (OxPhos), focusing on acute inflammatory conditions, in particular sepsis and experimental sepsis models. We discuss mitochondrial alterations, specifically suppression of oxidative metabolism and the role of mitochondrial reactive oxygen species in disease pathology. Several signaling pathways including metabolic, proliferative, and cytokine signaling affect mitochondrial function and appear to be important in inflammatory disease conditions. Cytochrome c oxidase (COX) and cytochrome c, the latter of which plays a central role in apoptosis in addition to mitochondrial respiration, serve as examples for the entire OxPhos system since they have been studied in more detail with respect to cell signaling. We propose a model in which inflammatory signaling leads to changes in the phosphorylation state of mitochondrial proteins, including Tyr304 phosphorylation of COX catalytic subunit I. This results in inhibition of OxPhos, a reduction of the mitochondrial membrane potential, and consequently a lack of energy, which can cause organ failure and death as seen in septic patients.
Keywords: Cell signaling, cytochrome c, cytochrome c oxidase, electron transport chain, haplogroup, inflammation, mitochondria, oxidative phosphorylation, oxygen, reactive oxygen species, sepsis, tyrosine kinase
1. Introduction
Sepsis is an acute systemic condition that is sometimes referred to as blood poisoning in the non-medical literature, but whose definition has changed to reflect the inflammatory response of the body, including the development of multiple organ dysfunction syndrome (MODS) [1]. About 10% of all patients in intensive care units (ICU) have severe sepsis [2] with a higher incidence in blacks than in whites [3]. This life-threatening condition develops in 750,000 people annually in the United States with more than 210,000 deaths, making it the leading cause of mortality in ICU patients [4]. Apparently, the combination of a pathogenic infection with a maladaptive immune response can cause organ dysfunction and eventual death. A major hurdle is the lack of markers that allow early diagnosis and that can predict outcome. A recent review of the literature reports 178 biomarkers including microbiological cultures to identify the pathogen, and markers of inflammation such as C-reactive protein and procalcitonin analyzed from blood [5]. In the future, panels of markers will likely be used to increase sensitivity and specificity, such as multiplexed quantitative PCR [6], which can be easily employed with a fast readout.
A possible explanation for the lack of robust markers is the inherent problem of defining sepsis due to the large heterogeneity of the patient population that presents with acute infection. Sepsis can originate from any site in the body in combination with unspecific responses such as tachycardia and tachypnea all the way to organ dysfunction and failure [7]. Severe sepsis is characterized by acute organ dysfunction, which can develop during the course of an overwhelming infection, and septic shock is severe sepsis in combination with a dangerous drop in systolic blood pressure [8]. Sepsis is mostly caused by bacterial infections in addition to fungal infections originating at various sites in the body. Given the large heterogeneity of sepsis including the different stages, special care is needed when experimental data are interpreted and studies are compared.
To study acute inflammation in animals several models are commonly used, such as i.v. and i.p. injections of LPS (lipopolysaccharide, endotoxin), fecal peritonitis including cecal ligation and puncture (CLP), and injection of live bacteria. Most studies are performed in rats and mice but other animals are commonly used too, such as pigs. It should be noted that animal models used by researchers have certain limitations and sometimes only reflect certain aspects of the condition. For example, researchers often administer large doses of bacteria or endotoxin as a single bolus, whereas in patients there is a gradual increase in pathogen load over time. Therefore, a bolus administration of bacteria or endotoxin to a healthy animal has no clinical correlate [9]. The latter models produce a profound and fast hypodynamic response, a decrease in cardiovascular function and cardiac output leading to death within hours, whereas septic patients show a hyperdynamic response [9]. If lower amounts of endotoxin are administered a short hyperdynamic response can be seen followed by the hypodynamic response [10]. The fecal peritonitis model including the CLP model may better reflect human sepsis due to the temporal worsening along with an extended hyperdynamic cardiovascular response [9]. Differences between models such as bolus administration of LPS or bacteria versus fecal peritonitis may at least in part explain some data in the literature which at first sight seem to be conflicting. In addition, experimental shortcomings to realistically reflect sepsis may explain why there has been relatively little success in the past in translating promising findings from preclinical animal studies into the clinic [7]. These experimental limitations have to be taken into consideration at the planning stage of preclinical studies and when comparing experimental results using different models.
2. Role of oxidative phosphorylation in acute inflammation
Several lines of evidence, genetic and functional, suggest that the mitochondrial OxPhos system is a primary site of action during acute inflammation and that it is a central determinant of clinical outcome. Inflammatory responses are key for the outcome of sepsis and may affect cellular function at a very basic level, i.e., the mitochondrial OxPhos system, whose role in sepsis is still somewhat controversial. Most studies we are aware of reported decreased ATP levels in animal models of sepsis and in human patients [11–17]. For example, a human comparative study found that the average ATP/ADP ratio in muscle tissue was 7.44 in septic survivors versus 4.39 in non-survivors [11]. Using a rat fecal peritonitis model, the same group later reported a drop of ATP and an increase in AMP levels in skeletal muscle and liver tissue starting at 24 hours and 48 hours in liver and muscle tissue, respectively [13]. Applying a single bolus of LPS, the Kozlov lab reported a 70% reduction in liver ATP levels after 8 hours which improved at later time points in surviving animals [14]. However, there are some reports that found no changes in in cellular energy levels [18–21], likely because OxPhos was still able to support cellular function at the time of measurement or because overall metabolism and energy utilization are reduced [22]. Even if changes in cellular energy levels were not detected in the aforementioned studies, metabolic changes did occur including increased lactate production [19, 21], suggesting that increased glycolytic flux can maintain cellular energetics at least for some time.
In addition to differences between animal models and their ability to reflect sepsis as discussed in the previous section, bioenergetic discrepancies between some of the published reports may also be explained, at least in part, by utilization of assay protocols that do not preserve posttranslational modifications, since almost all older protocols for mitochondria isolation or mitochondrial assays do not utilize phosphatase inhibitor cocktails. We have thus modified existing protocols to maintain the physiological phosphorylation state of mitochondrial proteins and described the methods in detail for COX [23] and Cytc [24], which has allowed us to map and study over 10 phosphorylation sites on the two proteins in different animals and tissues [23–30]. Such modifications, in particular cytokine-mediated phosphorylations in the context of sepsis, may be easily lost when enzymes, i.e., phosphatases, that reverse such modifications are not inhibited. Therefore, most studies that involve mitochondria isolation followed by mitochondrial activity measurements have to be interpreted in light of the possible event that protein phosphorylations and perhaps other posttranslational modifications have been lost.
2.1 The energy metabolism crisis model
A recent study conducted by Kingsmore and colleagues analyzed the plasma metabolome (i.e., the composition of metabolites such as amino acids, Krebs cycle intermediates, and acyl-carnitines) and proteome (i.e., the protein complement expressed in cells, tissues, or bodily fluids) of sepsis survivors and non-survivors [31]. The study revealed several interesting findings. First, survivors of sepsis, severe sepsis, and septic shock showed no prominent differences in their metabolome and proteome. Second, there were no major differences among patients infected with three different pathogens, S. pneumoniae, S. aureus, or E. coli. In contrast, there were significant differences between sepsis survivors and non-survivors. For example, nine proteins involved in fatty acid transport were decreased in sepsis non-survivors suggesting a defect in β-oxidation. The non-uptake and non-utilization of fatty acids by the mitochondria led to an accumulation of acyl-carnitines in the plasma, another predictive marker for outcome established by the study. Glycolysis and gluconeogenesis were also markedly different. Sepsis survivors showed decreased levels of citrate, malate, glycerol, glycerol 3-phosphate, phosphate, and glucogenic and ketogenic amino acids whereas non-survivors showed increased levels of citrate, malate, pyruvate, dihydroxyacetone, lactate, phosphate, and gluconeogenic amino acids [31]. This data suggests that non-survivors cannot effectively utilize common metabolites to generate energy through the aerobic mitochondrial pathway. Another study reported that mitochondria are dysfunctional in human skeletal muscle of septic patients, but this effect is not due to altered mitochondrial biogenesis since in vivo protein synthesis and expression of mitochondrial genes is indistinguishable from controls [32]. These studies demonstrate that cellular metabolism is altered and affects several metabolic pathways in sepsis and suggest that metabolic enzyme levels might not be the culprit. In this article, we put forth the proposal that inflammatory signaling suppresses the activity of key metabolic enzymes leading to metabolic dysfunction.
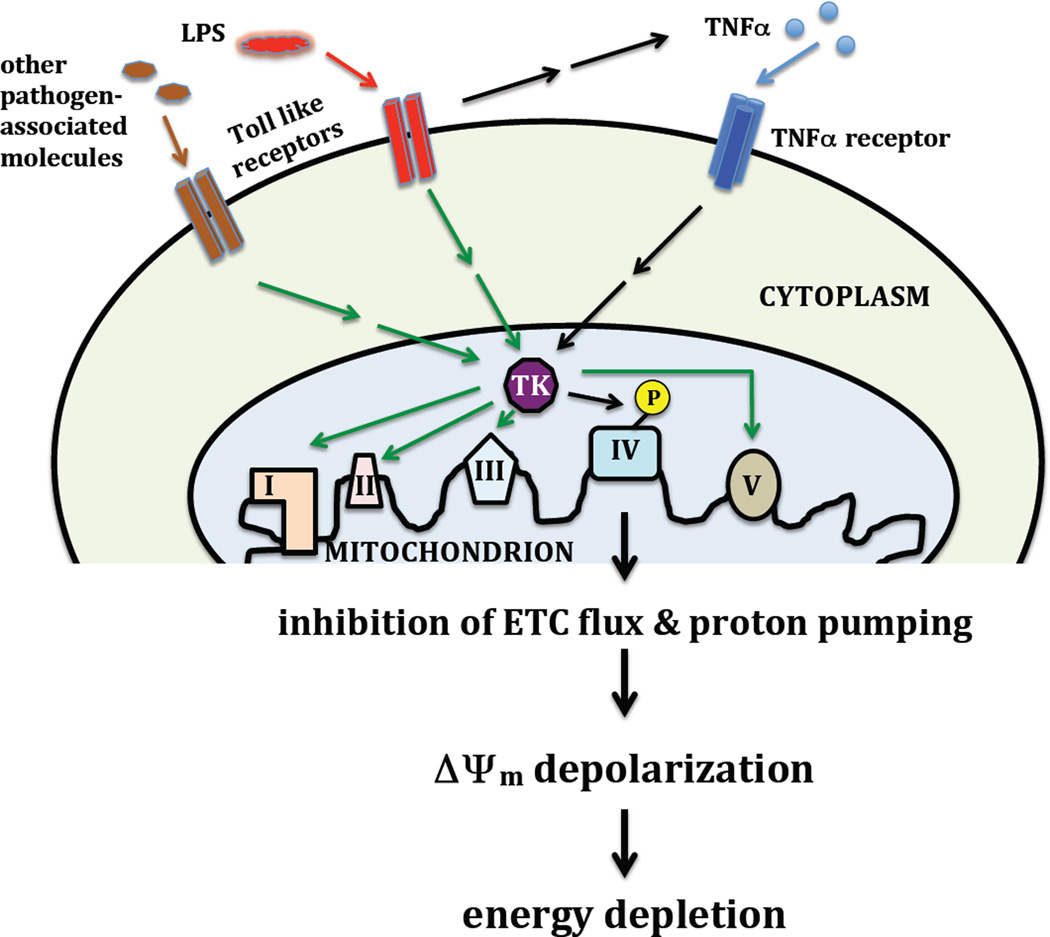
We propose that an important functional target of inflammatory signaling is the mitochondrial OxPhos machinery. It has long been known that direct systemic delivery of oxygen during the course of sepsis does not improve disease outcome in septic patients [33]. Rats subjected to CLP had muscle tissue oxygen levels similar to the sham control and septic animals after 6 hours, but the septic animals had significantly (19%) reduced ATP levels [34]. This suggests that oxygen utilization but not delivery is impaired, a condition referred to as cytopathic hypoxia [35]. This may further suggest that COX as the terminal acceptor of oxygen might be functionally different during the course of sepsis. In this and the following sections, we develop a model (Fig. 1), in which inflammatory signaling leads to inhibition of COX via tyrosine phosphorylation followed by the depolarization of the mitochondrial membrane potential, impaired ATP production and finally energy failure.
Figure 1.
Proposed sequence leading to mitochondrial dysfunction and energy crisis during acute inflammation. Pathogen associated molecules such as LPS are recognized by Toll-like receptors triggering inflammatory responses including the production of TNFα. TNFα leads to activation of an unknown downstream tyrosine kinase (TK) that phosphorylates COX on Tyr304 leading to strong enzyme inhibition, a critical reduction in the mitochondrial membrane potential ΔΨm, and a drop in energy levels in target organs that can lead organ failure and death (black arrows). It remains to be shown if Toll-like receptor signaling can directly activate the downstream TK and if there are OxPhos complexes other than COX that are also targeted for phosphorylation (green arrows).
Together with the other OxPhos complexes, the reaction catalyzed by COX and Cytc generates energy to drive all cellular functions. In addition, Cytc regulates cellular survival and executes apoptosis. It is thus not surprising that both COX and Cytc have been implicated in the pathology of sepsis. In a recent editorial, Dolganiuc concludes that “how and why excessive COX inhibition becomes detrimental and limits the survival during sepsis may be among the top research priorities of this area” [36]. A preliminary answer to this question may already be at hand, via phosphorylation of COX, as we will discuss at the end of this section.
2.2 Genetic evidence: nuclear polymorphisms and the mitochondrial haplogroup as predictive markers
A small number of non-mitochondrial genetic markers for the outcome of sepsis have been proposed. A polymorphism (11367C) identified in the gene of toll-like receptor 4 (TLR4), which is important for the activation of the innate immune response and recognizes LPS, was associated with a better prognosis [37], likely due to a decreased immune response including lower levels of cytokines such as TNFα. The same concept is supported by another study showing that a polymorphism in the TNFα gene, which results in higher circulating TNFα concentrations, is associated with higher rates of MODS [38]. Other examples with various levels of predictive power are polymorphic variants in the 3’-UTR of the human leukocyte antigen G (HLA-G) gene [39], polymorphisms in the leptin and leptin receptor genes [40], a polymorphism in the promoter of the macrophage migration inhibitory factor (MIF) gene [41], the 372 T/C polymorphism of tissue inhibitor of matrix metalloproteinase (TIMP)-1 in which the T allele was associated with 16% higher mortality [42], and the 1595C/T polymorphism in the interleukin-1-receptor-associated kinase 1 (IRAK1), which was associated with the need for prolonged mechanical ventilation and decreased survival [43]. These genetic markers suggest that a dysregulation of the immune response, specifically an overactivation, is associated with decreased survival.
Interestingly, the composition of mitochondrial DNA, the so-called haplogroups have also been suggested as predictors of survival after sepsis. Mitochondrial haplogroups are defined by a relatively small number of mitochondrial DNA polymorphisms that are preserved in various populations, and these subtle changes have been associated with certain phenotypes, such as longevity, male fertility, cardiomyopathy, and neurodegenerative diseases [44–46]. A study conducted in England revealed that Europeans who are haplogroup H carriers have a more than twofold increased chance of survival after sepsis compared to patients with other haplotypes [47]. Thus, mitochondrial haplogroups may behave as susceptibility factors for the outcome of certain diseases including sepsis. Another recent study conducted in Spain showed that the JT haplotype is also associated with higher survival rates and that patients with this haplotype have on average a 14% higher COX activity and 51% increased COX protein levels normalized to citrate synthase activity in platelets at the time of diagnosis [48]. At days 4 and 8 after diagnosis, average COX amount was statistically significantly increased (52% and 49%, respectively). The authors suggested that while COX levels in platelets and thus platelet function is unlikely to be a factor determining survival, it might reflect differences in mitochondrial function in other tissues and organs [48]. It is not fully clear at this point what functional and mechanistic effects different haplogroups have and how they relate to improved survival. However, there are studies that have concluded that there are changes in mitochondrial function depending on the haplogroup. For example, using cybrid methodology, which allows studying mitochondria derived from different haplogroups in the same cellular (i.e., nuclear DNA) background, Gomez-Duran and colleagues showed increased mitochondrial membrane potential and cytochrome c oxidase (COX) activity in H versus Uk haplogroup mitochondria [49]. It is therefore possible that COX activity and amount could serve as a predictive blood-based marker for the outcome of sepsis. Recent studies in C57BL/6 and C3H/HeN mice strains and strains generated by replacing the endogenous mitochondria with the mitochondria of the other strain indicated that the mitochondrial haplogroup determines state 3 respiration rates of isolated cardiac mitochondria as well as basal membrane potential and ROS levels [50]. It is unclear, however, how important ROS are as a determinant of outcome after acute inflammation versus ATP, as discussed in section 3.
2.3 Oxidative phosphorylation - temporal changes during acute inflammation
Acute inflammation causes both short-term and longer-term effects and adaptations at the level of OxPhos. Crouser and colleagues observed a 40% reduction of COX activity in cats 4 h after LPS administration in combination with partial uncoupling of mitochondrial OxPhos [51]. Another study found that electron transport chain (ETC) complexes I, II, and COX were down-regulated in the diaphragm within 24 h after LPS administration in rats, both at the mRNA and protein level, and state 3 respiration declined by 48% [52]. Proteomic analysis of cat liver mitochondria 4 h after LPS administration revealed 14 proteins that showed differences in protein levels. Among them was one OxPhos member, ATP synthase α subunit, which was 41% reduced in the septic animals [53]. Early during septic shock using the CLP model, rat liver mitochondria showed significantly reduced mitochondrial respiration rates and reduced COX levels [54]. Interestingly, Lu and colleagues observed significantly increased and decreased mitochondrial ATP synthase activity in rats after applying 5 mg/kg LPS at the early and late stages of endotoxic shock, respectively [55]. These findings may be explained by the temporal development in this model, with a hyperdynamic followed by a hypodynamic response, utilizing a relatively low LPS bolus. At the level of COX, early in sepsis after CLP, oxidation of Cytc by COX was competitively and reversibly inhibited in the hearts of mice, whereas at a later time point (48 h), it became noncompetitive and irreversible [22]. At that time point animals with fulminant sepsis showed about 38% reduced Vmax of COX measured spectrophotometrically. After LPS injection rats showed a time-dependent increase of Cytc in the cytosolic fractions in the heart along with increased apoptotic markers [56, 57], suggesting that Cytc release from the mitochondria and the execution of apoptosis become more prevalent in the late stage of sepsis in the animal model. In the brain of septic rats after CLP, certain cell types, such as the hippocampal CA1 region, the choroid plexus, and Purkinje cells of the cerebellum, showed increased susceptibility levels to the induction of apoptosis [58]. The authors found that among the apoptotic markers tested, Cytc release was the only one with prognostic power. It is important to note, however, that the finding of increased apoptosis as observed in several animal studies might be a shortcoming of the models, since apoptosis does not seem to be the key determinant in patients with sepsis. In contrast to organs such as the heart or brain shown to be affected in the animal models, only a few cell types including lymphocytes and gastrointestinal epithelial cells die through an apoptotic process in septic patients [59]. In lymphocytes, the release of Cytc from the mitochondria would trigger apoptosis and also augment OxPhos dysfunction due to interruption of electron flux in the ETC. This may contribute to a failure of the immune system since prevention of lymphocyte apoptosis was shown to improve survival [59]. Interestingly, the Levy group was able to restore mitochondrial respiration in the hearts of mice subjected to cecal ligation and puncture by i.v.-injection of cow heart Cytc [60, 61], apparently without triggering apoptosis. The treatment led to an uptake of Cytc into the cardiomyocytes, and survival increased from 15% for the sepsis control group to about 50% in mice that were also injected with Cytc. The uptake might be facilitated by an interesting feature of Cytc; it contains a cell-penetrating peptide sequence located in the C-terminus of the protein [62], enabling it to cross cellular membranes in a non-traditional fashion.
Taken together, these studies suggest that mitochondrial dysfunction via a decrease of OxPhos function and an increase in apoptotic activity in selected cell types may contribute to the septic phenotype. The role of inflammatory signaling discussed below and temporal changes thereof of may be important to understand the different phases during the course of sepsis and the point of no return when mitochondrial failure cannot be reversed any longer, leading to death.
2.4 Tyrosine 304 phosphorylation on cytochrome c oxidase subunit I as a metabolic switch to strongly inhibit OxPhos in acute inflammation
LPS administration in experimental sepsis leads to a TLR4-mediated burst of pro-inflammatory cytokines, including tumor necrosis factor α (TNFα). In a rat model of endotoxic shock TNFα expression peaked early after LPS injection and led to a 70% decrease in cellular ATP levels [14] (Fig. 1). In septic patients plasma levels of TNFα were significantly higher in patients that died compared to the surviving group [63], making TNFα an interesting target for therapy. Neutralizing it with an antibody fragment against TNFα in experimental sepsis resulted in significantly increased survival rates [64]. However, a similar treatment only slightly improved survival in patients with sepsis [65], suggesting that there is redundancy in inflammatory signaling. Nevertheless, TNFα alone is sufficient to induce metabolic changes similar to those seen in sepsis. For example, TNFα induces the generation of lactate in vitro and in vivo [66, 67], pointing to an impairment of aerobic energy metabolism.
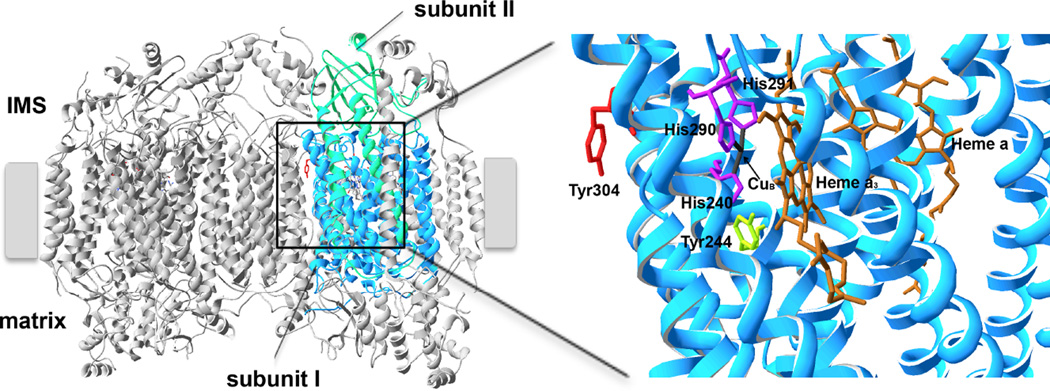
In order to investigate the effect of inflammatory signaling at a more mechanistic level we analyzed the effect of TNFα on liver tissue from cow and mouse as well as mouse hepatocytes in culture. TNFα treatment of liver tissue homogenates resulted in a fast (within 5 min) 60% reduction of COX activity [68]. Purification of cow COX after TNFα treatment, using protocols that preserve phosphorylations, following Western analysis suggested tyrosine phosphorylation on catalytic subunit I. Further analysis with a phospho-epitope-specific antibody revealed phosphorylation of tyrosine 304, the same site that we earlier mapped by mass spectrometry after activating the cAMP-dependent pathway in liver [25]. In cultured mouse H2.35 hepatocytes TNFα treatment for 5 min had a profound diminishing effect on the mitochondrial membrane potential and resulted in a 64% reduction of cellular ATP levels [68] (Fig. 1). Tyr304 is located on helix VIII of catalytic subunit I, close to the intermembrane space (Fig. 2). Helix VIII is in contact with the heme a3-CuB reaction center where oxygen is reduced to water (Fig. 2, right), an ideal site for COX regulation. Tyr304 and the surrounding epitope are highly conserved in eukaryotes [25]. Interestingly, the COX substrate Cytc has a similar epitope surrounding Tyr97 with 5 out of 10 amino acids being identical and this site is phosphorylated in heart tissue under normal conditions, although it is unclear to what extent [26]. Because Cytc Tyr97 phosphorylation leads to a shift of the Km of COX for Cytc from 2.5 µM for dephosphorylated Cytc to 5.5 µM for phosphorylated Cytc, and because the epitopes in COX and Cytc are similar, TNFα-mediated phosphorylation of Cytc would augment the effect of COX Tyr304 phosphorylation, a concept that we will explore in future work.
Figure 2.
TNFα leads to phosphorylation of Tyr304 of COX catalytic subunit I. Crystal structure data of cow heart COX [119] were used and processed with the program Swiss-PDBViewer 4.1. Left, overview of COX with catalytic subunits I and II highlighted in blue and green, respectively (IMS, intermembrane space). Tyr 304, which is phosphorylated in a TNFα-dependent manner, is highlighted in red. Right, higher magnification of the catalytic center in subunit I showing the close proximity of Tyr304 with the binuclear CuB-heme a3 oxygen binding site. Histidines 240, 290, and 291, which bind CuB and Tyr244, which is involved in the catalytic cycle of the enzyme, are shown in sticks.
It should be noted that OxPhos components other than COX (and perhaps Cytc) may also be affected in a similar way by posttranslational modifications. For example, decreased activity has been reported in several animal sepsis models as well as in patients for one or more of the other ETC complexes [13, 16, 69–73], but the molecular mechanism still has to be elucidated. Over 200 phosphorylation sites have been mapped on OxPhos complexes but for almost all of them the functional effects and the signaling pathways involved, including kinases and phosphatases, remain unknown (reviewed in [74]).
Other proposals to explain reduced OxPhos activity exist. For example, one study reported selective degradation of some subunits of the OxPhos complexes I, III, IV, and V in the diaphragm after experimental sepsis using 2D blue native gel electrophoresis [75]. However, the authors also reported strong upregulation of COX subunit IV. Given the usually assumed 1:1 stoichiometry of the OxPhos subunits, additional follow-up experiments including direct Western blotting after 1D SDS PAGE would be needed to strengthen or disprove such a model. Later, using the same experimental approach, the authors reported downregulation of all OxPhos subunits analyzed 48 h after LPS administration [52]. More recently and in agreement with the model put forth in this review, the authors favored a mechanism involving cell signaling and identified p38 mitogen activated protein kinase and double-stranded RNA-dependent protein kinase as kinases that determine the extent of the execution of apoptosis [76, 77]. They also showed that the stress enzyme Jun N-terminal kinase (JNK) was activated in the LPS sepsis model of diaphragmatic dysfunction, and that its pharmacologic inhibition prevented caspase 8 activation and diaphragm weakness in septic mice [78]. Under conditions of stress, activated JNK translocates to the mitochondria, inhibits mitochondrial respiration, and can induce apoptosis via a permeability transition pore dependent or independent mechanism, resulting in the release of proapoptotic factors from the mitochondria such as Cytc [79, 80]. As expected, suppression of JNK translocation to the mitochondria protects mitochondrial integrity and function [81]. It was shown that JNK translocates to the mitochondrial outer membrane where it phosphorylates pyruvate dehydrogenase, leading to enzyme inhibition and thus limited substrate delivery for the Krebs cycle and OxPhos [82]. It is possible that JNK activates other kinases within the mitochondria, such as the as-of-yet unknown tyrosine kinase that phosphorylates COX subunit I Tyr304 (Fig. 1).
In addition to kinases that act on mitochondrial enzymes, protein phosphatases are equally interesting therapeutic targets. For example, in a rat sepsis heart failure model it was shown that LPS administration leads to protein phosphatase 2A activation and a concomitant reduction of mitochondrial calcium retention capacity [83]. PP2A is an interesting phosphatase candidate to dephosphorylate and thus regulate mitochondrial proteins because it localizes to the mitochondria in many tissues, including the heart and brain [84]. PP2A is a multifunctional heterotrimeric serine/threonine protein phosphatase composed of a scaffolding A subunit, a catalytic C subunit, and a regulatory B subunit, the latter of which is expressed as several distinct isoforms. For example, among B subunit isoforms, the brain-specific splice isoform Bβ2, upon neurotoxic stress, translocates from the cytosol to the outer mitochondrial membrane, triggered by phosphorylation of three N-terminal residues [85], to induce mitochondrial fission and to execute apoptosis [86], a process which is accompanied by increased ROS production [87]. Mitochondrial PP2A also provides a direct link to autophagy, which is induced in several disorders including neurodegenerative disease due to mitochondrial dysfunction or cellular stress and can further enhance cellular demise [88]. The protein may also localize to the inner mitochondrial compartments including the intermembrane space and the matrix as predicted by the localization prediction tool Psort II [89]. If so, due to its broad specificity, it could also act on OxPhos.
Taken together there is clear evidence that OxPhos is targeted by cell signaling pathways. However, depending on the animal model and experimental procedures utilized there are some conflicting data regarding OxPhos activity and enzyme levels. Both enzyme activities of individual complexes and reported protein levels may be affected by phosphorylations. For example, in the latter case it is possible that the epitope(s) recognized by antibodies are masked by phosphorylation, preventing antibody binding and thus erroneously suggesting changes in protein amount. Although it is possible that protein degradation is increased during sepsis, in general, under normal conditions mitochondrial protein turnover is slow, with a half life of about 17 and 4 days averaged across several COX subunits in mouse heart and liver, respectively (calculated from [90]). In patients with sepsis where the condition develops over longer periods of time mitochondrial protein degradation may be a more important contributing factor to mitochondrial dysfunction compared to animal studies in which protein levels are analyzed after a few hours. In any case, it might be worth revisiting protein levels in the various models by comparing results using mitochondrial isolation buffers which lack and contain phosphatase inhibitors, respectively.
3. Reactive oxygen species
Increased levels of reactive oxygen species and (ROS) have long been implicated in sepsis [72, 91, 92]. ROS cause tissue damage and they can also trigger apoptosis. Therefore, antioxidants and radical scavengers have been proposed as a possible therapy and have shown some efficacy in sepsis models [71, 93–95]. More recently, ROS scavengers especially targeted to the mitochondria, such as MitoQ, SkQ1, MitoE, and Tempol conjugates, which accumulate in the mitochondria manifold raising their effective concentration at the sub-cellular sites needed, were proposed as a novel strategy (reviewed in [96]). In addition to a direct protective effect by detoxifying ROS, scavengers may also affect the communication between immune cells. For example, SkQ1, a mitochondria targeted plastoquinone derivative developed by the Skulachev lab [97], affects levels of certain immune cells, including CD8(+) T cells, naïve T cells, and memory T cells [98]. However, the compound still has to be tested in a sepsis animal model.
Mitochondria-generated ROS may serve as signaling molecules to communicate with the other cellular compartments. Complex III may play a key role in this communication process because it generates ROS, which are released into the mitochondrial intermembrane space and thus the cytosol, usually at high mitochondrial membrane potentials or in the presence of complex III inhibitors [99]. It is possible, however, that different types of immune cells respond differently to ROS. For example, it was recently shown that complex III-generated ROS are required for T cell activation [100]. In contrast, a study using in vitro culture of neutrophils in the presence of LPS and inhibitors of complex III concluded that increased ROS levels inhibit inflammatory responses required for production of cytokines such as TNFα and macrophage inflammatory protein 2 (MIP-2) [101]. Another important source of mitochondrial ROS is via the p66shc pathway [102]. p66shc is intimately linked to OxPhos because it accepts electrons from Cytc and transfers them to oxygen, generating superoxide. It is activated by phosphorylation and was strongly induced in rodent models of burn trauma and sepsis [103]. Since Cytc is also targeted and regulated by phosphorylation [24, 26, 30, 104, 105] it is possible that changes in the phosphorylation state of Cytc during sepsis may affect its interaction with p66shc. Finally, in addition to mitochondrial ROS production, NADPH oxidase-dependent ROS generation may significantly contribute to the total ROS load during sepsis and could be therapeutically targeted [106]. Although ROS are clearly part of the septic sequence their specific role and importance relative to the energy crisis component has to be further examined.
4. Conclusion
Our proposed model emphasizes the energetic aspect during acute inflammation. We propose that inflammatory signaling leads to phosphorylation of COX and other mitochondrial targets followed by suppression of mitochondrial function, reduced mitochondrial membrane potentials, and energy failure (Fig. 1). Inhibition of mitochondrial energy metabolism during sepsis and its life-threatening consequences may, at first sight, not seem reasonable. However, such a systemic condition is very rare in comparison to daily events that generate small wounds and areas of inflammation locally. Here, containing the affected area by locally inhibiting mitochondrial function makes sense because some pathogens seize the host energetic infrastructure and energy production system. For example, chlamydiae express a number of nucleotide transporters facilitating the acquisition of molecules such as ATP [107]. As a result, inhibiting mitochondria locally at the site of infection might counteract pathogenic growth because important metabolites are no longer generated by the host. However, in the rare condition of a systemic inflammatory response, this response, i.e., the inhibition of mitochondria, might lead to energy depletion, MODS, and death.
Future therapeutic approaches should target OxPhos and could be combined with strategies already in place that show some efficacy. Several mitochondria-targeted therapies have been tested including treatment with mitochondrial substrates (e.g., carnitine, succinate, MgCl2-ATP), cofactors (e.g., coenzyme Q, α-lipoic acid), antioxidants and ROS scavengers (e.g., MitoQ, SkQ, phenyl-tert-butylnitrone, N-acetylcysteine, Tempol), and membrane stabilizers (e.g., cyclosporine A, melatonin), which restore mitochondrial function to some extent (reviewed in [108]). However, interfering with inflammatory signaling at the level of the mitochondria might result in better outcomes. For example, the synthetic cortisol homolog dexamethasone, which affects several signaling pathways including Wnt/β-cateninin, NFκB, MAPK/Erk, and PI3K signaling, was shown to partially rescue COX function 24 h after cecal ligation and puncture in the cortex and outer stripe of the outer medulla of mouse kidneys [109]. Additional signaling pathways have been shown to target COX (reviewed in [110]), which might be explored to boost aerobic activity. Among those, non-receptor tyrosine kinase Src and protein kinase Cε (PKCε) have been identified as positive regulators of COX. In addition to its primary localization in the cytosol Src was found in the mitochondrial intermembrane space [111]. Here it was shown to phosphorylate COX catalytic subunit II in osteoblasts on a yet-to-be identified residue leading to enzyme activation [112]. Treatment of rat neonatal cardiac myocytes with PKC activators diacylglycerol or 4β-phorbol 12-myristate 13-acetate (4β-PMA) resulted in the phosphorylation of an 18 kDa protein and a two- to fourfold increase in COX activity [113, 114]. It was further shown that PKCε co-immunoprecipitated with COX and that the 18 kDa band contained COX subunit IV as identified by mass spectrometry although the precise phosphorylation site still has to be identified. A third kinase that leads to COX activation is matrix-localized carbon dioxide/bicarbonate-regulated adenylyl cyclase which phosphorylates COX subunits I and IV [115]. There is also evidence that protein tyrosine phosphatase Shp2, which is part of the Ras pathway, directly or indirectly activates COX. Similarly to Src, Shp2 also localizes to the mitochondrial intermembrane space [116]. In mouse cell lines with mutations in the Shp-2 encoding gene PTPN11 leading to constitutively active Shp2 [117] and in human lymphoblasts with activating mutations we found about 74% and 63% increased COX activities, respectively [118].
In conclusion, a better understanding of the multifaceted regulation of OxPhos might provide strategies in the future to activate aerobic energy metabolism during acute inflammation by selectively targeting downstream kinases and phosphatases that regulate the activity of the OxPhos complexes including the examples discussed above for COX. An important goal should be the identification of kinases and phosphatases that directly act on OxPhos during acute inflammation, which might allow direct control of OxPhos activity and restoration of its functionality to levels allowing cell survival and maintenance of cellular functions during sepsis in order to decrease mortality.
Highlights.
-
-
Acute inflammation as in sepsis is the leading cause of mortality in intensive care units.
-
-
In sepsis oxygen utilization by the mitochondria is dysfunctional, but not oxygen delivery.
-
-
Mitochondrial DNA composition and cytochrome c oxidase (COX) activity are predictors for outcome.
-
-
In our model, inflammatory signaling targets COX, and causes a strong inhibition of OxPhos and an energy crisis.
-
-
We propose targeting of OxPhos as a future therapy to improve patient outcome.
Acknowledgements
We thank Dr. Jeffrey Doan for comments of the manuscript. This work was supported by a grant from the National Institutes of Health (GM089900), the Center for Molecular Medicine and Genetics, and the Cardiovascular Research Institute, Wayne State University School of Medicine, Detroit.
Abbreviations
- Cytc
Cytochrome c
- COX
cytochrome c oxidase
- ETC
electron transport chain
- EGFR
epidermal growth factor receptor
- ICU
intensive care units
- JNK
Jun N-terminal kinase
- LPS
lipopolysaccharide
- mtDNA
mitochondrial DNA
- MODS
multiple organ dysfunction syndrome
- OxPhos
oxidative phosphorylation
- PP2A
protein phosphatase 2A
- ROS
reactive oxygen species
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
References
- 1.Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin. 2010;26:567–575. doi: 10.1016/j.ccc.2010.04.007. x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care. 2004;8:222–226. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dick A, Liu H, Zwanziger J, Perencevich E, Furuya EY, Larson E, Pogorzelska-Maziarz M, Stone PW. Long-term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432. doi: 10.1186/1472-6963-12-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Sankar V, Webster NR. Clinical application of sepsis biomarkers. J Anesth. 2013;27:269–283. doi: 10.1007/s00540-012-1502-7. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Reinhart K. Molecular diagnostics of sepsis--where are we today? Int J Med Microbiol. 2010;300:411–413. doi: 10.1016/j.ijmm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. Preclinical models of shock and sepsis: what can they tell us? Shock. 2005;24(Suppl 1):1–6. doi: 10.1097/01.shk.0000191383.34066.4b. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- 11.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 12.Omachi A, Sharma AC, Alden KJ, Sam AD, Ferguson JL. Induction of peritoneal sepsis increases the susceptibility of isolated hearts to a calcium paradox-mediated injury. Shock. 2002;17:193–198. doi: 10.1097/00024382-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–R497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 14.Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Hüttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest. 2008;88:70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- 15.Regueira T, Djafarzadeh S, Brandt S, Gorrasi J, Borotto E, Porta F, Takala J, Bracht H, Shaw S, Lepper PM, Jakob SM. Oxygen transport and mitochondrial function in porcine septic shock, cardiogenic shock, and hypoxaemia. Acta Anaesthesiol Scand. 2012;56:846–859. doi: 10.1111/j.1399-6576.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 16.Correa TD, Vuda M, Blaser AR, Takala J, Djafarzadeh S, Dunser MW, Silva E, Lensch M, Wilkens L, Jakob SM. Effect of treatment delay on disease severity and need for resuscitation in porcine fecal peritonitis. Crit Care Med. 2012;40:2841–2849. doi: 10.1097/CCM.0b013e31825b916b. [DOI] [PubMed] [Google Scholar]
- 17.Patil NK, Parajuli N, Macmillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Song SK, Neil JJ, Chen RD, Manchester JK, Karl IE, Lowry OH, Ackerman JJ. Sepsis does not impair tricarboxylic acid cycle in the heart. Am J Physiol. 1991;260:C50–C57. doi: 10.1152/ajpcell.1991.260.1.C50. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Karl IE. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA. 1992;267:1503–1510. [PubMed] [Google Scholar]
- 20.Solomon MA, Correa R, Alexander HR, Koev LA, Cobb JP, Kim DK, Roberts WC, Quezado ZM, Scholz TD, Cunnion RE, et al. Myocardial energy metabolism and morphology in a canine model of sepsis. Am J Physiol. 1994;266:H757–H768. doi: 10.1152/ajpheart.1994.266.2.H757. [DOI] [PubMed] [Google Scholar]
- 21.Levy B, Mansart A, Bollaert PE, Franck P, Mallie JP. Effects of epinephrine and norepinephrine on hemodynamics, oxidative metabolism, and organ energetics in endotoxemic rats. Intensive Care Med. 2003;29:292–300. doi: 10.1007/s00134-002-1611-0. [DOI] [PubMed] [Google Scholar]
- 22.Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome c oxidase in sepsis. Shock. 2004;21:110–114. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- 23.Lee I, Salomon AR, Yu K, Samavati L, Pecina P, Pecinova A, Hüttemann M. Isolation of regulatory-competent, phosphorylated cytochrome c oxidase. Methods Enzymol. 2009;457:193–210. doi: 10.1016/S0076-6879(09)05011-3. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Lee I, Salomon AR, Yu K, Hüttemann M. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim Biophys Acta. 2008;1777:1066–1071. doi: 10.1016/j.bbabio.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 26.Lee I, Salomon AR, Yu K, Doan JW, Grossman LI, Hüttemann M. New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry. 2006;45:9121–9128. doi: 10.1021/bi060585v. [DOI] [PubMed] [Google Scholar]
- 27.Helling S, Hüttemann M, Kadenbach B, Ramzan R, Vogt S, Marcus K. Discovering the phosphoproteome of the hydrophobic cytochrome c oxidase membrane protein complex. Methods Mol Biol. 2012;893:345–358. doi: 10.1007/978-1-61779-885-6_21. [DOI] [PubMed] [Google Scholar]
- 28.Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012;1817:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helling S, Hüttemann M, Ramzan R, Kim SH, Lee I, Muller T, Langenfeld E, Meyer HE, Kadenbach B, Vogt S, Marcus K. Multiple phosphorylations of cytochrome c oxidase and their functions. Proteomics. 2012;12:950–959. doi: 10.1002/pmic.201100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson TH, Mahapatra G, Pecina P, Ji Q, Yu K, Sinkler C, Varughese A, Kumar R, Bukowski MJ, Tousignant RN, Salomon AR, Lee I, Hüttemann M. Cytochrome c is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS One. 2013;8:e78627. doi: 10.1371/journal.pone.0078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langley RJ, Tsalik EL, Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, Freeman DH, Wang M, You J, Wulff J, Thompson JW, Moseley MA, Reisinger S, Edmonds BT, Grinnell B, Nelson DR, Dinwiddie DL, Miller NA, Saunders CJ, Soden SS, Rogers AJ, Gazourian L, Fredenburgh LE, Massaro AF, Baron RM, Choi AM, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG, Jr, Rivers EP, Woods CW, Kingsmore SF. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013;5:195ra195. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredriksson K, Tjader I, Keller P, Petrovic N, Ahlman B, Scheele C, Wernerman J, Timmons JA, Rooyackers O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One. 2008;3:e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. New Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 34.Astiz M, Rackow EC, Weil MH, Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock. 1988;26:311–320. [PubMed] [Google Scholar]
- 35.Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolganiuc A. Cytochrome c oxidase predicts the toll of sepsis. Crit Care Med. 2011;39:1552–1554. doi: 10.1097/CCM.0b013e3182148a24. [DOI] [PubMed] [Google Scholar]
- 37.Duan ZX, Gu W, Zhang LY, Du DY, Hu P, Huang J, Liu Q, Wang ZG, Hao J, Jiang JX. Clinical relevance of the TLR4 11367 polymorphism in patients with major trauma. Arch Surg. 2009;144:1144–1148. doi: 10.1001/archsurg.2009.211. [DOI] [PubMed] [Google Scholar]
- 38.Stuber F, Petersen M, Bokelmann F, Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-alpha concentrations and outcome of patients with severe sepsis. Crit Care Med. 1996;24:381–384. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Graebin P, Veit TD, Alho CS, Dias FS, Chies JA. Polymorphic variants in exon 8 at the 3' UTR of the HLA-G gene are associated with septic shock in critically ill patients. Crit Care. 2012;16:R211. doi: 10.1186/cc11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bracho-Riquelme RL, Loera-Castaneda V, Torres-Valenzuela A, Loera-Castaneda GA, Sanchez-Ramirez JP. Leptin and leptin receptor polymorphisms are associated with poor outcome (death) in patients with non-appendicular secondary peritonitis. Crit Care. 2011;15:R227. doi: 10.1186/cc10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann LE, Book M, Hartmann W, Weber SU, Schewe JC, Klaschik S, Hoeft A, Stuber F. A MIF haplotype is associated with the outcome of patients with severe sepsis: a case control study. J Transl Med. 2009;7:100. doi: 10.1186/1479-5876-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorente L, Martin M, Plasencia F, Sole-Violan J, Blanquer J, Labarta L, Diaz C, Borreguero-Leon JM, Jimenez A, Paramo JA, Orbe J, Rodriguez JA, Salido E. The 372 T/C genetic polymorphism of TIMP-1 is associated with serum levels of TIMP-1 and survival in patients with severe sepsis. Crit Care. 2013;17:R94. doi: 10.1186/cc12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toubiana J, Courtine E, Pene F, Viallon V, Asfar P, Daubin C, Rousseau C, Chenot C, Ouaaz F, Grimaldi D, Cariou A, Chiche JD, Mira JP. IRAK1 functional genetic variant affects severity of septic shock. Crit Care Med. 2010;38:2287–2294. doi: 10.1097/CCM.0b013e3181f9f9c7. [DOI] [PubMed] [Google Scholar]
- 44.Herrnstadt C, Howell N. An evolutionary perspective on pathogenic mtDNA mutations: haplogroup associations of clinical disorders. Mitochondrion. 2004;4:791–798. doi: 10.1016/j.mito.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Zaragoza MV, Brandon MC, Diegoli M, Arbustini E, Wallace DC. Mitochondrial cardiomyopathies: how to identify candidate pathogenic mutations by mitochondrial DNA sequencing, MITOMASTER and phylogeny. Eur J Hum Genet. 2011;19:200–207. doi: 10.1038/ejhg.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baudouin SV, Saunders D, Tiangyou W, Elson JL, Poynter J, Pyle A, Keers S, Turnbull DM, Howell N, Chinnery PF. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366:2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]
- 48.Lorente L, Martin MM, Lopez-Gallardo E, Iceta R, Sole-Violan J, Blanquer J, Labarta L, Diaz C, Jimenez A, Lafuente N, Hernandez M, Mendez F, Medina N, Ferrer-Aguero JM, Ferreres J, MC LL, Mora ML, Lubillo S, Sanchez-Palacios M, Montoya J, Ruiz-Pesini E. Platelet cytochrome c oxidase activity and quantity in septic patients. Crit Care Med. 2011;39:1289–1294. doi: 10.1097/CCM.0b013e31820ee20c. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 50.Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, Sammy MJ, Johnson M, Dunham-Snary KJ, Cao X, Bradley WE, Zhang J, Wei CC, Chacko B, Schurr TG, Kesterson RA, Dell'italia LJ, Darley-Usmar VM, Welch DR, Ballinger SW. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume - overload. Biochem J. 2013;455:157–167. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med. 2002;30:276–284. doi: 10.1097/00003246-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Callahan LA, Supinski GS. Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol. 2005;99:1120–1126. doi: 10.1152/japplphysiol.01157.2004. [DOI] [PubMed] [Google Scholar]
- 53.Crouser ED, Julian MW, Huff JE, Mandich DV, Green-Church KB. A proteomic analysis of liver mitochondria during acute endotoxemia, Intensive. Care Med. 2006;32:1252–1262. doi: 10.1007/s00134-006-0224-4. [DOI] [PubMed] [Google Scholar]
- 54.Eyenga P, Roussel D, Morel J, Rey B, Romestaing C, Teulier L, Sheu SS, Goudable J, Negrier C, Viale JP. Early septic shock induces loss of oxidative phosphorylation yield plasticity in liver mitochondria. J Physiol Biochem. 2014 doi: 10.1007/s13105-013-0280-5. [DOI] [PubMed] [Google Scholar]
- 55.Lu SM, Song SM, Liu JC, Yang HM, Li P, Wang ZG. Changes of proton transportation across the inner mitochondrial membrane and H(+)-ATPase in endotoxic shock rats. Chin J Traumatol. 2003;6:292–296. [PubMed] [Google Scholar]
- 56.Li L, Hu BC, Chen CQ, Gong SJ, Yu YH, Dai HW, Yan J. Role of mitochondrial damage during cardiac apoptosis in septic rats. Chin Med J (Engl) 2013;126:1860–1866. [PubMed] [Google Scholar]
- 57.Chopra M, Sharma AC. Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction. Life Sci. 2007;81:306–316. doi: 10.1016/j.lfs.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messaris E, Memos N, Chatzigianni E, Konstadoulakis MM, Menenakos E, Katsaragakis S, Voumvourakis C, Androulakis G. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med. 2004;32:1764–1770. doi: 10.1097/01.ccm.0000135744.30137.b4. [DOI] [PubMed] [Google Scholar]
- 59.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 60.Piel DA, Deutschman CS, Levy RJ. Exogenous cytochrome C restores myocardial cytochrome oxidase activity into the late phase of sepsis. Shock. 2008;29:612–616. doi: 10.1097/SHK.0b013e318157e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piel DA, Gruber PJ, Weinheimer CJ, Courtois MR, Robertson CM, Coopersmith CM, Deutschman CS, Levy RJ. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit Care Med. 2007;35:2120–2127. doi: 10.1097/01.ccm.0000278914.85340.fe. [DOI] [PubMed] [Google Scholar]
- 62.Jones S, Holm T, Mager I, Langel U, Howl J. Characterization of bioactive cell penetrating peptides from human cytochrome c: protein mimicry and the development of a novel apoptogenic agent. Chem Biol. 2010;17:735–744. doi: 10.1016/j.chembiol.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Takakuwa T, Endo S, Nakae H, Kikichi M, Suzuki T, Inada K, Yoshida M. Plasma levels of TNF-alpha, endothelin-1 and thrombomodulin in patients with sepsis. Res Commun Chem Pathol Pharmacol. 1994;84:261–269. [PubMed] [Google Scholar]
- 64.Newham P, Ross D, Ceuppens P, Das S, Yates JW, Betts C, Reens J, Randall KJ, Knight R, McKay JS. Determination of the safety and efficacy of therapeutic neutralization of tumor necrosis factor-alpha (TNF-alpha) using AZD9773, an anti-TNF-alpha immune Fab, in murine CLP sepsis. Inflamm Res. 2013 doi: 10.1007/s00011-013-0683-3. [DOI] [PubMed] [Google Scholar]
- 65.Rondon E, Venkataraman R. Afelimomab led to a modest mortality benefit in patients with severe sepsis and elevated interleukin-6 levels. Crit Care. 2005;9:E20. doi: 10.1186/cc3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MD, Zentella A, Vine W, Pekala PH, Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987;84:2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tracey KJ, Lowry SF, Fahey TJ, Albert JD, Fong Y, Hesse D, Beutler B, Manogue KR, Calvano S, Wei H, Cerami A, Shires GT. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164:415–422. [PubMed] [Google Scholar]
- 68.Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gellerich FN, Trumbeckaite S, Hertel K, Zierz S, Muller-Werdan U, Werdan K, Redl H, Schlag G. Impaired energy metabolism in hearts of septic baboons: diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock. 1999;11:336–341. [PubMed] [Google Scholar]
- 70.Li CM, Chen JH, Zhang P, He Q, Yuan J, Chen RJ, Cheng XJ, Tan HZ, Yang Y. Continuous veno-venous haemofiltration attenuates myocardial mitochondrial respiratory chain complexes activity in porcine septic shock. Anaesth Intensive Care. 2007;35:911–919. doi: 10.1177/0310057X0703500609. [DOI] [PubMed] [Google Scholar]
- 71.Choumar A, Tarhuni A, Letteron P, Reyl-Desmars F, Dauhoo N, Damasse J, Vadrot N, Nahon P, Moreau R, Pessayre D, Mansouri A. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15:2837–2854. doi: 10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- 72.Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 73.Peruchi BB, Petronilho F, Rojas HA, Constantino L, Mina F, Vuolo F, Cardoso MR, Goncalves CL, Rezin GT, Streck EL, Dal-Pizzol F. Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res. 2011;167:e333–e338. doi: 10.1016/j.jss.2010.11.893. [DOI] [PubMed] [Google Scholar]
- 74.Covian R, Balaban RS. Cardiac mitochondrial matrix and respiratory complex protein phosphorylation. Am J Physiol Heart Circ Physiol. 2012;303:H940–H966. doi: 10.1152/ajpheart.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med. 2005;172:861–868. doi: 10.1164/rccm.200410-1344OC. [DOI] [PubMed] [Google Scholar]
- 76.Supinski GS, Callahan LA. Double-stranded RNA-dependent protein kinase activation modulates endotoxin-induced diaphragm weakness. J Appl Physiol. 2011;110:199–205. doi: 10.1152/japplphysiol.01203.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Supinski GS, Ji XY, Callahan LA. p38 Mitogen-activated protein kinase modulates endotoxin-induced diaphragm caspase activation. Am J Respir Cell Mol Biol. 2010;43:121–127. doi: 10.1165/rcmb.2008-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R825–R834. doi: 10.1152/ajpregu.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J. 2003;372:359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chambers JW, Howard S, LoGrasso PV. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J Biol Chem. 2013;288:1079–1087. doi: 10.1074/jbc.M112.421354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 83.Neviere R, Hassoun SM, Decoster B, Bouazza Y, Montaigne D, Marechal X, Marciniak C, Marchetti P, Lancel S. Caspase-dependent protein phosphatase 2A activation contributes to endotoxin-induced cardiomyocyte contractile dysfunction. Crit Care Med. 2010;38:2031–2036. doi: 10.1097/CCM.0b013e3181eedafb. [DOI] [PubMed] [Google Scholar]
- 84.Nagase T, Murakami T, Nozaki H, Inoue R, Nishito Y, Tanabe O, Usui H, Takeda M. Tissue and subcellular distributions, and characterization of rat brain protein phosphatase 2A containing a 72-kDa delta/B" subunit. J Biochem. 1997;122:178–187. doi: 10.1093/oxfordjournals.jbchem.a021726. [DOI] [PubMed] [Google Scholar]
- 85.Merrill RA, Slupe AM, Strack S. N-terminal phosphorylation of protein phosphatase 2A/Bbeta2 regulates translocation to mitochondria, dynamin-related protein 1 dephosphorylation, and neuronal survival. FEBS J. 2013;280:662–673. doi: 10.1111/j.1742-4658.2012.08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dagda RK, Merrill RA, Cribbs JT, Chen Y, Hell JW, Usachev YM, Strack S. The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem. 2008;283:36241–36248. doi: 10.1074/jbc.M800989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang K, Li HF, Hsieh CH, Li DY, Song DC, Cheng WT, Guo ZX. Differential autophagic cell death under stress with ectopic cytoplasmic and mitochondrial-specific PPP2R2B in human neuroblastoma cells. Apoptosis. 2013;18:627–638. doi: 10.1007/s10495-013-0809-7. [DOI] [PubMed] [Google Scholar]
- 88.Cheng WT, Guo ZX, Lin CA, Lin MY, Tung LC, Fang K. Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B (Bbeta2) BMC Cell Biol. 2009;10:91. doi: 10.1186/1471-2121-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 90.Kim TY, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–1594. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor DE, Ghio AJ, Piantadosi CA. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys. 1995;316:70–76. doi: 10.1006/abbi.1995.1011. [DOI] [PubMed] [Google Scholar]
- 92.Nethery D, DiMarco A, Stofan D, Supinski G. Sepsis increases contraction-related generation of reactive oxygen species in the diaphragm. J Appl Physiol. 1999;87:1279–1286. doi: 10.1152/jappl.1999.87.4.1279. [DOI] [PubMed] [Google Scholar]
- 93.Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC, Streck EL, Dal-Pizzol F. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion. 2008;8:211–218. doi: 10.1016/j.mito.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1095–R1102. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rocha M, Herance R, Rovira S, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets. 2012;12:161–178. doi: 10.2174/187152612800100189. [DOI] [PubMed] [Google Scholar]
- 96.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 97.Skulachev VP. A biochemical approach to the problem of aging: "megaproject" on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc) 2007;72:1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y, Karakhanova S, Soltek S, Werner J, Philippov PP, Bazhin AV. In vivo immunoregulatory properties of the novel mitochondria-targeted antioxidant SkQ1. Mol Immunol. 2012;52:19–29. doi: 10.1016/j.molimm.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 99.Rottenberg H, Covian R, Trumpower BL. Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J Biol Chem. 2009;284:19203–19210. doi: 10.1074/jbc.M109.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zmijewski JW, Lorne E, Banerjee S, Abraham E. Participation of mitochondrial respiratory complex III in neutrophil activation and lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L624–L634. doi: 10.1152/ajplung.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 103.Mascarenhas DD, Elayadi A, Singh BK, Prasai A, Hegde SD, Herndon DN, Finnerty CC. Nephrilin peptide modulates a neuroimmune stress response in rodent models of burn trauma and sepsis. Int J Burns Trauma. 2013;3:190–200. [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao X, Leon IR, Bak S, Mogensen M, Wrzesinski K, Hojlund K, Jensen ON. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol Cell Proteomics. 2011:M110.000299. doi: 10.1074/mcp.M110.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, Hüttemann M. Phosphomimetic substitution of cytochrome c tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49:6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- 106.Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knab S, Mushak TM, Schmitz-Esser S, Horn M, Haferkamp I. Nucleotide parasitism by Simkania negevensis (Chlamydiae) J Bacteriol. 2011;193:225–235. doi: 10.1128/JB.00919-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dare AJ, Phillips AR, Hickey AJ, Mittal A, Loveday B, Thompson N, Windsor JA. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic Biol Med. 2009;47:1517–1525. doi: 10.1016/j.freeradbiomed.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 109.Choi HM, Jo SK, Kim SH, Lee JW, Cho E, Hyun YY, Cha JJ, Kang YS, Cha DR, Cho WY, Kim HK. Glucocorticoids attenuate septic acute kidney injury. Biochem Biophys Res Commun. 2013;435:678–684. doi: 10.1016/j.bbrc.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 110.Hüttemann M, Lee I, Grossman LI, Doan JW, Sanderson TH. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: respiration, apoptosis, and human disease. Adv Exp Med Biol. 2012;748:237–264. doi: 10.1007/978-1-4614-3573-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim Biophys Acta. 2002;1589:181–195. doi: 10.1016/s0167-4889(02)00174-x. [DOI] [PubMed] [Google Scholar]
- 112.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogbi M, Johnson JA. Protein kinase Cε interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cε function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salvi M, Stringaro A, Brunati AM, Agostinelli E, Arancia G, Clari G, Toninello A. Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria. Cell Mol Life Sci. 2004;61:2393–2404. doi: 10.1007/s00018-004-4211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 118.Lee I, Pecinova A, Pecina P, Neel BG, Araki T, Kucherlapati R, Roberts AE, Hüttemann M. A suggested role for mitochondria in Noonan syndrome. Biochim Biophys Acta. 2010;1802:275–283. doi: 10.1016/j.bbadis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]