Abstract
The dopamine D3 receptor (DRD3) gene has been implicated in schizophrenia, autism, and substance use-disorders and is related to emotion reactivity, executive functioning, and stress-responding, processes impaired in posttraumatic stress disorder (PTSD). This aim of this candidate gene study was to evaluate DRD3 polymorphisms for association with PTSD. The discovery sample was trauma-exposed white, non-Hispanic veterans and their trauma-exposed intimate partners (N = 491); 60% met criteria for lifetime PTSD. The replication sample was 601 trauma-exposed African American participants; 24% met criteria for lifetime PTSD. Genotyping was based on high-density bead chips. In the discovery sample, four single nucleotide polymorphisms (SNPs), rs2134655, rs201252087, rs4646996, and rs9868039, showed evidence of association with PTSD and withstood correction for multiple testing. The minor alleles were associated with reduced risk for PTSD (odds ratio range: 0.59 – 0.69). In the replication sample, rs2251177, located 149 base pairs away from the most significant SNP in the discovery sample, was nominally associated with PTSD in men (odds ratio: 0.32). Although the precise role of the D3 receptor in PTSD is not yet known, its role in executive functioning and emotional reactivity, and the sensitivity of the dopamine system to environmental stressors, could potentially explain this association.
Keywords: Dopamine receptor, DRD3, posttraumatic stress disorder, molecular genetics
The dopamine system is involved in incentive/reward motivation (Beninger, 1983; Berridge & Robinson, 1998), motor control, and impulsivity (Dalley et al., 2007) and has been linked to the pathophysiology of substance-related disorders (Heidbreder et al., 2005; Pierce & Kumaresan, 2006), depression (Weiss et al., 1981), schizophrenia, and response to anti-psychotic drugs (Schwartz et al., 2000). The functioning of the dopamine system is thought to be affected by environmental stressors, and this sensitivity may help explain the link between life stress and onset of psychotic symptoms (Furuyashiki, 2012). There are two main types of dopamine receptors: D1-like (D1 and D5) and D2-like (D2, D3, and D4); the former has stimulatory effects and the latter is inhibitory. The D3 receptor has particular relevance to psychiatric phenotypes because it is expressed in brain regions thought to govern emotion and emotional responses to stress, reward motivation, and executive function (Sokoloff et al., 1990), such as the nucleus accumbens, and, to a lesser extent, the anterior cingulate gyrus, amygdala, and hippocampus (Gurevich & Joyce, 1999; Pennartz et al., 1994). The D3 receptor has been associated with startle reactivity (Halberstadt & Geyer, 2009), sensorimotor gating (Bristow et al., 1996; Giakoumaki et al., 2007; Swerdlow et al., 2009), memory, social recognition and responding (Loiseau & Millan, 2009; Watson et al., 2012), and cognitive impairment (Millan et al., 2010).
Prior research suggests that the dopamine D3 receptor gene (DRD3) may play a role in the etiology of a wide range of psychopathology including schizophrenia (Crocq et al., 1992; Dominguez et al., 2007; Ishiguro et al., 2000; Kennedy et al., 1995; Talkowski et al., 2006; Williams et al., 1998; Zhang et al., 2011), autism spectrum disorders (de Krom et al., 2009; Staal & de Krom, 2012), alcohol craving (Agrawal et al., 2013), nicotine dependence (Wei et al., 2012), depression (Dikeos et al., 1999), impulsivity among violent offenders (Retz et al., 2003), and obsessive-compulsive personality disorder (Light et al., 2006). The association between DRD3 and multiple psychiatric disorders is consistent with the finding that genes exert shared effects across mental disorders (Smoller et al., 2013); this suggests the importance of evaluating genes shown to have association with one psychiatric phenotype in other psychiatric populations.
The D3 receptor’s relationship to psychiatric impairment may be mediated by the receptor’s role in stimulus and stressor-related social learning, memory, executive functioning, and emotional reactivity. These processes are particularly relevant to posttraumatic stress disorder (PTSD) as the disorder is typified by heightened fear responsivity to stressors (e.g., hyperarousal symptoms, emotional reactivity to trauma cues) and executive function deficits (e.g., concentration problems, difficulty regulating anger and other emotions). Given this, we sought to conduct the first candidate gene study of the association between DRD3 and PTSD using data from a sample of 491 white, non-Hispanic, trauma-exposed military veterans and their cohabitating trauma-exposed intimate partners and an independent replication sample of 601 trauma-exposed African American men and women who participated in the Detroit Neighborhood Health Study (Goldmann et al., 2011). Given prior evidence for sex differences in the prevalence of PTSD (Kessler et al., 1995), and genetic work suggesting that DRD3 may be related to alcohol dependence in men but not women (Wodarz et al., 2003), we also evaluated if sex moderated the association between DRD3 and PTSD.
Methods
Discovery Sample Participants
The complete discovery sample included 852 veterans and their intimate partners who participated in one of two research studies. Ancestry was determined with the program STRUCTURE, which performs a Bayesian clustering analysis to assign subjects to ancestry groups, using 10,000 randomly chosen markers with minor allele frequency (MAF) > .05 (Falush et al., 2003; Pritchard et al., 2000). This process identified three groups, the largest of which was 540 participants who self-identified as white non-Hispanic. This study was based on 491 of them (364 veterans and 127 non-veteran partners) who reported lifetime exposure to a traumatic event and had valid lifetime PTSD interview data. Trauma exposure histories as a function of sex are detailed in Wolf et al., (2013): men and women did not differ in total trauma exposure but men were more likely to report combat exposure and women more likely to report sexual trauma. There was no evidence of PTSD-associated population substructure in this sample (Logue et al., 2012). The majority of participants were male (65%), and the mean age was 51.95 years (range: 21 – 75). In this sample, 60.29% (n = 296, comprised of 251 veterans and 45 non-veteran partners) met DSM-IV diagnostic criteria for lifetime PTSD. In Logue et al. (2012), we also presented results of an African American subset of the VA sample (n = 84). We do not present results of this subsample here, as there was no evidence for association between DRD3 and PTSD, and this null effect could not be interpreted given the low power associated with such a small sample.
Measures
Clinician Administered PTSD Scale (CAPS; Blake et al., 1995)
The CAPS assesses the 17 DSM-IV PTSD symptoms on frequency and intensity scales (each ranging from 0-4). PTSD diagnosis was calculated using a commonly-used and validated scoring rule (Weathers et al., 1999) which required endorsement of at least 1 reexperiencing, 3 avoidance and numbing, and 2 hyperarousal symptoms, each at a frequency of 1 or greater and an intensity of 2 or greater. Inter-rater reliability for lifetime diagnosis, as determined by a second rater making independent ratings from videotaped recordings of approximately 25% of the total participant interviews was excellent (κ = .87).
Traumatic Life Events Questionnaire (TLEQ; Kubany et al., 2000)
The TLEQ is a self-report instrument that assesses history of exposure to 22 different types of traumatic experiences that meet the DSM-IV PTSD Criterion A1. The measure also assesses whether the experience met Criterion A2 and asks the respondent to indicate the number of times each event occurred on a 7-point scale ranging from “never” to “more than five times.” The TLEQ has shown good test-retest reliability and predictive validity with respect to PTSD diagnoses (Kubany et al., 2000).
Procedure
Participants completed one of two research protocols with identical interview assessment procedures and the data from the two studies were combined for these analyses. One study recruited trauma-exposed military veterans and their cohabitating intimate partners; the other recruited trauma-exposed military veterans who screened positive for PTSD over the telephone. Both studies included comprehensive structured diagnostic interviews of all participants that were digitally videotaped for purposes of quality control and evaluating inter-rater reliability. The studies were approved and reviewed annually by the appropriate institutional review boards. Additional procedural details are provided in Logue et al. (2012).
Genotyping
DNA was isolated from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents and samples normalized using PicoGreen assays (Invitrogen). Samples were run on an Illumina OMNI 2.5-8 array and scanned using an Illumina HiScan System according to the manufacturer’s protocol. Details of the quality control procedure are described in detail elsewhere (Logue et al., 2012). We restricted our attention to the 26 DRD3 single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) greater than 5%. None of these SNPs failed a test of Hardy-Weinberg Equilibrium (i.e., all p > .01). DRD3 is located on chromosome 3 and spans 70,756 bases between 113,847,499 and 113,918,254 base pair (bp); SNP locations were derived from the hg19 human-genome assembly (February 2009).
Data Analyses
SNPs in DRD3 were examined for association with lifetime PTSD diagnosis using the standard χ2 case/control test of association in PLINK; this is an allelic test which compares the frequency of the minor allele in cases and controls under the assumption of an additive model (Purcell et al., 2007). We corrected for multiple-testing across the gene using the MAX(T) permutation procedure with 5000 replications. We also stratified the sample by sex and ran the same analysis to evaluate potential sex differences and used the PLINK logistic regression test to evaluate if any of the SNPs interacted with sex. Linkage disequilibrium (LD) was evaluated using the program Haploview (Barrett et al., 2005).
Replication Sample
The replication sample comprised 601 trauma-exposed African American (per self-report and confirmed by multidimensional scaling analysis of genome-wide SNP data in PLINK) men and women living in Detroit, MI who participated in one of three waves of data collection in the Detroit Neighborhood Health Study (additional details provided in Goldmann et al., 2011; Logue et al., 2012; Uddin et al., 2010). The sample we evaluated was predominately female (57%), and the mean age was 52.59 years (range: 18 – 95 years). Participants had been exposed to one or more traumas (see Breslau et al., 1998). Twenty-four percent (n = 142) met criteria for lifetime PTSD as determined by structured interview administered over the telephone. The interview, using the PTSD Checklist, assessed the extent to which participants had been bothered by each of the 17 DSM-IV PTSD symptoms on a 1 to 5 scale (Weathers et al., 1993) in relation to a specific, stressful event. To meet criteria for the diagnosis, participants had to endorse DSM-IV PTSD Criteria A1 and A2, at least one reexperiencing, three avoidance and numbing, and two hyperarousal symptoms, each at a symptom rating of three (“moderately”) or higher, and endorse clinically significant impairment in functioning. In addition, the diagnosis required that the symptoms were present for at least a month. DNA was extracted from whole blood or saliva and evaluated on an Illumina HumanOmniExpress BeadChip. We evaluated all DRD3 SNPs available on this chip; 11 of these overlapped the SNPs evaluated in the discovery sample. The analytic approach paralleled those in the discovery sample.
Results
Discovery Sample
Main effects
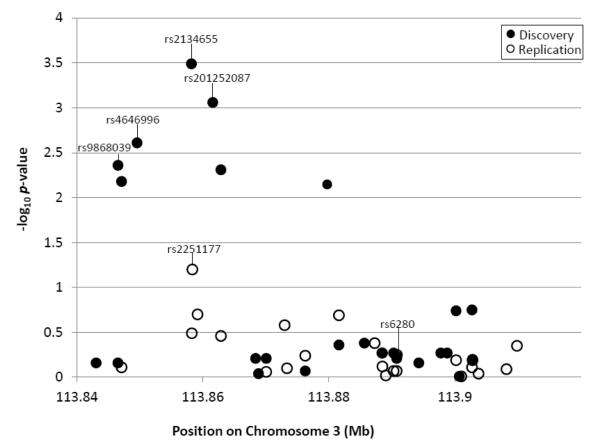
Six of the 26 SNPs in DRD3 showed nominal evidence of association (p < .05) with lifetime PTSD (see Table 1 and Figure 1). Four of these withstood correction for multiple testing: rs2134655, rs201252087, rs4646996, and rs9868039, with corrected p-values ranging from .005 to .049 (see Table 1). For each of these SNPs, the minor allele was less common among individuals with PTSD, suggesting a protective effect against risk for PTSD (odds ratios [ORs] ranged from 0.59 - 0.69; see Table 1). These four SNPs were in high LD with each other (see Table 2). Secondary data analyses revealed that results for these four SNPs were unchanged when we controlled for total trauma exposure and when we controlled for the non-independence of the 116 couples included in the analyses using a sandwich estimator to adjust the standard errors in Mplus 7.11 (Muthén & Muthén, 2012). The prevalence of lifetime PTSD among participants with one copy of the protective allele on rs2134655 (the most significant SNP) was 33%; it was 62% for those with no copies of the minor allele (there were no participants with two copies of the minor allele).
Table 1.
SNPs in DRD3 (Chromosome 3) with at Least Nominal Evidence of Association with Lifetime PTSD in the Discovery and/or Replication Samples
| Sample | SNP | bp | Minor Allele |
Freq Aff | Freq Unaff | OR |
p (uncorrected) |
p (corrected) |
|---|---|---|---|---|---|---|---|---|
| D | rs9868039 | 113846542 | A | 0.41 | 0.50 | 0.6883 | 0.004 | 0.049 |
| D | rs9817063 | 113847108 | G | 0.53 | 0.44 | 1.427 | 0.007 | 0.07 |
| D | rs4646996 | 113849565 | A | 0.44 | 0.54 | 0.6724 | 0.002 | 0.03 |
| D | rs2134655 | 113858201 | A | 0.28 | 0.32 | 0.5907 | 0.0003 | 0.005 |
| R | rs2251177 | 113858350 | G | 0.11 | 0.15 | 0.6733 | 0.06 | 0.60 |
| D | rs201252087 | 113861589 | G | 0.38 | 0.48 | 0.6447 | 0.0009 | 0.013 |
| D | rs963468 | 113862887 | A | 0.45 | 0.36 | 1.457 | 0.005 | 0.051 |
Note. DRD3 = dopamine receptor D3; PTSD = posttraumatic stress disorder; SNP = single nucleotide polymorphism; D = discovery sample; R = replication sample; bp = base pair; freq = frequency; aff = affected; unaff = unaffected; OR = odds ratio. SNPs that were significant after permutation testing are highlighted in bold font.
Figure 1.
The figure shows the nominal (uncorrected) p-values (in −log10) at each base pair location for all SNPs evaluated for association with PTSD in the discovery (filled circles) and replication (open circles) samples. The four SNPs that were significant after permutation testing in the discovery sample and the most significant SNP in the replication sample are identified, as is rs6280, a commonly studied DRD3 polymorphism.
Table 2.
Linkage Disequilibrium among the Four DRD3 SNPs showing the Strongest Association with PTSD in the Discovery Sample
| SNP | rs9868039 | rs4646996 | rs2134655 | rs201252087 |
|---|---|---|---|---|
| rs9868039 | -- | |||
| rs4646996 | D’ = .89 | -- | ||
| R2 = .68 | ||||
| rs2134655 | D’ = .99 | D’ = .99 | -- | |
| R2 = .42 | R2 = .36 | |||
| rs201252087 | D’ = .99 | D’ = .99 | D’ = .99 | -- |
| R2 = .86 | R2 = .75 | R2 = .75 |
Note. DRD3 = dopamine receptor D3; PTSD = posttraumatic stress disorder; SNP = single nucleotide polymorphism.
SNP by sex effects
We next evaluated these associations in men and women separately. In men (n = 339), the same six SNPs that were nominally significant in the full sample again showed nominally significant associations with PTSD. One of these (rs201252087) withstood correction for multiple testing (OR = .60, uncorrected p = .003, corrected p = .04). In women (n = 215), that same SNP (rs201252087) was not significantly associated with PTSD (OR = .69, uncorrected p = .10, corrected p = .57). Only one SNP (rs2134655) showed a nominally significant association with PTSD in women, but it did not withstand multiple testing correction (OR = .59, uncorrected p = .03, corrected p = .23); that SNP also evidenced a nominally significant association in the male subsample that did not withstand permutation testing (OR = .61, uncorrected p = .010, corrected p = .10). There was no evidence for a SNP × Sex interaction for rs201252087, as evaluated by a formal interaction test performed in PLINK (interaction term p = .67, smallest p for any of the other SNP × Sex interaction terms = .21). Given that men were more often exposed to combat and women more often exposed to sexual assault (see Wolf et al., in press), we conducted secondary analyses to determine if the potential differential strength of association between the SNPs and PTSD as a function of sex was actually attributable to these exposure variables instead. We found no evidence of SNP interactions with sexual assault history or combat exposure.
Replication Sample
The four SNPs showing evidence of association with PTSD in the discovery sample (rs2134655, rs201252087, rs4646996, and rs9868039) were not on the SNP array used in the replication sample. Of the 23 SNPs available for analysis, only rs9828046 and rs2251177 were located in close physical proximity (< 150 bp) to rs2134655, the most strongly associated SNP from the discovery sample. None of the SNPs in the replication sample evidenced a significant association with PTSD in the full sample; however, one SNP, rs2251177 located at 113,858,350 bp, just failed to meet the threshold for a nominal association (OR = 0.67, uncorrected p = .06, corrected p = .60; see Table 1). The prevalence of PTSD among those with two copies of the minor allele at this location was 21.4%; it was 17.3% for those with one copy and 25.7% for those with no copies. This SNP is 149 bp away from the most significant SNP identified in the discovery sample (rs2134655) and 3,239 bp away from the second most significant SNP identified in the discovery sample (rs201252087). Secondary data analyses controlling for trauma exposure revealed that this association became non-significant in the full sample (OR= .69, p = .11). We also stratified this sample by sex and evaluated the SNPs separately for men (n = 257) and women (n = 344). In the men, the G allele of rs2251177 showed evidence of a nominally significant association with PTSD (OR = 0.32, uncorrected p = .01) although the corrected p-value after permutation testing was.17. This SNP remained nominally significant when controlling for trauma (OR = .35, uncorrected p = .03). None of the SNPs were significant in the female subsample (association result for rs2251177: OR = 0.86, uncorrected p = .54, corrected p = .1.0). The test of the SNP × Sex interaction for rs2251177 failed to reach statistical significance (p = .09); none of the other SNPs evidenced a significant SNP × Sex interaction. We were unable to test for SNP interactions with trauma type in this sample as we did not have data on specific trauma types.
Discussion
DRD3 has previously been associated with a broad range of psychiatric disorders and the receptor encoded by the gene is important for executive functioning, emotional reactivity, and responding to environmental stressors. Given this, we examined the gene in relationship to PTSD. In our discovery sample of white, non-Hispanic trauma-exposed veterans and their spouses, four SNPs (rs2134655, rs201252087, rs4646996, and rs9868039) were associated with lifetime PTSD after adjustment for multiple testing. In an independent sample of trauma-exposed African Americans, one SNP (rs2251177), in close physical proximity to the most significant SNP identified in the discovery sample (rs2134655), evidenced a nominally significant association with PTSD in the male subsample; this same SNP just failed to meet the threshold for a nominal association with PTSD in the full replication sample. The minor alleles of these SNPs were protective against risk for PTSD, given trauma exposure. We would not expect rs2134655 (the most significant SNP identified in the discovery sample) to be significant in the replication sample because of differences in the MAF across Caucasian and African American populations (27% versus 6%, respectively, per the International HapMap Project). Similarly, we would not expect rs2251177 (the most significant SNP identified in the replication sample) to be significant in the discovery sample because that SNP is monomorphic (i.e., invariant) among the Caucasian population. Nevertheless, the physical proximity of these two SNPs across the two samples may suggest that a single risk locus in this region of DRD3 is implicated in risk for PTSD (i.e., these SNPs may be markers for the same functional variant).
The SNP that showed the strongest association with PTSD in the discovery sample (rs2134655) has also been implicated in prior work with other psychiatric disorders. In a study of white individuals living in the United States, it was nominally associated with schizophrenia (Talkowski et al., 2006) and, in samples of European ancestry, this SNP was part of a haplotype block associated with risk for schizophrenia with the T allele protective (Costas et al., 2009). That effect was consistent with the direction of effect in this study.
Other SNPs associated with PTSD in this study have been shown to be related to other psychiatric phenotypes. For example, rs9817063, which was nominally associated with PTSD in the discovery sample, was nominally associated with activity in the ventral striatum (as assessed via functional magnetic resonance imaging) and with treatment response to electroconvulsive therapy for depression (Dannlowski et al., 2011). In addition, rs963468, which just failed to meet the corrected p-value threshold for statistical significance in the discovery sample, was implicated as part of a haplotype block in risk for schizophrenia (Nunokawa et al., 2010) and also showed a nominal association with nicotine dependence (Huang et al., 2008).
The precise role of the D3 receptors in PTSD and associated disorders has not yet been elucidated, but the available evidence suggests it plays a role in at least two processes of relevance to PTSD: amygdala-mediated fear and anxiety processes and executive functioning. The receptor has been shown to be upregulated in basal, central, and lateral amygdaloid nuclei in individuals with depression (Klimek et al., 2002) and also appears to mediate anxiety following a social stressor (Hood et al., 2010). Imaging studies suggested that increased D2 and D3 receptor availability in prefrontal regions was associated with greater amgydala response to unpleasant images (Kobiella et al., 2009). Similarly, in animal models, the D3 receptor plays a role in behavioral responding to stressors (Xi et al., 2004). These studies have relevance to PTSD as imaging research implicates enhanced amygdala activation and fear responding in PTSD (Patel et al., 2012). Moreover, heightened negative emotional reactivity to trauma cues, anger, and hypervigilance are symptoms of PTSD that may be related to the functioning of the D3 receptors in the limbic brain regions governing emotional arousal and reactivity.
The prefrontal cortex and D3 receptor also play a role in working memory and executive function (Black et al., 2002). Evidence for this comes from research suggesting that D3 antagonists are potential therapeutic agents for the treatment of the cognitive dysfunction and confusion common to psychosis (Joyce & Millan, 2005) and from work showing the role of D3 receptors in working memory (Ersche et al., 2011). Moreover, the Ser9Gly DRD3 polymorphism has been shown to be associated with perseverative errors (Lane et al., 2008) as well as with other indices of executive functioning (Bombin et al., 2008). PTSD also is associated with executive function deficits (Polak et al., 2012), decreased activation of pre-frontal brain regions (Patel et al., 2012), and impaired concentration. Together, this raises the possibility that dysfunction at the site of D3 receptors at least partially accounts for the cognitive deficits seen in PTSD. More research is needed to directly test this possibility.
Finally, prior research suggests that the D3 receptors function differently in men compared to women. In one study, men expressed greater amounts of dopamine in striatal brain regions following exposure to amphetamine (Munro et al., 2006). D2/D3 receptor availability was also shown to confer risk for nicotine dependence in men but not women (Brown et al., 2012) and may be more strongly associated with the positive symptoms of schizophrenia in men compared to women (Glenthoj et al., 2006). Sex-specific effects of the gene have also been reported previously: the BA1I DRD3 polymorphism was associated with alcohol dependence among men, but not women, with a history of delirium tremens (Wodarz et al., 2003). In this study, we did not find evidence of statistically significant genotype by sex interactions; this issue would benefit from further investigation in larger samples that are adequately powered to detect potential interactive and sex-specific effects.
Limitations and Conclusions
Limitations of the study include the relatively small sample size for genetic association analysis and the inability to compare the exact same polymorphisms across the discovery and replication samples. It is difficult to conduct comparisons across different racial and ethnic groups due to differences in genetic variation (i.e., MAF) across these groups, thus our replication sample was not ideal for confirming results observed in the discovery sample. We were also unable to disentangle the potential effects of sex from those of trauma exposure history; moreover, we were under-powered to detect sex-specific effects thus our findings with respect to potential sex differences should be interpreted with caution and considered preliminary. In addition, we have previously reported results of a genome-wide genetic association study (i.e., an atheoretical, empirical test) with PTSD (Logue et al., 2012) in this sample and that study did not find an effect for DRD3 when genome-wide association standards (i.e., p < 5 × 10−8) were applied; however we think it is important to conduct a hypothesis-driven candidate gene study as this may identify weaker effects attributable only to a subset of the population that would otherwise be missed in genome-wide scans. This is the first study to report an association between DRD3 and PTSD and, as such, additional replication is needed.
In conclusion, the results of this study provide initial evidence that polymorphisms in DRD3, perhaps reflecting a single risk locus, may be associated with lifetime PTSD diagnosis. The findings are consistent with the results of prior genetic research on other psychiatric phenotypes and with studies of the role of the D3 receptor in emotional reactivity and executive functioning. The relationship between DRD3 and PTSD may also be a reflection of the sensitivity of the dopamine system to stress.
Acknowledgments
Funding for this study was provided by a National Institute on Mental Health (NIMH) award RO1 MH079806 and by a VA CSR&D Merit Award (application # 5I01CX000431-02) to Mark W. Miller. Erika J. Wolf’s contribution to this work was supported by a VA CSR&D Career Development Award. Karen S. Mitchell’s contribution to this work was supported by an NIMH award 1K01MH093750-01A1. Mark W. Logue is funded by NIMH award K01 MH076100.
The Detroit Neighborhood Health Study (PI: Allison Aiello) is funded by DA022720, DA 022720-S1, and MH 088283.
References
- Agrawal A, Wetherill L, Bucholz KK, Kramer J, Kuperman S, Lynskey MT, Bierut LJ. Genetic influences of craving for alcohol. Addictive Behaviors. 2013;38:1501–1508. doi: 10.1016/j.addbeh.2012.03.021. doi: 10.1016/j.addbeh.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Research. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research: Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. A possible substrate for dopamine-related changes in mood and behavior: Prefrontal and limbic effects of a D3-preferring dopamine agonist. Proceedings from the National Academy of Sciences of the United States of America. 2002;99:17113–17118. doi: 10.1073/pnas.012260599. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler R, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. doi:10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Bristow LJ, Cook GP, Gay JC, Kulagowski JJ, Landon L, Murray F, Hutson PH. The behavioural and neurochemical profile of the putative dopamine D3 receptor agonist, (+)-PD 128907, in the rat. Neuropharmacology. 1996;35:285–294. doi: 10.1016/0028-3908(96)00179-7. 10.1016/0028-3908(96)00179-7. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. International Journal of Neuropsychopharmacology. 2012;15:989–994. doi: 10.1017/S1461145711001957. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombin I, Arango C, Mayoral M, Castro-Fornieles JC, Gonzalez-Pinto A, Gonzalez-Gomez C, Patiño-Garcia A. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2008;147B:873–879. doi: 10.1002/ajmg.b.30710. doi: 10.1002/ajmg.b.30710. [DOI] [PubMed] [Google Scholar]
- Costas J, Carrera N, Dominguez E, Vilella E, Martorell L, Valero J, Carracedo A. A common haplotype of DRD3 affected by recent positive selection is associated with protection from schizophrenia. Human Genetics. 2009;124:607–613. doi: 10.1007/s00439-008-0584-7. doi: 10.1007/s00439-008-0584-7. [DOI] [PubMed] [Google Scholar]
- Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, Schwartz JC. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. Journal of Medical Genetics. 1992;60:558–562. doi: 10.1136/jmg.29.12.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Britchard L, Robinson ESJ, Theobald DEH, Lääne K, Robbins TW. Nucleus Accumbens D2/3 Receptors Predict Trait Impulsivity and Cocaine Reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Domschke K, Birosova E, Lawford B, Young R, Voisey J, Zwanzger P. Dopamine D3 receptor gene variation: Impact on electroconvulsive therapy response and ventral striatum responsiveness in depression. International Journal of Neuropsychopharmacology. 2011;18:1–17. doi: 10.1017/S1461145711001659. doi: 10.1017/S1461145711001659. [DOI] [PubMed] [Google Scholar]
- de Krom M, Staal WG, Ophoff RA, Hendriks J, Buitelaar J, Franke B, van Ree JM. A common variant in DRD3 receptor is associated with autism spectrum disorder. Biological Psychiatry. 2009;65:625–630. doi: 10.1016/j.biopsych.2008.09.035. doi: 10.1016/j.biopsych.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Dikeos DG, Papadimitriou GN, Avramopoulos D, Karadima G, Daskalopoulou EG, Souery D, Stefanis CM. Association between the dopamine D3 receptor gene locus (DRD3) and unipolar affective disorder. Psychiatric Genetics. 1999;9:189–195. doi: 10.1097/00041444-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Loza MI, Padin F, Gesteira A, Paz E, Paramo M, Costas J. Extensive linkage disequilibrium mapping at HTR2A and DRD3 for schizophrenia susceptibility genes in the Galician population. Schizophrenia Research. 2007;90:123–129. doi: 10.1016/j.schres.2006.09.022. doi: /10.1016/j.schres.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Lucas M, Domenici E, Robbins TW, Bullmore ET. Peripheral biomarkers of cognitive response to dopamine receptor agonist treatment. Psychopharmacology. 2011;214:779–789. doi: 10.1007/s00213-010-2087-1. doi: 10.1007/s00213-010-2087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyashiki T. Roles of dopamine and inflammation-related molecules in behavioral alterations caused by repeated stress. Journal of Pharmacological Sciences. 2012;120:63–69. doi: 10.1254/jphs.12r09cp. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Frangou S, Bitsios P. Disruption of prepulse inhibition of the startle reflex by the preferential D(3) agonist ropinirole in healthy males. Psychopharmacology. 2007;194:289–295. doi: 10.1007/s00213-007-0843-7. doi: 10.1007/s00213-007-0843-7. [DOI] [PubMed] [Google Scholar]
- Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, Videbaek C. Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biological Psychiatry. 2006;60:621–629. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: The Detroit Neighborhood Health Study. Journal of Traumatic Stress. 2011;24:747–751. doi: 10.1002/jts.20705. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce DN. Distribution of dopamine D3 recpetor expressing neurons in the human forebrain: Comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. doi:10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: Opposite influences of dopamine D1 and D2-family receptors. Neurobiology of Learning and Memory. 2009;92:243–248. doi: 10.1016/j.nlm.2008.05.015. doi: 10.1016/j.nlm.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-H, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: A review of pharmacological evidence. Brain Research. Brain Research Reviews. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood SD, Potokar JP, Davies SJC, Hince DA, Morris K, Seddon KM, Argyropoulos SV. Dopaminergic challenges in social anxiety disorder: Evidence for dopamine D3 desensitisation following successful treatment with serotonergic antidepressants. Journal of Psychopharmacology. 2010;24:709–716. doi: 10.1177/0269881108098144. doi: 10.1177/0269881108098144. [DOI] [PubMed] [Google Scholar]
- Huang W, Payne TJ, Ma JZ, Li MD. A functional polymorphism, rs6280, in DRD3 is significantly associated with nicotine dependence in European-American smokers. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2008;147B:1109–1115. doi: 10.1002/ajmg.b.30731. doi: 10.1002/ajmg.b.30731. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Okuyama Y, Toru M, Arinami T. Mutation and association analysis of the 5′ region of the dopamine D3 receptor gene in schizophrenia patients: Identification of the Ala38Thr polymorphism and suggested association between DRD3 haplotypes and schizophrenia. Molecular Psychiatry. 2000;5:433–438. doi: 10.1038/sj.mp.4000731. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discovery Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. doi: 10.1016/S1359-6446(05)03491-4, [DOI] [PubMed] [Google Scholar]
- Kennedy JL, Billett EA, Macciardi FM, Verga M, Parsons TJ, Meltzer HY, Buchanan JA. Association study of dopamine D3 receptor gene and schizophrenia. American Journal of Medical Genetics. 1995;60:558–562. doi: 10.1002/ajmg.1320600615. doi: 10.1002/ajmg.1320600615. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. doi:10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: A postmortem study. Biological Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. doi: 10.1016/S0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Vollstadt-Klein S, Buhler M, Graf C, Buchholz H-G, Bernow N, Smolka MN. Human dopamine receptor D2/D3 availability predicts amygdala reactivity to unpleasant stimuli. Human Brain Mapping. 2009;31:716–726. doi: 10.1002/hbm.20900. doi: 10.1002/hbm.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. doi: 10.1037/1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Chang YC, Wu PL, Huang HC, Tsai GE. RGS4 polymorphisms predict clinical manifestations and responses to risperidone treatment in patients with schizophrenia. Journal of Clinical Psychopharmacology. 2008;1:64–68. doi: 10.1097/jcp.0b013e3181603f5a. doi: 10.1097/jcp.0b013e3181603f5a. [DOI] [PubMed] [Google Scholar]
- Light KJ, Joyce PR, Luty SE, Mulder RT, Frampton CMA, Joyce LRM, Kennedy MA. Preliminary evidence for an association between a dopamine D3 receptor gene variant and obsessive-compulsive personality disorder in patients with major depression. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2006;141B:409–413. doi: 10.1002/ajmg.b.30308. doi: 10.1002/ajmg.b.30308. [DOI] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, Miller MW. A genome-wide association study of posttraumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.113. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau F, Millan MJ. Blockade of dopamine D(3) receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: Similar actions of D(1) receptor agonists, but not of D(2) antagonists. European Neuropsychopharmacology. 2009;19:23–33. doi: 10.1016/j.euroneuro.2008.07.012. doi: 10.1016/j.euroneuro.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Buccafusco JJ, Loiseau F, Watson DJ, Decamp E, Fone KC, Schneider JS. The dopamine D3 receptor antagonist, S33138, counters cognitive impairment in a range of rodent and primate procedures. International Journal of Neuropsychopharmacology. 2010;13:035–1051. doi: 10.1017/S1461145710000775. doi: 10.1017/S1461145710000775. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Nunokawa A, Watanabe Y, Kaneko N, Sugai T, Yazaki S, Arinami T, Someya T. The dopamine D3 receptor (DRD3) gene and risk of schizophrenia: Case-control studies and an updated meta-analysis. Schizophrenia Research. 2010;116:61–67. doi: 10.1016/j.schres.2009.10.016. doi: 10.1016/j.schres.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience Biobehavioral Research. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological, and anatomical data. Progressive Neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience Biobehavioral Research. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. doi: 10.1016/j.neubiorev.2005.04.016, [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders. 2012;141:11–21. doi: 10.1016/j.jad.2012.01.001. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. doi: 10.3410/f.1162373.622875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retz W, Rösler M, Supprian T, Retz-Junginger P, Thome J. Dopamine D3 receptor gene polymorphism and violent behavior: Relation to impulsiveness and ADHD-related psychopathology. Journal of Neural Transmissions. 2003;110:561–572. doi: 10.1007/s00702-002-0805-5. doi 10.1007/s00702-002-0805-5. [DOI] [PubMed] [Google Scholar]
- Schwartz J-C, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D3 receptor in schizophrenia and in antipsychotic drug actions. Brain Research. Brain Research Reviews. 2000;31:277–287. doi: 10.1016/s0165-0173(99)00043-0. doi: 10.1016/S0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;20:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. doi:10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Staal WG, de Krom M. Brief report: The dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in Autism Spectrum Disorder (ASD) Journal of Autism and Developmental Disorders. 2012;42:885–888. doi: 10.1007/s10803-011-1312-z. doi: 10.1007/s10803-011-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Lelham SA, Owens ANS, Chang W-L, Sassen SDT, Talledo JA. Pramipexole effects on startle gating in rats and normal men. Psychopharmacology. 2009;205:689–698. doi: 10.1007/s00213-009-1577-5. doi: 10.1007/s00213-009-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Mansour H, Chowdari KV, Wood J, Butler A, Varma PG, Nimgaonkar VL. Novel, replicated associations between dopamine D3 receptor gene polymorphisms and schizophrenia in two independent samples. Society of Biological Psychiatry. 2006;60:570–577. doi: 10.1016/j.biopsych.2006.04.012. doi: 10.1016/j.biopsych.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: A key role for the prefrontal cortex. Neuropsychopharmacology. 2012;37:770–786. doi: 10.1038/npp.2011.254. doi:10.1038/npp.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the 9th Annual Conference of the ISTSS; San Antonio, TX. 1993. [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment. 1999;11:124–133. doi: 10.1037/1040-3590.11.2.124. [Google Scholar]
- Wei J, Chu C, Wang Y, Yang Y, Wang Q, Li T, Ma X. Association study of 45 candidate genes in nicotine dependence in Han Chinese. Addictive Behaviors. 2012;37:622–626. doi: 10.1016/j.addbeh.2012.01.009. doi: 10.1016/j.addbeh.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Research. Brain Research Reviews. 1981;3:167–205. doi: 10.1016/0165-0173(81)90005-9. [Google Scholar]
- Williams J, Spurlock G, Holmans P, Mant R, Murphy K, Jones L, Owen MJ. A meta-analysis and transmission disequilibrium study of association between the dopamine D3 receptor gene and schizophrenia. Molecular Psychiatry. 1998;3:141–149. doi: 10.1038/sj.mp.4000376. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Mitchell KS, Logue MW, Baldwin CT, Reardon AF, Humphries DE, Miller MW. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depression and Anxiety. doi: 10.1002/da.22176. (in press) doi: 10.1002/da.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz N, Bobbe G, Eichhammer P, Weijers HG, Wiesbeck GA, Johann M. The candidate gene approach in alcoholism: Are there gender-specific differences? Archives of Women’s Mental Health. 2003;6:225–230. doi: 10.1007/s00737-003-0011-y. doi: 10.1007/s00737-003-0011-y. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Hagan JJ, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Fan H, Xu Y, Zhang K, Huang X, Zhu Y, Liu P. Converging evidence implicates the dopamine D3 receptor gene in vulnerability to schizophrenia. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2011;156B:613–619. doi: 10.1002/ajmg.b.31203. doi: 10.1002/ajmg.b.31203. [DOI] [PubMed] [Google Scholar]