Abstract
Subcutaneous mycoses are chronic fungal infections of the skin and subcutaneous tissues caused by variety of fungal agents and usually occur following trauma with vegetative matter. We report a case of subcutaneous mycoses caused by rare fungus belonging to the genus Rhytidhysteron, in an immunocompetent male who presented with a subcutaneous nodule on left foot. This unusual species was identified and confirmed by molecular methods.
Keywords: Subcutaneous mycoses, Rhytidhysteron species, Immunocompetent, Non-sporulating, India, Sequencing
1. Introduction
Subcutaneous mycoses include a heterogeneous group of fungal infections that develop at the site of transcutaneous trauma and can occur in both immunocompetent and immunocompromised patients. Infection evolves slowly as the etiological agent survives and adapts to the adverse host tissue environment. The main subcutaneous fungal infections include sporotrichosis, chromoblastomycosis, mycetoma, lobomycosis, rhinosporidiosis, subcutaneous zygomycosis, and subcutaneous phaeohyphomycosis [1]. The common fungal etiologic agents like Sporothrix schenkii, Cladophialophora carrionii, Fonsecaea pedrosoi, Phialophora verrucosa, Rhinocladiella aquaspersa, Exophiala jeanselmei, Exophiala spinifera, Wangiella dermatitidis, Acremonium spp., Conidiobolus coronatus and Basidiobolus ranarum have been associated with subcutaneous mycotic infections [2–5]. The subcutaneous mycoses caused by rare fungi such as Colletotrichum species, Diaporthe, Fusarium subglutinans, Chaetomium funicola etc. have been described in the literature so far [6–9]. We report a case of subcutaneous mycoses in an immunocompetent male caused by a dematiaceous fungus belonging to genus Rhytidhysteron.
2. Case
A 65-year old, hawker by profession, resident of Delhi, presented with a painless subcutaneous nodule on tendoachilles region in the left foot with duration of 10 years. The nodule was about 4×4 cm2 in size, well circumscribed, indurated, nontender, and the overlying skin was hard and blackish in color (Fig. 1). There was no discharging sinus, grains or any ulceration. Patient was healthy otherwise, with no evidence of immunosuppression or diabetes. There was no history of travel or treatment. However, history of trauma could not be excluded owing to bare feet walking in his profession. All investigations were done on the day 0 when patient first presented to the hospital. The routine laboratory investigations which included complete blood counts, random blood glucose, urine analysis, liver and renal function test and X-ray chest were normal. Patient was seronegative for HIV.
Fig. 1.
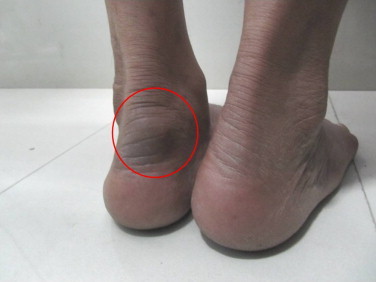
Swelling measuring 4×4 cm2 on tendoachilles in left foot in a 65-year-old male.
Fine needle aspiration cytology (FNAC) was done from the nodule and aspirate was sent for analysis. Direct microscopic examination of aspirate after digestion with 10 percent potassium hydroxide (KOH) demonstrated thick brown hyphae. No spherical or sclerotic bodies were seen, ruling out the diagnosis of chromoblastomycosis. FNAC smears revealed septate and branching pigmented fungal hyphae which were PAS positive and surrounded by intense neutrophilic reaction (Fig. 2). Acid fast stains for aspirate was negative. Fungal cultures were performed on Sabouraud׳s Dextrose Agar with and without cycloheximide and incubated at 25 °C. After 2 weeks grayish black, floccose colonies with black reverse were observed on culture tubes (Fig. 3). Standard Tease Mounts using Lacto Phenol Cotton Blue and slide cultures using Potato Dextrose Agar (PDA) and Malt Extract Agar (MEA) were prepared to observe the microscopic morphology and sporulation respectively. Microscopically, it showed thick, brown septate hyphae but the isolate was non-sporulating (Fig. 4).
Fig. 2.
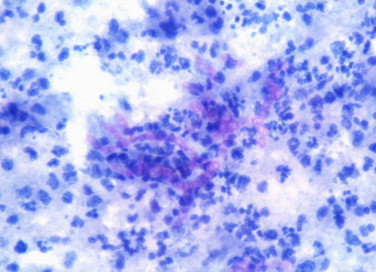
Fine needle aspirate from the foot swelling shows branching septate hyphae, which are Periodic Acid Schiff (PAS) positive and surrounded by intense neutrophilic reaction (400×).
Fig. 3.
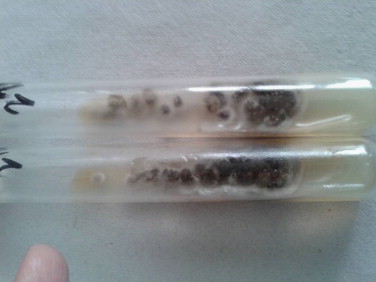
Culture shows fluffy, black colonies Sabouraud’s dextrose Agar at 25 °C.
Fig. 4.
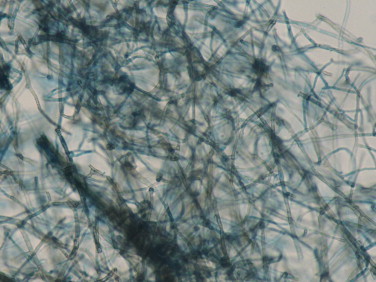
Lacto phenol Cotton Blue mount shows branching hyphae having black cell walls with tiny conidia sticking to them (400×).
Based on the macroscopic and microscopic observations, the isolate could not be identified, hence, was subjected to molecular methods for identification. The culture grown on SDA slant was subjected to PCR amplification and sequencing using the panfungal primers ITS1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS4 (3′ TCCTCCGCTTATTGATATGC 5′) to amplify the internal transcribed spacer (ITS) region of 18S rRNA in an outsourced laboratory (CromDx Solutions, India). A homology search of all the sequences was carried out using BLAST (National Center for Biotechnology Information, Washington, DC). The isolate was identified as Rhytidhysteron species, with the closest link being Rhytidhysteron rufulum with 97% identity to the GenBank accession number AM711974.1. However, due to considerable uncertainty of species classification in the genus and lack of sufficient molecular data, species classification cannot be achieved unequivocally. The GenBank accession number of the small subunit sequence is KM052345. The isolate was deposited to the National Reference and Research Laboratory, Mycology division; Post Graduate Institute of Medical Education Research (PGIMER), Chandigarh, India for further confirmation.
The patient was treated with oral terbinafine (250 mg/day) and itraconazole (100 mg/day) and the swelling partially regressed after 3 months. He has been advised to continue treatment for 1 year and is on regular follow-up.
3. Discussion
Rhytidhysteron genus was described initially in the poorly known family of Ascomycetes, Patellariaceae [10]. It is characterized by hysteriform ascomata that become discoidal at maturity and paraphyses covered by a gelatinous layer (pseudoepithecium). The ascospores are thick walled and deeply pigmented. The genus comprised two species: R. rufulum and R. hysterinum. Recently on the basis of aminoacid and sequence data provided by Boehm et al. in 2009, the Rhytidhysteron genus, previously placed in the Patellariaceae, has been transferred to the Hysteriaceae [11].
Rhytidhysteron spp. are common in the tropics and subtropics where it grows as a saprobe or weak parasite on wood of a variety of dicotyledonous plants. These are rare human pathogens. Very few reports of mycoses caused by Rhytidhysteron sp. have been described in literature [12,13]. In our report, the patient was immunocompetent and we hypothesize that walking bare footed might have led to a penetrating injury from plants resulting in entry of this fungus as it is a saprobe with a diverse geographical distribution in tropical countries. This suggests that Rhytidhysteron sp. is not an opportunistic pathogens and can cause infection in both immunocompetent and immunocompromised patients with different clinical presentations. As described by Chowdhary et al. [12], our isolate also failed to sporulate on laboratory media and was identified by ITS sequencing, highlighting the necessity of molecular techniques for identification of non-sporulating fungus. As the previous cases of human infections caused by this fungus have been reported only from India, presence of this fungus in Indian environment is speculated. The fungus in the genus Rhytidhysteron are not known pathogens in humans and currently no specific management protocols exist, so the patient was treated with antifungal combination of itraconazole and terbinafine considered appropriate for management of subcutaneous mycoses caused by dematiceous fungus. Since experience with this fungus has been limited so far, further case reports and studies may be helpful in establishing the epidemiology, morphological features and management protocol for this group of mycoses.
In conclusion, subcutaneous mycoses can be caused by both common and rare fungal species. Apart from routine fine needle aspirate cytology and fungal cultures, molecular methods for identification of non-sporulating fungus should be included in the diagnostic workup of such cases. Further characterization of an unusual fungal species and its antifungal susceptibility profile should be performed to guide the clinicians so that they can optimize the accurate treatment.
Conflict of interest
None.
Source of funding
None.
Acknowledgments
The authors are thankful to Dr. Arunaloke Chakraborty, Professor Department of Medical Microbiology, and Centre of Advanced Research in Medical Mycology, Post Graduate Institute of Medical Education and Research, Chandigarh, India for confirming our isolate species.
References
- 1.Queiroz-Telles F., McGinnis M.R., Salkin I., Graybill J.R. Subcutaneous mycoses. Infect Dis Clin N Am. 2003;17(1):59–85. doi: 10.1016/s0891-5520(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 2.McGinnis M.R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1–6. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 3.Rippon J.W. WB Saunders; Philadelphia: 1988. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes; pp. 276–296. [Google Scholar]
- 4.Kwon-Chung K.J., Bennett J.E. Lea and Febiger; Philadelphia: 1992. Medical mycology; pp. 337–355. [Google Scholar]
- 5.Hospenthal D.R. Agents of chromoblastomycosis. In: Mandell G.L., Bennet J.E., Dolin R., editors. Principles and practice of infectious disease. 7th ed. Churchill Livingstone; Philadelphia: 2010. pp. 2643–2654. [Google Scholar]
- 6.Guarro J., Svidzinski T.E., Zaror L., Forjaz M.H., Gene´ J., Fischman O. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. J Clin Microbiol. 1998;36(10):3060–3065. doi: 10.1128/jcm.36.10.3060-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iriart X., Binois R., Fior A., Blanchet D., Berry A., Cassaing S. Eumycetoma caused by Diaporthe phaseolorum (Phomopsis phaseoli): a case report and a mini-review of Diaporthe/Phomopsis spp invasive infections in humans. Clin Microbiol Infect. 2011;17(10):1492–1494. doi: 10.1111/j.1469-0691.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Macías P., Arenas-Guzmán R., Hernández-Hernández F. Fusarium subglutinans: a new eumycetoma agent. Med Mycol Case Rep. 2013;2:128–131. doi: 10.1016/j.mmcr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepenbring M., Mendez O.A.C., Espinoza A.A.E., Kirschner R., Schöfer H. Chromoblastomycosis casued by Chaetomium funicola: a case report from Western Panama. Br J Dermatol. 2007;157:1025–1029. doi: 10.1111/j.1365-2133.2007.08091.x. [DOI] [PubMed] [Google Scholar]
- 10.Samuels G.J., Muller E. Life-history studies of Brazilian Ascomycetes. Rhytidhysterium rufulum and the genus Eutryblidiella. Sydowia. 1979;32:277–292. [Google Scholar]
- 11.Boehm E.W.A., Schoch C.L., Spatafora J.W. On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycol Res. 2009;113:461–479. doi: 10.1016/j.mycres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhary A., Guarro J., Randhawa H.S., Gené J., Cano J., Jain R.K. rare case of chromoblastomycosis in a renal transplant recipient caused by a non-sporulating species of Rhytidhysteron. Med Mycol. 2008;46:163–166. doi: 10.1080/13693780701630420. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan V.K., Sharma V., Prabha N., Thakur K., Sharma N.L., Rudramurthy S.M. A rare case of subcutaneous phaeohyphomycosis caused by a Rhytidhysteron species: a clinico-therapeuticexperience. Int J Dermatol. 2014;5 doi: 10.1111/ijd.12529. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]


