Abstract
Background
Children with craniofacial disorders are at increased risk for obstructive sleep apnea syndrome (OSAS). Methods for diagnosing OSAS in this population remain controversial. Sleep studies are the gold standard but are impractical for all patients. The utility of OSAS questionnaires like the Pediatric Sleep Questionnaire (PSQ) is unknown in children with craniofacial disorders. We hypothesized that the PSQ would be a sensitive tool for detecting OSAS in children with craniofacial abnormalities.
Methods
A retrospective review of consecutive children with diagnosed craniofacial disorders who both completed the PSQ and had a polysomnogram (PSG) was performed. Demographics, PSQ score, and PSG data were recorded. Statistical analysis included calculation of sensitivity, specificity, positive predictive value (PPV), and negative predictive values (NPV) for the PSQ.
Results
83 children 2–18 years old were included in the study. Of these, 44 (53.0%) screened positive on the PSQ and 23 (27.7%) had PSG evidence of OSAS, but the sensitivity of the PSQ for detecting OSAS in this sample was only 0.57 and the specificity was 0.48. PPV and NPV were 0.30 and 0.74, respectively. The correlation between the apnea hypopnea index and PSQ score was 0.152 (p=0.17).
Conclusions
A substantial portion of craniofacial patients referred for PSG was found to have OSAS. However, the PSQ is not a good screening tool for OSAS in children with craniofacial conditions. More research is needed to determine which patients with craniofacial disorders should be evaluated for OSAS by PSG or other means.
LEVEL OF EVIDENCE
Diagnostic, III
BACKGROUND
Obstructive sleep apnea syndrome (OSAS) is common in children, with a prevalence ranging from 1.2 to 5.8%, depending on the population studied and the criteria used to define OSAS 1,2. Adverse sequelae of untreated OSAS in children are significant, including neurocognitive impairment, behavioral problems, failure to thrive, hypertension, and cardiac dysfunction 3. While the prevalence of OSAS in children with craniofacial abnormalities remains unknown, studies have demonstrated high rates of OSAS symptoms, particularly in patients with underlying syndromes such as Pierre Robin sequence 4,5. Additional studies have demonstrated high rates of OSAS in children with craniofacial conditions studied with polysomnography (PSG) 6, which is the gold standard for diagnosis of OSAS in children 7.
A screening questionnaire with good sensitivity and specificity for OSAS could potentially avoid children. The Pediatric Sleep Questionnaire (PSQ) is a 22-item survey that asks questions related to snoring and observed apnea, daytime sleepiness and inattentiveness, and other symptoms characteristic of childhood OSAS 8. This questionnaire, which was validated with a sensitivity of 0.85 and specificity of 0.87 in otherwise healthy children, is among the best screening tools for pediatric OSAS 9. A re-validation of the PSQ in a cohort of school age children undergoing adenotonsillectomy found that it was useful in predicting OSAS both before and after surgery 10. The PSQ has been applied to many pediatric populations. In the past year alone, it has been used to evaluate Estonian children who were overweight or underweight 11, children in an orthodontic clinic 12, children with nocturnal enuresis 13, and teenagers being re-evaluated for OSAS after being treated 14, among others. While developed as a research tool, the PSQ is often used clinically 14,15.
Screening for OSAS in children with craniofacial conditions is problematic. In these patients, upper airway obstruction often has a different etiology than in children with adenotonsillar hypertrophy or obesity, and typical symptoms like nighttime snoring may not be present 16. Additionally, children with craniofacial disorders may be at increased risk for hearing deficits, learning disorders, and chronic illness with multiple hospitalizations that may make evaluating daytime symptoms of OSAS less reliable. While previous studies have evaluated the prevalence of OSAS in children with cleft palate 17, to our knowledge, none have attempted to correlate the results from screening questionnaires like the PSQ with PSG data in this population. This study evaluated the relationship between PSQ score and PSG findings to determine the utility of the PSQ in detecting OSAS in children with craniofacial conditions. Due to differences between children evaluated in a craniofacial clinic and the otherwise healthy children for whom the PSQ was originally developed, we hypothesized that the PSQ would be less sensitive for detecting OSAS in children with craniofacial conditions, but that it would still be an effective screening tool.
PATIENTS AND METHODS
Study group and study design
As part of an effort to standardize history-taking with regard to obstructive sleep apnea, the PSQ was given to parents of patients being seen in the Cleft and Craniofacial Clinic of The Children’s Hospital of Philadelphia as part of routine clinical care. Parents completed the questionnaire during their clinic visit. Children between 2 and 18 years of age who completed the PSQ were eligible for inclusion in this retrospective cohort study as this is the age range for the PSQ. To be eligible for inclusion, patients also had to have an in-laboratory PSG within two years of completion of the PSQ and could not have had any surgical intervention between completing the PSQ and obtaining the PSG. Patients with tracheostomies and those using non-invasive positive pressure ventilation were excluded. Patients were referred for PSG as per the clinical decision of the individual attending surgeon. The medical record of each patient completing the PSQ was reviewed. This study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia, which waived consent for this retrospective study. PSQ data was collected from January 2011 through August 2013.
Polysomnograms
All overnight PSGs were conducted in sleep laboratories and scored according to the American Academy of Sleep Medicine pediatric specifications 18. The apnea hypopnea index (AHI) was determined by dividing the total number of obstructive apneas and hypopneas by the number of hours of total sleep time. An AHI > 5/hour was considered positive for OSAS as this was the cutoff used in the PSQ validation. Analysis using an AHI of 2/hour was also performed as this is commonly considered the cutoff for diagnosis of OSAS in children 19. Other variables, including oxyhemoglobin saturation (SpO2) nadir and time with saturation < 90%, time with end-tidal CO2 > 50 torr, and arousal index (total number of arousals from sleep divided by total sleep time) were also assessed. Body mass index (BMI) was calculated at the time of PSG and normalized for patient age (BMI z score).
Pediatric Sleep Questionnaire
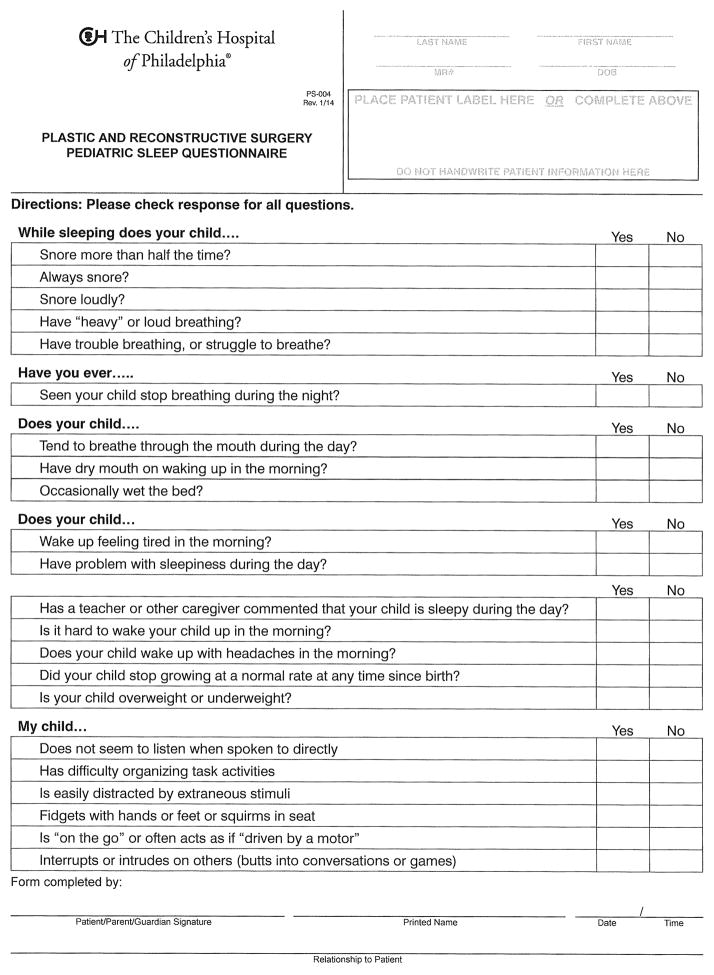
The Pediatric Sleep Questionnaire is composed of 22 “yes” or “no” items evaluating signs and symptoms of sleep-disordered breathing, including snoring, mouth breathing, daytime sleepiness, and inattentiveness (Figure 1). The ratio of questions answered in the affirmative divided by the total number of questions completed is the PSQ score. PSQ validation studies have found a score of 0.33 to be the cutoff for detecting an AHI > 5/hr in otherwise healthy children 8. Paper copies of the PSQ were completed by parents of patients being seen in the Cleft and Craniofacial Clinic, and scores were tallied by clinical staff.
Figure 1.
Pediatric Sleep Questionnaire.
Data analysis
PSQ score, BMI z score, AHI, and other PSG variables were evaluated for normality of distribution using histograms and the Kolmogorov-Smirnov test for normal distribution. When not normally distributed, logarithmic transformations were performed, which normalized AHI and arousal index, but not the other PSG variables. Pearson correlation was performed to evaluate the relationship between PSQ score and log transformed AHI, while Spearman correlations were performed between PSQ score and PSG variables with skewed distributions that could not be normalized with transformations and to evaluate the relationship between BMI z score and AHI. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The above statistical analysis of the data was conducted with IBM SPSS Statistics for Windows, Version 20.0 (Released 2011. Armonk, NY: IBM Corp.). Data are shown as mean ± standard deviation if normally distributed and the median (range) if not normally distributed, unless otherwise specified. A p value of ≤ 0.05 was considered statistically significant.
RESULTS
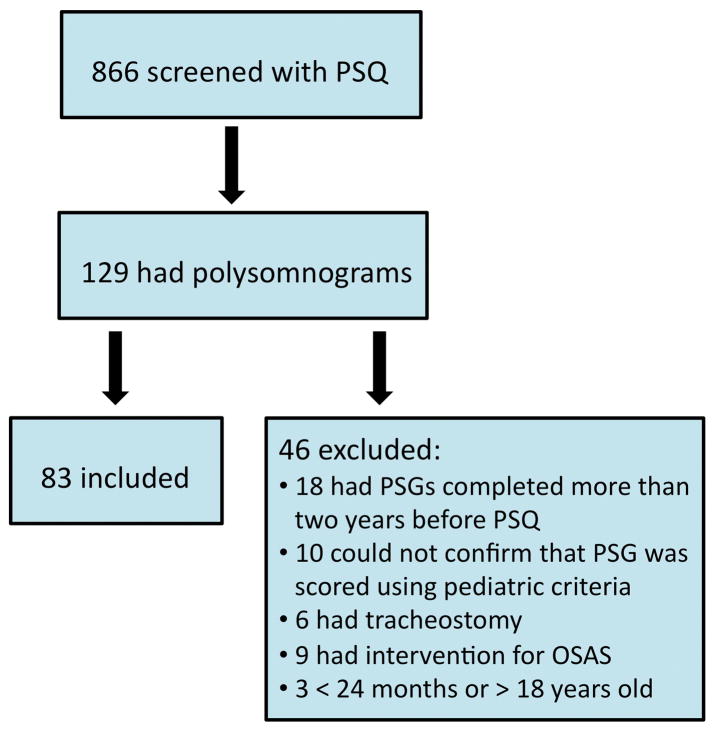
The PSQ was completed for a total of 866 children. 83 patients (9.6%) met inclusion criteria for this study (Figure 2). Age at the time of PSQ completion for those meeting inclusion criteria was 7.8 ± 4.2 years. Diagnosis was highly variable, but included children with velopharyngeal insufficiency, isolated cleft lip/palate and a variety of craniofacial syndromes (Table 1).
Figure 2.
Study diagram of eligible children.
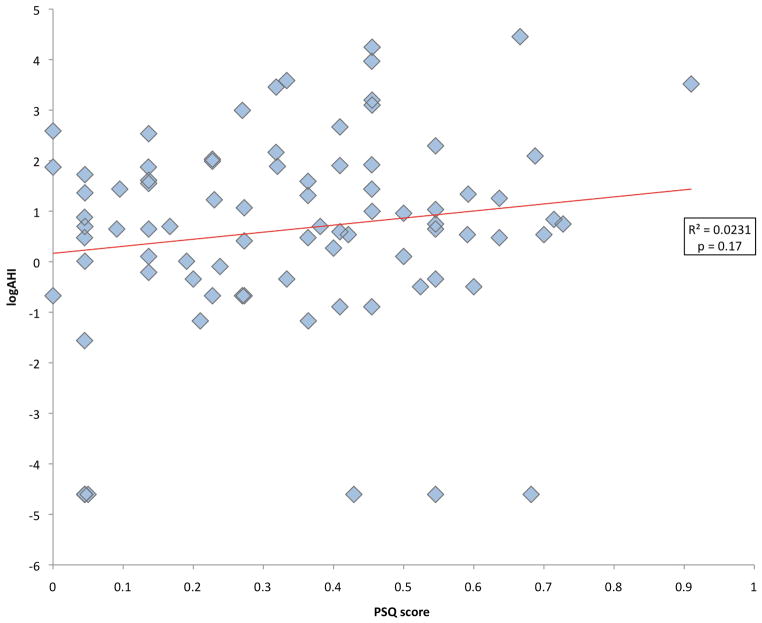
The PSQ score was normally distributed. The mean PSQ was 0.34 ± 0.21 and 44 patients (53.0%) had a positive PSQ. As has been shown in other studies of OSAS 19, the AHI by PSG was not normally distributed because the results were skewed toward zero, with most patients having a low AHI but a few patients having a very high AHI. Median AHI was 2/hour (range 0 to 86). 23 patients (27.7%) had PSGs with an AHI > 5/hour (Table 2). 44 patients (53.0%) had an AHI ≥ 2/hour. Using a cutoff of 5/hour, the sensitivity of the PSQ for detecting OSAS in this sample was 0.57 and the specificity was 0.48. The PPV of the PSQ was 0.30 and the NPV was 0.74. Using a cutoff of 2/hour, the PSQ had a sensitivity of 0.57, a specificity of 0.51, and PPV and NPV were 0.57 and 0.51, respectively. Log-transformed AHI and PSQ score were not significantly correlated (r=0.152, p=0.17) (Figure 3). None of the other PSG variables evaluated were normally distributed. There was not a significant correlation between the PSQ score and the other PSG variables evaluated (Table 3). Even after log transformation, all other PSG variables other than arousal index remained skewed. BMI z score and logAHI were not correlated (r=−0.05, p=0.68). See the supplemental table (insert link) for complete raw data for each subject included.
Figure 3.
Correlation between the Pediatric Sleep Questionnaire (PSQ) score and the apnea hypopnea index (AHI).
As isolated cleft lip/palate is common, we evaluated this subgroup separately. The cohort of patients with cleft lip/palate (n=19) had similar findings to the overall group. For this group, the median AHI was 2/hour (range 0 to 36.1) and 4 subjects (21.0%) had an AHI > 5/hour (57.9% using a cutoff of 2/hour). 10 subjects (52.6%) had a positive PSQ and the mean PSQ score for the cleft palate/lip group was 0.30 ± 0.23. The positive and negative predictive values of the PSQ were 0.50 and 0.47, respectively.
DISCUSSION
Our data shows that the Pediatric Sleep Questionnaire is not an adequate tool for screening children with craniofacial conditions for obstructive sleep apnea. Although this instrument has been validated in otherwise healthy children, apparent phenotypic differences in children with craniofacial conditions make the PSQ unreliable in this population. The PSQ was neither sensitive nor specific in our cohort of children with craniofacial conditions and the score on the PSQ did not correlate with apnea hypopnea index or other measures of severity of OSA in children.
OSAS is a common condition in children which has consequences that can be avoided if it is identified and properly treated. In some children with craniofacial disorders, OSAS can be successfully treated with surgeries such as adenotonsillectomy 20 or selective craniofacial procedures such as mandibular distraction osteogenesis 21. In other cases, continuous positive pressure airway pressure (CPAP) can be used to effectively treat OSAS 22 and in extreme cases, tracheostomy may be necessary.
Because of their abnormal upper airway anatomy, children with craniofacial disorders are at increased risk for OSAS 23. However, there is currently no standardized means of identifying the risk of individual children within this population. The appropriate age to screen for OSAS is also not clear for these patients as the effect of growth on the airway is unknown. It is impractical to screen all children using PSG, which is costly and not always readily available. Ideally, a screening tool would evaluate which patients are at high risk, and those patients should be referred for evaluation.
Many questionnaires exist to evaluate sleep in children, most of which lack validation, standardization, or both. Because the Pediatric Sleep Questionnaire has been validated for the evaluation of sleep disordered breathing in children, it is considered to be among the best instruments for assessing OSAS 9. Aside from questionnaires, a variety of other modalities exist to screen for OSAS in children. These include audio/video recordings, nocturnal oximetry, ambulatory PSG, and nap PSG. Some studies have found these more limited tools to be adequate 24,25, while others found these more limited tests and in-laboratory PSG were poorly correlated 26–28. These instruments have not been sufficiently studied in children with craniofacial conditions.
There are several reasons why the PSQ score may not have been associated with PSG findings in our cohort. First, children with craniofacial conditions may have different symptoms than the otherwise healthy children for which the PSQ was validated. For example, the site of upper airway obstruction may be different in patients with craniofacial conditions than other children who have OSAS, resulting in differences in snoring or mouth breathing, which are major portions of the PSQ. Additionally, parental perceptions and expectations may be different for children with craniofacial conditions. As some of the children in our cohort have had a lifetime of surgical and medical interventions, some parents may have a heightened vigilance toward symptoms of sleep-disordered breathing while other parents may not consider them significant. Furthermore, because children with craniofacial conditions are more likely to have developmental delays or hearing deficits, symptoms of distractibility and the inability to organize tasks and listen attentively may be less likely related to OSAS than in otherwise healthy children. Unlike other populations, our cohort did not show a relationship between degree of obesity and severity of obstructive sleep apnea. This may be related to the underlying complex medical conditions in this cohort, not limited to difficulties with feeding.
This is the first study to our knowledge to evaluate the efficacy of a screening tool for OSAS in a pediatric craniofacial population. In our analysis, we did not separate subjects into sub-groups based on diagnosis for two reasons. First, due to individual variation and overlap of clinical features between syndromes, deciding how to create sub-groups is inherently problematic and arbitrary. Second, our goal was to evaluate the use of this tool in a real-world clinical setting, where patients may have a variety of relatively uncommon conditions.
As a retrospective analysis, this study has several limitations. First, there could be referral bias as the only children who had PSG were those who were referred clinically. Since only 53% of patients who had PSG screened positive with the PSQ, however, it is less likely that the results are skewed toward patients presenting with more severe symptoms. We chose a two-year window of time between completing the PSQ and obtaining a PSG for inclusion, with no intervention in between. As children can grow significantly and have changes in symptoms during that time period, this could also have skewed our results. Adenotonsillar hypertrophy is a risk factor for OSAS in children, but because this was not assessed systematically in this study, we were unable to include it as a covariate in our analysis. Finally, we evaluated a population of children seen in craniofacial clinic as a single group; it is possible that the PSQ may be more accurately utilized in screening patients with specific craniofacial syndromes. We did not find a substantial difference when evaluating the cleft lip/palate cohort separately.
While doing post-hoc analyses of individual items on a screening questionnaire is problematic in that it undermines the validity of the tool, our data suggest that some items on the PSQ were more sensitive and specific than others. Future studies are needed to determine whether a new questionnaire using a subset of the PSQ items would be useful for diagnosis of OSAS, and to validate it prospectively in an independent cohort of craniofacial patients undergoing PSG.
In conclusion, a significant proportion of patients with craniofacial disorders in our cohort had symptoms consistent with OSAS based on the PSQ, and many of those clinically referred for PSG were found to have OSAS. However, the sensitivity, specificity, and negative and positive predictive values of the PSQ were all low. Thus, the PSQ is not a good screening tool for OSAS in children with craniofacial abnormalities. More research is needed to determine which patients with craniofacial conditions need to be evaluated for OSAS by PSG or other means, and to develop improved screening techniques. A prospective study evaluating consecutive patients with PSG would be an important way to confirm the findings of this study.
Supplementary Material
Footnotes
Preliminary data from this study was presented in poster presentation format at the American Thoracic Society 2013 International Conference. This data has not been published elsewhere.
Institutional Review Board
This study was reviewed and approved by the Institutional Review Board of the Children’s Hospital of Philadelphia
Author contribution
Christopher M. Cielo: study design, data analysis and interpretation, manuscript preparation
Carole L. Marcus: study design, data analysis and interpretation, manuscript preparation
Jason Silvestre: data analysis and interpretation and manuscript preparation
J. Thomas Paliga: data analysis and interpretation and manuscript preparation
Meg Maguire: study design, data collection, and manuscript preparation
Paul R. Gallagher: data analysis and interpretation and manuscript preparation
Jesse A. Taylor: study conception, study design, data interpretation, and manuscript preparation
Financial Disclosures:
Christopher M. Cielo has no conflicts to disclose and received support for this project through grants NIH T32HL7953 and NIH UL1RR024134
Jason Silvestre has no conflicts to disclose and received support for this project through grant R25 - HL084665.
J. Thomas Paliga has no conflicts to disclose and has not received grant support for this project.
Meg Maguire has no conflicts to disclose and has not received grant support for this project.
Paul R. Gallagher has no conflicts to disclose and has not received grant support for this project
Carole L. Marcus has no conflicts to disclose and received support for this project through grant NIH R01 HL58585
Jesse A. Taylor has no conflicts to disclose and has not received grant support for this project.
Contributor Information
Christopher M. Cielo, Division of Pulmonary Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Jason Silvestre, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
J. Thomas Paliga, Division of Plastic and Reconstructive Surgery, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Meg Maguire, Division of Plastic and Reconstructive Surgery, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Paul R. Gallagher, Clinical and Translational Research Center, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Carole L. Marcus, Perelman School of Medicine at the University of Pennsylvania, Sleep Center, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Jesse A. Taylor, Perelman School of Medicine at the University of Pennsylvania, Division of Plastic and Reconstructive Surgery, The Children’s Hospital of Philadelphia, Philadelphia, PA.
References
- 1.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009 Jun;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010 Nov;65(11):991–997. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 4.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008 Feb;118(2):348–353. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 5.Maclean JE, Waters K, Fitzsimons D, Hayward P, Fitzgerald DA. Screening for obstructive sleep apnea in preschool children with cleft palate. Cleft Palate Craniofac J. 2009 Mar;46(2):117–123. doi: 10.1597/07-215.1. [DOI] [PubMed] [Google Scholar]
- 6.MacLean JE, Fitzsimons D, Hayward P, Waters KA, Fitzgerald DA. The identification of children with cleft palate and sleep disordered breathing using a referral system. Pediatr Pulmonol. 2008 Mar;43(3):245–250. doi: 10.1002/ppul.20763. [DOI] [PubMed] [Google Scholar]
- 7.Marcus CL, Brooks LJ, Draper KA, Gozal D. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):1–9. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 8.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000 Feb 1;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 9.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011 Feb;15(1):19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007 Mar;133(3):216–222. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 11.Vaher H, Kasenomm P, Vasar V, Veldi M. A survey of parentally reported sleep health disorders in estonian 8–9 year old children. BMC Pediatr. 2013 Dec 4;13(1):200. doi: 10.1186/1471-2431-13-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katyal V, Pamula Y, Daynes CN, et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2013 Dec;144(6):860–871. doi: 10.1016/j.ajodo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe-Christensen C, Kovacevic LG, Mirkovic J, Lakshmanan Y. Lower health related quality of life and psychosocial difficulties in children with monosymptomatic nocturnal enuresis--is snoring a marker of severity? J Urol. 2013 Oct;190(4 Suppl):1501–1504. doi: 10.1016/j.juro.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 14.Guilleminault C, Huang YS, Quo S, Monteyrol PJ, Lin CH. Teenage sleep-disordered breathing: recurrence of syndrome. Sleep Med. 2013 Jan;14(1):37–44. doi: 10.1016/j.sleep.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Pena-Zarza JA, Osona-Rodriguez de Torres B, Gil-Sanchez JA, Figuerola-Mulet J. Utility of the pediatric sleep questionnaire and pulse oximetry as screening tools in pediatric patients with suspected obstructive sleep apnea syndrome. Sleep Disord. 2012;2012:819035. doi: 10.1155/2012/819035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLean JE, Hayward P, Fitzgerald DA, Waters K. Cleft lip and/or palate and breathing during sleep. Sleep Med Rev. 2009 Oct;13(5):345–354. doi: 10.1016/j.smrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Robison JG, Otteson TD. Increased prevalence of obstructive sleep apnea in patients with cleft palate. Arch Otolaryngol Head Neck Surg. 2011 Mar;137(3):269–274. doi: 10.1001/archoto.2011.8. [DOI] [PubMed] [Google Scholar]
- 18.The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013 Jun 20;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Aziz M. The effectiveness of tonsillectomy and partial adenoidectomy on obstructive sleep apnea in cleft palate patients. Laryngoscope. 2012 Nov;122(11):2563–2567. doi: 10.1002/lary.23507. [DOI] [PubMed] [Google Scholar]
- 21.Rachmiel A, Emodi O, Aizenbud D. Management of obstructive sleep apnea in pediatric craniofacial anomalies. Ann Maxillofac Surg. 2012 Jul;2(2):111–115. doi: 10.4103/2231-0746.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006 Mar;117(3):e442–451. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 23.Maclean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012 Dec;97(12):1058–1063. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003 Apr;142(4):383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 25.Bannink N, Mathijssen IM, Joosten KF. Use of ambulatory polysomnography in children with syndromic craniosynostosis. J Craniofac Surg. 2010 Sep;21(5):1365–1368. doi: 10.1097/SCS.0b013e3181ec69a5. [DOI] [PubMed] [Google Scholar]
- 26.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):e714–755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 27.Kirk VG, Bohn SG, Flemons WW, Remmers JE. Comparison of home oximetry monitoring with laboratory polysomnography in children. Chest. 2003 Nov;124(5):1702–1708. doi: 10.1378/chest.124.5.1702. [DOI] [PubMed] [Google Scholar]
- 28.Zucconi M, Calori G, Castronovo V, Ferini-Strambi L. Respiratory monitoring by means of an unattended device in children with suspected uncomplicated obstructive sleep apnea: a validation study. Chest. 2003 Aug;124(2):602–607. doi: 10.1378/chest.124.2.602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.