Abstract
Fracture stabilization in the diabetic patient is associated with higher complication rates, particularly infection and impaired wound healing, which can lead to major tissue damage, osteomyelitis, and higher amputation rates. With an increasing prevalence of diabetes and an aging population, the risks of infection of internal fixation devices are expected to grow. Although numerous retrospective clinical studies have identified a relationship between diabetes and infection, currently there are few animal models that have been used to investigate postoperative surgical site infections associated with internal fixator implantation and diabetes. We therefore refined the protocol for inducing hyperglycemia and compared the bacterial burden in controls to pharmacologically induced type-1 diabetic rats after undergoing internal fracture plate fixation and Staphylococcus aureus surgical site inoculation. Using an initial series of streptozotocin doses, followed by optional additional doses to reach a target blood glucose range of 300–600 mg/dl, we reliably induced diabetes in 100% of the rats (n=16) who maintained a narrow hyperglycemic range 14 days after onset of diabetes (466 ± 16 mg/dl, mean ± SEM; coefficient of variation = 0.15). With respect to our primary endpoint, we quantified a significantly higher infectious burden in inoculated diabetic animals (median 3.2 × 1010 CFU/mg dry tissue) when compared to inoculated non-diabetics (7.2 × 104 CFU/mg dry tissue). These data support our hypothesis that uncontrolled diabetes adversely affects the immune system’s ability to clear S. aureus associated with internal hardware.
Keywords: biomedical implants, wound healing, bioburden
1. Introduction
Diabetes mellitus affects 26 million individuals in the United States1, causing a growing incidence of non-healing ulcers with limb and digit amputations. Diabetic patients are at increased risk of bone fractures, often requiring internal hardware2–4.
Over two million orthopedic procedures are performed annually involving implantable materials5. Indwelling devices are associated with a variety of complications, most importantly infection causing soft tissue damage and osteomyelitis4,8,9. These infections are generally difficult to manage and necessitate prolonged antibiotic therapy and revision surgery5–7. The overall infection rate for implanted hardware is about 5% with 40% of these infections caused by Staphylococcus aureus8.
Diabetic patients undergoing orthopedic surgery have increased rates of peri-operative complications9,10. Several large-scale case series studied predictability of post-operative infection11–15, with infection rates in diabetic patients of 7–10% compared to 1% in non-diabetics. Currently, there are few experimental models to study post-operative surgical site infections in diabetic animals after hardware implantation, limiting quantitative evaluation of new therapies. Therefore, we investigated an experimental model for type-1 diabetes in rats that allows quantification of the bacterial burden after hardware implantation and inoculation with S. aureus.
2. Materials and Methods
Fixation plates
Stainless steel fracture plates (2.0 mm, Synthes, Paoli, IL) were cut into 1cm segments. Self-tapping stainless steel cortical screws were cut to a length of 4–5mm.
Bacterial Husbandry
A methicillin-sensitive strain of S. aureus derived from a previously resistant strain of Colindale (termed COL, thought to be strain 9204) was provided by Dr. Vance Fowler, Section of Infectious Diseases, Duke University. Stationary phase cultures of S. aureus were grown from −80°C stock overnight at 37°C in an 8ml TSB tube. Aliquots of 100μl were transferred into a fresh 8ml TSB tubes and incubated at 37°C for 5hr to obtain a log phase culture. This yielded 2 × 108 CFU/ml as estimated by comparison to the 1.0 McFarland standard. Serial dilution and culture plating were used to confirm reproducibility.
In Vivo Studies
This protocol was approved by the Duke University Animal Care and Use Committee. Male CD Rats (150–200g) were obtained from Charles River Laboratories (Raleigh, NC). Rats were given streptozotocin (STZ; VWR, Radnor, PA) injections of 40 mg/kg in citrate buffer consecutively for 3 days, with a fasting period of 8 hours on Day 1 prior to injection3,7. Rats were given water supplemented with 15 g/l sucrose for 48h to protect from STZ-induced insulin release. Forty-eight hours after the third injection, 3-hour fasting blood glucose measurements were taken via tail vein using a standard glucometer (One Touch Ultra, LifeScan, Milpitas, CA).
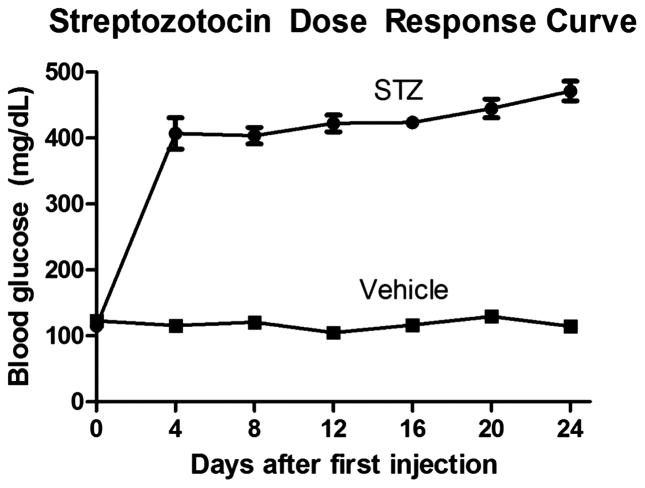
Rats with a fasting blood glucose level on Day 5 of <350 mg/dl received a fourth dose of STZ. This procedure was repeated every other day until the target blood glucose was achieved. Rats in the non-diabetic group received three vehicle injections1,11. Blood glucose was measured over the duration of the experiment (Figure 1). Rats were given food and water ad libitum. Body weight and water intake were assessed.
Figure 1.
Average blood glucose (mg/dl) for both the STZ (n=16, q2 days) and Vehicle (n=8, q4 days) rats. There was a significant increase in the mean blood glucose for the STZ group when compared to the Vehicle group for all days except day 0 (p< 0.0001*).
Femur Implant and Inoculation
Fourteen days after becoming diabetic, rats were anesthetized with 2% isoflurane in oxygen. The right hindlimb skin was shaved, prepped with chlorhexidine and 70% ethanol, and draped. A 2.5cm incision was made on the lateral aspect of the right thigh parallel to the femur between the hip and the knee. The plane between the flexor and extensor musculature was sharply and bluntly dissected. The antero-lateral aspect of the femur was exposed and a pilot hole was drilled through the proximal cortex of the femoral diaphysis using a 1.5mm diameter bit. The sterilized fracture plate was screwed onto the femur. The right femur and implant were inoculated by pipetting 10μl of S. aureus at 2 × 108 CFU/ml onto the fracture plate. The wound was then closed in two layers suturing the fascia with running 4-0 Maxon™, then suturing the skin with interrupted polypropylene. The procedure was repeated for the left hindlimb without bacterial inoculation. All rats received a subcutaneous dose of 2.5 mg/Kg flunixin at the time of surgery and then daily for three days.
Explantation
Seven days after implantation, rats were anesthetized and the skin was shaved and prepped as above. The left hindlimb sutures were removed and a new incision was made directly over the previous wound. The femur was then exposed using blunt dissection. The vastus lateralis muscle directly overlying the implant was excised in line with the ends of the plate roughly approximating the size and shape of the fracture plate. The muscle was then divided with the proximal segment used to calculate a wet:dry weight and the distal segment for bacterial quantification. The screw and plate were removed and placed in a sterile container for biofilm quantification. This procedure was repeated for the right side.
Explant Quantitative Microbiology
The proximal muscle was weighed wet then dried at 50 °C for >24 h. The specimen taken for culture was weighed wet, minced with dissection scissors, and then homogenized by adding a volume of sterile PBS buffer equal to ten times the specimen weight (10X dilution v:w). The homogenate underwent 6 serial 10-fold dilutions and 100μl of each dilution was plated on TSA plates and incubated for 72 h at 37 °C. After 72 h, the plates containing between 30–300 colonies were counted for number of CFUs.
Biofilm Assay
Biofilm formation on the explanted hardware was quantified using the technique of Christensen et al16 and modified by Antoci et al17. Explanted stainless steel implants and cortical screws were transferred into a sterile Falcon 24-well plate and washed 6 times with sterile PBS. The hardware was transferred into a glass Vacutainer containing 1ml TSB + 1% glucose and sonicated for 5 min at 37 °C. Three 300μl aliquots of the sonicated broth were transferred into separate wells on the Falcon plate and incubated overnight at 37°C. Broth was aspirated and each well was washed 3 times with sterile PBS. Crystal Violet (300μl of 1% w:v in PBS) was added and incubated for 5 min at room temperature. The stain was aspirated and wells were washed three times with sterile PBS. Finally, 300μl of glacial acetic acid was added to the wells to re-suspend the crystal violet stain. Absorbance was measured at 595nm using a Genios spectrophotometer running Magellan software (Tecan, Männedorf, Switzerland).
Study Design
Twenty-four rats were divided into two experimental groups, diabetic (n=16) and non-diabetic (n=8). All animals received bilateral femur implants and the right hindlimb of each rat was inoculated with S. aureus, resulting in four rat hindlimb groups. The non-diabetic (ND) non-inoculated (N) data served to quantify infectious burden without direct inoculation in non-diabetic rats. The diabetic (DM)-N data were compared to the ND-N data to determine whether having diabetes alone in the absence of direct inoculation increases infectious susceptibility. The ND-inoculated (I) data served as a control to see if bacterial inoculation produces measurable infection in a non-diabetic animal. The DM-I data were compared to the ND-I group to see if having diabetes has an effect on the immune system’s ability to fight and clear infection. Additionally, we implanted hardware bilaterally into control rats without bacterial inoculation. These data were compared to the ND-N data to see if measurable crossover of bacteria occurs between the inoculated and non-inoculated hindlimbs.
Statistical Analysis
The median, 25th, and 75th quartile CFU/mg tissue for the diabetic and non-diabetic rats were calculated using JMP software (SAS, Cary, NC). Since the microbiologic and biofilm data were not normally distributed, they were compared using a Wilcoxon Rank Sum Test. Blood glucose and body weight data were compared using a Student’s t-test at day 0 and every 4th day.
3. Results
Blood Glucose
Fasting blood glucose measurements for the STZ (diabetic) and vehicle (non-diabetic) groups were averaged for each day (Figure 1). We observed a significant difference in the mean blood glucose levels between both groups for all days except day 0 (p < 0.001*). Average blood glucose for the diabetic group 14 days past initial hyperglycemic measurement was 466 ± 16 mg/dl (mean ± SEM). The non-diabetic group remained normoglycemic at 105 ± 11 mg/dl 12 days after receiving the initial vehicle injection (Figure 1).
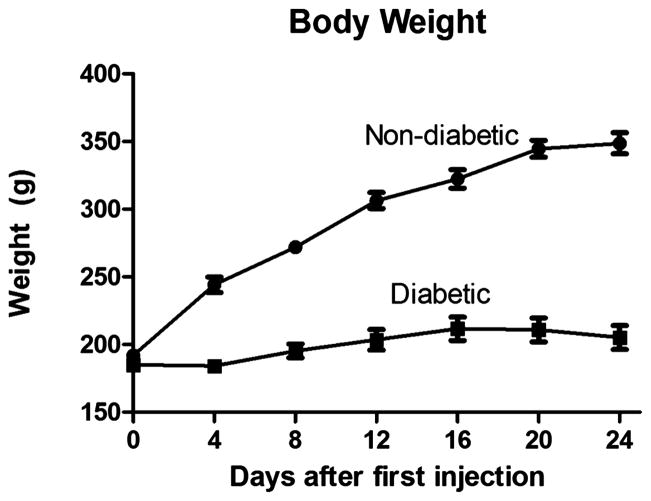
Body Weight
Body weight was averaged by day and group (Figure 2). We observed a significant difference in the mean body weight for all days except day 0 (p < 0.001*). Average body weight on day 24 was 349 ± 8 g for the non-diabetic group and 205 ± 9 for the diabetic group (Figure 2).
Figure 2.
Average body weight (g) for both the diabetic (n=16, q2 days) and non-diabetic (n=8, q4 days) rats. There was a significant difference in the mean body weight between the diabetic and non-diabetic groups for all days except day 0 (p< 0.0001*).
Quantitative Microbiology
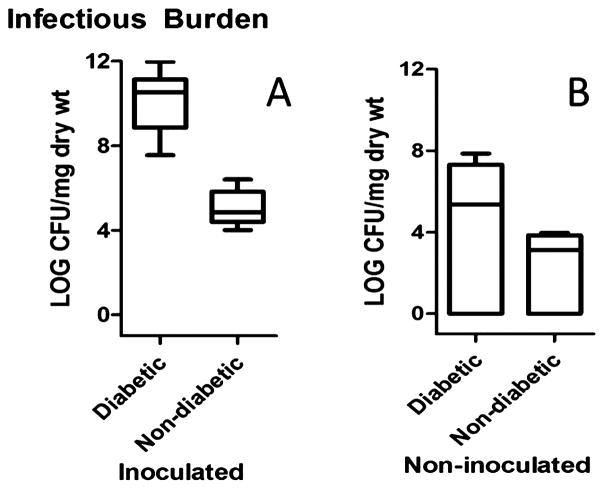
Infectious burden was measured by quantifying CFU of S. aureus 72h after muscle explantation (Figures 3 & 4). We observed a significantly greater infectious burden (CFU/mg dry tissue) in the inoculated hindlimb for the diabetic versus non-diabetic group (p = 0.0003*, Figure 3). The median number of CFU/mg dry tissue in the diabetic group was approximately six orders of magnitude greater than in the non-diabetic group (Table 1). The median CFU/mg dry tissue for the non-inoculated limb in the diabetic rats was consistently above the non-diabetic, however this difference was not significant (Figure 3, Table 1). As expected, when we compared the bacterial burden for the inoculated vs. non-inoculated hindlimbs within each group, we detected a significant increase in the CFU/mg dry tissue for the inoculated limb (Table 1). A significant difference in the CFU/mg dry tissue for the non-inoculated hindlimb in the diabetic inoculated group when compared to the control (diabetic non-inoculated) group (p = 0.64, Figure 4) was not observed, indicating lack of bacterial crossover.
Figure 3.
Infectious burden in the inoculated (A) and non-inoculated hindlimb (B) for both the diabetic (n=14) and non-diabetic (n=7) groups quantified by plate culture method. Box plot data represent median, 25th, 75th percentiles, with whiskers representing minimum and maximum values. Vertical axis is logarithmic base 10 scale with values representing power of the base. There was a significant difference in the concentration of bacteria cultured for the inoculated hindlimb between the diabetic and non-diabetic groups (p = 0.0003*). There was not a significant difference detected in the infectious burden for the non-inoculated hindlimb between the diabetic and non-diabetic groups (p = 0.0682).
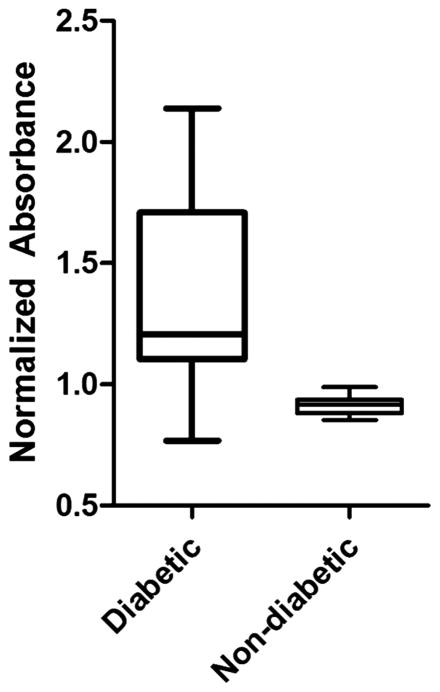
Figure 4.
Normalized absorbance of hardware as measured by indirect staining of biofilm adherent to the fracture plate and screws in both the diabetic and non-diabetic groups. Box plot data represent median, 25th, 75th percentiles, with whiskers representing ± 1.5 times the interquartile range. Data represent values for the hardware of the inoculated hindlimb of each group after normalization to the hardware of non-inoculated hindlimb. Dashed red line has a value of 1 and represents the non-inoculated hindlimb absorbance data. There was a significant increase in the amount of biofilm detected in the inoculated hindlimb of the diabetic rats (p = 0.0017*).
Of the 24 rats in this study, one diabetic rat had to be euthanized prematurely following unintended femur fracture and one non-diabetic rat died prematurely of unknown etiology. Additionally, the culture plates of one rat in the diabetic group became contaminated and those plates were not used for data analysis. Consequently, data from 14 diabetic and 7 non-diabetic animals were analyzed, respectively.
Biofilm Formation
We observed a significant difference in the normalized absorbance between the diabetic, 1.37 (1.11, 2.06), and non-diabetic groups, 0.92 (0.88, 0.94) (p = 0.0017*; Figure 4). Data are presented as median (+ 25th, − 75th percentile). Differences in variability in the diabetic and non-diabetic groups can most likely be attributed to the severity of the disease state in diabetic animals, in particular variable levels of hyperglycemia, which influences bacterial adherence and biofilm formation.
4. Discussion
Streptozotocin and Diabetes
Streptozotocin causes selective pancreatic beta cell toxicity via GLUT-2 receptors-associated uptake18. Single large doses produce diabetes by direct cellular toxicity, whereas multiple lower doses produce diabetes via secondary autoimmune insulitis, better mimicking human type-1 diabetes. Importantly, at low doses (40mg/kg) STZ is not broadly cytotoxic and is not known to directly cause immune dysfunction.
We induced diabetes in 100% of the rats, exceeding results in the literature of 50–90% for single dose or multiple low-dose regimens22. The rats tolerated the IP injections well with none exhibiting direct drug toxicity. No rats exceeded 600 mg/dL or required exogenous insulin.
With the variety of diabetic models available, choosing an experimental model requires careful consideration. Generally, drug-induced type-1 diabetes are the most reproducible and efficient means of generating a stable diabetes23,24. Type-1 diabetes can also be selected for through inbreeding, i.e. with “non-obese diabetic” (NOD) mice19. However, spontaneous models are fairly heterogeneous and contain numerous genes related to autoimmune susceptibility19,20. Type-2 Diabetes is defined by insulin resistance with or without impaired insulin secretion due to obesity, genetic, and environmental factors19–21. Because of the complexity of human type-2 Diabetes, no single animal model is currently capable of representing all aspects of the disease. The specific genotype and phenotype selected for the disease may differ greatly and confound experimental results. Thus, a type-1 diabetic model seemed the most suitable for this investigation. The choice of chemical induction with streptozotocin is due to its efficacy, reproducibility, and controllability19.
Our type-1 diabetic model is a very helpful tool for “diabetic” interrogations. However, type-2 Diabetes mellitus represents the bigger portion of the diabetic population. Therefore, the development of a reliable type-2 model needs to be goal of future diabetes research.
Body Weight
Trends in body weight were monitored to ensure no animals experienced wasting secondary to severe hyperglycemia. The weight gain in the control group was significantly greater in comparison to the modest gain seen in the diabetic group. We attribute this small gain to the stresses of the disease state.
Bacterial Quantification
We consistently reproduced our primary endpoint of quantifying S. aureus infections at the surgical site seven days after plate implantation and bacterial inoculation. Showing a decreased ability to fight post-operative infections, our data aligns with the abundant literature on diabetic pathophysiology in rats. Secondary manifestations of diabetes such as immune dysfunction and impaired wound healing explain the increased infectious burden seen in our study.
With respect to immune dysfunction, diabetes can best be thought of as a mild immunocompromised state, with most immunologic defects occurring in the cellular innate immune system22. Multiple dysfunctions in both neutrophils and macrophages exist (Table 2). Neutrophil dysfunction includes altered activation, increased cell adhesion, altered chemotaxis, and decreased phagocytosis23. Macrophage dysfunction includes decreased cell count, immunogenicity, phagocytic rate, and antigen presenting ability24. With respect to wound healing, our group recently published a review addressing the impaired deep tissue healing at the site of implantation in diabetic animals25. To date, over 100 known pathophysiological factors have been discovered that contribute to impaired wound healing in individuals with diabetes26. These include but are not limited to decreased or impaired growth factor secretion27–29, angiogenic response29,30, collagen accumulation, quality of granulation tissue29, and delayed bone healing31–36. Delayed bone healing can lead to mal-union and subsequently hardware fracture requiring revision surgery. Decreased blood flow to the site of implantation predisposes infection. Immunologic dysfunction and delayed wound healing contribute to the increased postoperative infectious burden of the diabetic patient. Importantly, the rodent immunologic dysfunction observed here must be cautiously interpreted since the immune response to infection seen in humans could differ37,38.
Although our surgical model is novel, we are not the first group to look at in vivo infection susceptibility to S. aureus in diabetic rodents. S. aureus injection in the hind paw of both type-1 and type-2 diabetic rodents causes a significantly higher mean bacterial burden when compared to non-diabetics39. Importantly, this study also showed that strict glucose regulation improved immune function in the diabetic animals. Although the degree of immune dysfunction seems proportional to blood glucose, further research is necessary to investigate whether control of blood glucose levels with exogenous insulin affects the infectious burden in our model.
Biofilm Formation
Biofilm formation is of interest to our study as its presence makes treating orthopedic implant infections extremely difficult. Due to S. aureus’ ability to generate biofilm on hardware, we indirectly quantified the degree of biofilm formation on our implants. Our results showed a significant increase in the ability of S. aureus to form a biofilm on the implanted hardware in the diabetic animals. It is known that the majority of biofilm formation occurs during the early post-operative period. Thus, immediately after bacterial inoculation becomes the critical period when the body must prevent bacterial adherence to the implanted hardware. However, the early immune response to infection is dominated by neutrophils, and without an intact innate cellular immune response S. aureus is able to freely colonize the implant. Importantly, normalizing the absorbance values was required due to intra-experimental variability in the absolute absorbance values detected by our spectrophotometer. We believe the relative values obtained are reliable because the inoculated and non-inoculated sides for a given animal were analyzed simultaneously.
Study Limitations
In our study, we are limited in drawing conclusions about the infectious susceptibility of our diabetic animals when compared to non-diabetics. Insight into infectious susceptibility comes from the bacterial quantification data in the non-inoculated hindlimb. One would assume that the non-inoculated hindlimbs of both groups would have approximately the same degree of bacterial burden if their infectious susceptibility was the same. However, this was not the case, as the diabetic group had increased bacterial burden in the non-inoculated hindlimb when compared to the non-diabetic group. Even in the absence of direct inoculation, the diabetic group was more susceptible to a more severe post-operative infection than the non-diabetic group. This trend is consistent with recent reports in the literature, which point to a variety of factors that contribute to infection susceptibility14,25, including decreased inflammatory response, poor angiogenic response, and nitric oxide deficiency. Insufficient power is likely why the two The fact that a significant difference could not be detected between the two groups is groups could not be distinguished, as the sample size for our study was small. Additonally, the duration of the study may affect the bioburden as it is possible the infection for the diabetic animals would worsen over time while the infection in control subjects may clear. In contrast, the difference between the bioburden of the inoculated and non-inoculated non-diabetic rats was statistically significant. This discrepancy may be attributed to the variability of the infectious burden in the diabetic non-inoculated group. While some diabetic wounds exhibited considerable infection in the absence of inoculation, others had none. In contrast, data obtained from non-diabetic rats was much less variable. The severity of the disease state in the diabetic rats may thus influence the variability. Rats with more severe hyperglycemia (>500 mg/dl) may exhibit greater immune deficiency resulting in increased infectious burden in comparison to rats with moderate hyperglycemia (350–500 mg/dl). Importantly, we attribute the bacterial growth in the non-inoculated hindlimb of both groups to normal bacterial burden from surgery, even though every effort was made to remain sterile for the entirety of the operations.
Our study was limited in its ability to quantify total infectious burden at the surgical site as we harvested only the muscle directly overlying the fracture plate. Although it is likely that the infections were not localized, the technique we employed provided the accuracy and precision needed to detect statistically significant differences via tissue plate quantification method. Lastly, our model of directly inoculating the surgical site with concentrated bacteria does not mimic real-life acquisition of a surgical infection.
5. Conclusion
The extensive literature on immune dysfunction and delayed wound healing in diabetic patients emphasizes the need to study post-operative infections in diabetic animals. The novel outcomes-based STZ dosing regimen presented here induced diabetes in 100% of the rats and achieved consistent hyperglycemic values. Diabetic rats demonstrated elevated infection proclivity above non-diabetics after S. aureus inoculation in the presence of implanted hardware. These data support the hypothesis that uncontrolled type-1 diabetes adversely affects the immune system’s ability to fight and clear bacteria in the post-operative period.
Supplementary Material
Table 1: Infectious burden in the diabetic and non-diabetic groups for the inoculated and non-inoculated hindlimbs (data represent median CFU/mg dry tissue). We detected a significant increase in the infectious burden in the diabetic group for the inoculated hindlimb when compared to the non-diabetic (p = 0.003*). No significant difference in the infectious burden between groups for the non-inoculated side was detected (p = 0.0682). We detected a significant difference in infectious burden between the inoculated and non-inoculated side for both diabetic and non-diabetic groups (p < 0.0001* and p = 0.0017*, respectively).
Table 2: Summary of the immune dysfunctions found in diabetic patients (adapted from FEMS Immunology and Medical Microbiology 1999; 26:259–265). Up and down arrows represent increased or decreased function and or quantity. An equal sign represents no significant difference between diabetics and non-diabetics.
Acknowledgments
This work was funded in part by NIH grants EB000708, by NIH grant T32 GM08555, and by the Robert Jones Fund.
The authors thank Dr. Howie Levinson, Dr. Scott Nichols, Ms. Julia Skettini, and Ms. Reema Sil, whose help and suggestions were greatly appreciated. This work was funded in part by NIH grants EB000708, by NIH grant T32 GM08555, and by the Robert Jones Fund.
Footnotes
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.National diabetes fact sheet: general information and national estimates on diabetes in the United States. U.S. Department of Health and Human Services; 2007. [Accessed 09/27/2010]. at http://www.cdc.gov/diabetes/pubs/factsheet07.htm. [Google Scholar]
- 2.Botushanov NP, Orbetzova MM. Bone mineral density and fracture risk in patients with type 1 and type 2 diabetes mellitus. Folia Med (Plovdiv) 2009;51:12–7. [PubMed] [Google Scholar]
- 3.Khazai NB, Beck GR, Jr, Umpierrez GE. Diabetes and fractures: an overshadowed association. Curr Opin Endocrinol Diabetes Obes. 2009;16:435–45. doi: 10.1097/MED.0b013e328331c7eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz AV. Diabetes Mellitus: Does it Affect Bone? Calcif Tissue Int. 2003;73:515–9. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche R. Treatment of infections associated with surgical implants. New England Journal of Medicine. 2004;350:1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 6.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–9. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein RA, Darouiche RO. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clinical Infectious Diseases. 2001;33:1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 8.Arciola C, An Y, Campoccia D, Donati M, Montanaro L. Etiology of implant orthopedic infections: a survey on 1027 clinical isolates. International Journal of Artificial Organs. 2005;28:1091–100. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- 9.Moon HK, Han CD, Yang IH, Cha BS. Factors Affecting Outcome after Total Knee Arthroplasty in Patients with Diabetes Mellitus. Yonsei Med J. 2008;49:129–37. doi: 10.3349/ymj.2008.49.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalavras C, Christensen T, Rigopoulos N, Holtom P, Patzakis M. Infection Following Operative Treatment of Ankle Fractures. Clinical Orthopaedics and Related Research®. 2009;467:1715–20. doi: 10.1007/s11999-009-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication Rates Following Open Reduction and Internal Fixation of Ankle Fractures. J Bone Joint Surg Am. 2009;91:1042–9. doi: 10.2106/JBJS.H.00653. [DOI] [PubMed] [Google Scholar]
- 12.SooHoo N, Farng E, Lieberman J, Chambers L, Zingmond D. Factors That Predict Short-term Complication Rates After Total Hip Arthroplasty. Clinical Orthopaedics and Related Research®. 2010;468:2363–71. doi: 10.1007/s11999-010-1354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460–5. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Anderson M, Cheng W, Wongworawat M. Diabetes Associated with Increased Surgical Site Infections in Spinal Arthrodesis. Clinical Orthopaedics and Related Research®. 2009;467:1670–3. doi: 10.1007/s11999-009-0740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchant MH, Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The Impact of Glycemic Control and Diabetes Mellitus on Perioperative Outcomes After Total Joint Arthroplasty. J Bone Joint Surg Am. 2009;91:1621–9. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 16.Gordon Christensen AS, Younger Janara, Baddour Larry, Barrett Fred, Melton Dennis, Beachey Edwin. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: a Quantitiative Model for Adherence of Staph to Medial Devices. Journal of Clinical Microbiology. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoci V, Jr, Adams CS, Parvizi J, et al. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684–90. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 2007;12:261–6. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 19.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359–70. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 20.von Herrath M, Nepom GT. Animal models of human type 1 diabetes. Nat Immunol. 2009;10:129–32. doi: 10.1038/ni0209-129. [DOI] [PubMed] [Google Scholar]
- 21.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–39. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunology and Medical Microbiology. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 23.Alba-Loureiro TC, Munhoz CD, Martins JO, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037–44. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 24.Ma H, Liu G, Ding W, Wu Y, Cai L, Zhao Y. Diabetes-induced alteration of F4/80+ macrophages: a study in mice with streptozotocin-induced diabetes for a long term. J Mol Med. 2008;86:391–400. doi: 10.1007/s00109-008-0304-8. [DOI] [PubMed] [Google Scholar]
- 25.Nga N Le MBR, Levinson Howard, Klitzman Bruce. Implant Healing in Experimental Animal Models of Diabetes. Journal of Diabetes Science and Technology. 2011;5:605–6. doi: 10.1177/193229681100500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galkowska HUW, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal epithelial cells in the margin of chronic diabetic food ulcers. Wound Repair Regen. 2006;14:558–65. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 28.Goren I, Müller E, Pfeilschifter J, Frank S. Severely Impaired Insulin Signaling in Chronic Wounds of Diabetic ob/ob Mice: A Potential Role of Tumor Necrosis Factor-[alpha] The American Journal of Pathology. 2006;168:765–77. doi: 10.2353/ajpath.2006.050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366:1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 30.Galiano RD, Tepper OM, Pelo CR, et al. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. The American Journal of Pathology. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1988:210–6. [PubMed] [Google Scholar]
- 32.Follak N, Klöting I, Merk H. Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats. Diabetes/Metabolism Research and Reviews. 2005;21:288–96. doi: 10.1002/dmrr.537. [DOI] [PubMed] [Google Scholar]
- 33.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasahara T, Imai S, Kojima H, et al. Malfunction of bone marrow-derived osteoclasts and the delay of bone fracture healing in diabetic mice. Bone. 2010;47:617–25. doi: 10.1016/j.bone.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azad V, Breitbart E, Al-Zube L, Yeh S, O’Connor JP, Lin SS. rhBMP-2 enhances the bone healing response in a diabetic rat segmental defect model. J Orthop Trauma. 2009;23:267–76. doi: 10.1097/BOT.0b013e31819f290e. [DOI] [PubMed] [Google Scholar]
- 36.Ogasawara A, Nakajima A, Nakajima F, Goto K-i, Yamazaki M. Molecular basis for affected cartilage formation and bone union in fracture healing of the streptozotocin-induced diabetic rat. Bone. 2008;43:832–9. doi: 10.1016/j.bone.2008.07.246. [DOI] [PubMed] [Google Scholar]
- 37.Roep BO. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann N Y Acad Sci. 2007;1103:1–10. doi: 10.1196/annals.1394.018. [DOI] [PubMed] [Google Scholar]
- 38.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 39.Park S, Rich J, Hanses F, Lee JC. Defects in Innate Immunity Predispose C57BL/6J-Leprdb/Leprdb Mice to Infection by Staphylococcus aureus. Infect Immun. 2009;77:1008–14. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Infectious burden in the diabetic and non-diabetic groups for the inoculated and non-inoculated hindlimbs (data represent median CFU/mg dry tissue). We detected a significant increase in the infectious burden in the diabetic group for the inoculated hindlimb when compared to the non-diabetic (p = 0.003*). No significant difference in the infectious burden between groups for the non-inoculated side was detected (p = 0.0682). We detected a significant difference in infectious burden between the inoculated and non-inoculated side for both diabetic and non-diabetic groups (p < 0.0001* and p = 0.0017*, respectively).
Table 2: Summary of the immune dysfunctions found in diabetic patients (adapted from FEMS Immunology and Medical Microbiology 1999; 26:259–265). Up and down arrows represent increased or decreased function and or quantity. An equal sign represents no significant difference between diabetics and non-diabetics.