Summary
Using a sample of 2925 stroke-free participants drawn from a national population-based study, we examined cross-sectional associations of obstructive sleep apnea risk (OSA) with cognition and quality of life and whether these vary with age, while controlling for demographics and co-morbidities. Included participants from the REasons for Geographic And Racial Differences in Stroke Study were aged 47-93. OSA risk was categorized as high or low based on responses to the Berlin Sleep Questionnaire. Cognitive function was assessed with standardized fluency and recall measures. Depressive symptoms were assessed with the four-item Center for Epidemiologic Studies Depression Scale. Health-related Quality of Life (HRQoL) was assessed with the Medical Outcomes Study Short Form-12 (SF-12). MANCOVA statistics were applied separately to the cognitive and quality of life dependent variables while accounting for potential confounders (demographics, co-morbidities). In fully adjusted models, those at high risk for OSA had significantly lower cognitive scores (Wilks’ Lambda = 0.996, F(3, 2786) = 3.31, p < .05) and lower quality of life (depressive symptoms and HRQoL) (Wilks’ Lambda = 0.989, F(3, 2786) = 10.02, p < .0001). However, some of the associations were age-dependent. Differences in cognition and quality of life between those at high and low obstructive sleep apnea risk were most pronounced during middle age, with attenuated effects after age 70.
Keywords: Obstructive sleep apnea, Berlin Sleep Questionnaire, cognitive function, depression, health related quality life, age differences
Recent studies have shown an association between obstructive sleep apnea (OSA) and cognitive impairment. Research examining cognition in OSA has shown impairments in general intellectual functioning, attention/concentration, vigilance, memory, and particularly executive functioning (Beebe et al., 2003; Sateia, 2003). Frontal lobe (i.e., executive) dysfunction has been proposed as the primary manifestation of OSA (Beebe & Gozal, 2002). This hypothesis is also supported by imaging studies (Zimmerman & Aloia, 2006). Factors often associated with OSA, such as psychological distress and co-morbid medical conditions, are also well-established correlates of executive dysfunction (O’Hara et al., 2006; Waldstein et al., 2001), and may substantially contribute to cognitive dysfunction in OSA. In addition, demographic characteristics may function as risk or protective factors for cognitive decline in this population. Although these factors have been suggested as possible contributors to this relationship, few studies have adequately controlled for these factors in analyses of OSA and cognition.
It is of particular interest to determine whether the cognitive and quality of life sequelae of OSA vary with age. Indeed, a recent review of OSA in adults reported that the increased risk of morbidity and mortality associated with OSA peaks at approximately 55 years of age (Lurie, 2011). However, a recent longitudinal study with an older population (mean age = 82) also found increased risk of mild cognitive impairment or dementia in participants with sleep-disordered breathing versus those without this condition (Yaffe et al., 2011). The study adjusted for demographic characteristics (i.e., age, race, education), comorbid conditions (i.e., diabetes, hypertension), and antidepressant use, but the results were limited to older women, and there was no examination of self-reported quality of life.
The purpose of the present study was to examine OSA and its association with depressive symptoms, quality of life, and cognitive function while controlling for other factors that may contribute to this association (i.e., age, sex, education, race, diabetes, dyslipidemia). This study extends prior research by utilizing a large national sample of black and white individuals from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, thus allowing simultaneous control of several variables while ensuring sufficient statistical power. We posited that, after controlling for potential confounders, cognitive performance would be lower, depressive symptoms would be more severe, and health-related quality of life (HRQoL) would be poorer in those at high versus low risk for OSA.
Methods
Design and Procedures
The REGARDS study aims to determine the causes of higher stroke mortality among black Americans and residents of the southern Stroke Belt (i.e., Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee). Mail and telephone contact methods were used to recruit the cohort of US adults 45 years of age or older from lists of U.S. residents purchased from Genesys, Inc. A total of 30,239 participants were recruited from January 2003 to October 2007. The institutional review boards on human research at all participating institutions approved the study. At baseline, participants answered demographic and medical history questions during a computer-assisted telephone interview. During a home visit they provided appropriate written informed consent, anthropometric measurements, blood pressure, blood and urine, medication inventory, and an electrocardiogram. Participants are followed by telephone twice per year. If a participant reports a suspected stroke, his/her medical records are obtained and adjudicated by study physicians to confirm or refute stroke incidence.
Data for this report were drawn from a quarterly data freeze on 10/01/2010. For the current analyses of sleep apnea risk, cognitive function, and quality of life variables (depression and HRQoL), we restricted our analyses to participants who had completed (1) the sleep apnea module, which was administered to each participant beginning in September 2008 at the first available six-month follow up call, (2) quality of life measurements assessed at baseline interview, and (3) an expanded cognitive battery including verbal fluency and word list learning and recall. The components of this expanded battery were implemented into the REGARDS follow-up call sequence over time. The battery was not administered as a unit until May, 2008, during participants’ scheduled follow up calls at months 18, 42, 66, and every 2 years thereafter (with timing depending upon the date of initial enrollment). For our purposes, we were interested in using concurrently administered cognitive assessments that were temporally contiguous to administration of the sleep apnea module (defined as within 365 days). To meet this criterion, we included only those participants who had completed the expanded cognitive battery in May 2008 or later and the sleep apnea module in September 2008 or later. The median number of days between these assessments was 192, and the mean was 162.6 (s.d. 138.3). With these restrictions, as well as the exclusion of participants with self-reported stroke at baseline and/or with confirmed incident stroke prior to their first expanded cognitive assessment in 2008 or later, 2925 participants were available for inclusion in these analyses.
Measures
Demographics
Participants’ age, sex, race (black, white), and educational attainment (less than high school, high school graduate, some college or vocational training, or college graduate) were obtained by self-report.
Obstructive Sleep Apnea (OSA)
The Berlin Sleep Questionnaire (BSQ; Netzer et al., 1999) includes 3 symptom categories of risk for OSA: (1) snoring (5 items), (2) daytime sleepiness (3 items), and (3) hypertension/body mass index (BMI) (2 items). Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or self-reported use of antihypertensive medications. BMI was calculated from measured height and weight. Per the standard protocol, participants were placed into two dependent variable categories based on their responses: (1) High risk for OSA diagnosis and (2) Low risk for OSA diagnosis. Respondents were considered high risk for OSA if they met criteria in 2 of the 3 symptom categories (Netzer et al., 1999), as indicated by ≥2 positive responses in symptom category 1, ≥2 in category 2, and ≥1 in category 3 (or a BMI >30). Those with sufficient symptoms in none or only one of the symptom categories were considered low risk for OSA. The BSQ has been found to predict elevated respiratory distress indices in high risk respondents with a sensitivity of 0.86, specificity of 0.77, and a positive predictive value of 0.89 (Netzer et al., 1999). Preceding the BSQ questions was a question regarding diagnosis of sleep apnea, followed by questions about treatment for those who reported having been given the diagnosis. Because studies have shown that Continuous Positive Airway Pressure (CPAP) treatment may be associated with improvement in cognition (Ferini-Strambi et al., 2003; Feuerstein et al., 1997), depressive symptoms (Kawahara et al., 2005; Means et al., 2003), and HRQoL (Siccoli et al., 2008), we examined the impact of self-reported OSA treatment as a modifier of outcomes among those who self-reported a physician diagnosis of sleep apnea.
Cognitive Function
Verbal Memory (Word List Delayed Recall)
Word List Delayed Recall is a component of Word List Learning from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery. The list learning portion consists of three learning trials of 10 semantically-unrelated words which are presented in a fixed order that varies across the three trials (Morris et al., 1989). In REGARDS, this measure is administered according to the standard protocol, with two modifications for telephone administration: (1) no visual presentation of the stimuli and (2) participants are instructed not to write anything down. For delayed recall, participants are asked to freely recall as many of the ten words as possible after an approximate 5-minute delay. Non-cognitive interview questions are asked during the delay. Scores range from 0 to 10.
Executive Function (Animal Fluency and Letter Fluency)
The fluency measures were administered according to standardized scripts (Strauss et al., 2006) which require participants to name as many words beginning with the letter ‘F’ as they can, and subsequently, to name as many animals as they can. The time allotted for each measure is 1 minute. Raw scores on each consist of the total number of valid responses produced by each participant in 60 seconds. Responses were recorded on audio. WAV files that were later scored by trained college-educated scorers with computer-assisted scoring programs developed for this purpose. Inter-scorer agreement on scores for these measures was excellent (kappas >.95) for both.
Quality of Life
For the purposes of this study, we included depressive symptoms and mental and physical aspects of HRQoL under the broader designation “quality of life”.
Depressive Symptoms
The 4-item Center for Epidemiological Studies - Depression Scale (CES-D-4; Melchior et al., 1993) was derived from the original 20-item CES-D (Radloff, 1977) to identify the presence of depressive symptoms. Reliability and validity are similar to that of the original instrument (Melchior et al., 1993). Responses to each item are recorded on a 4-point (0-3) scale for symptoms experienced in the preceding week. Scores range from 0 to 12, with higher scores indicating endorsement of more depressive symptoms, and a score of ≥ 4 indicating elevated psychological distress (Melchior et al., 1993). For the present study, scores were analyzed on a continuum from 0 to 12.
Health-related Quality of Life (HRQoL)
HRQoL was assessed utilizing responses to The Medical Outcomes Study Short Form-12 (SF-12). Responses to the SF-12 produce two scales, the Mental Component Summary (MCS-12) indicating mental health and well-being, and the Physical Component Summary (PCS-12) indicating physical health and well-being (Ware et al., 1996). The scores for each scale range from 0 to 100, with higher scores indicative of better functioning. The MCS-12 and PCS-12 scores have been found to closely approximate their equivalents in the SF-36 (Ware et al., 1996).
Diabetes and Dyslipidemia
Participants were categorized as diabetic if they met one or more of the following criteria: fasting glucose of ≥ 126 mg/dL, non-fasting glucose of ≥ 200 mg/dL, or use of diabetic medication or insulin. Dyslipidemia was defined by one or more of the following: total cholesterol of ≥ 240 mg/dL, LDL ≥ 160 mg/dL, HDL ≤ 40 mg/dL, or use of lipid modifying medication. REGARDS baseline BMI and hypertension measurements were not included as comorbid conditions, as these conditions were also assessed as part of the BSQ definition of risk for OSA.
Statistical Analyses
Pearson chi-square tests were used to assess differences of proportions in categorical variables across BSQ groups, and t-tests were used to identify differences in means for continuous variables. The three cognitive outcomes were analyzed simultaneously in incremental MANOVA/MANCOVA models, as were the three quality of life outcome variables (i.e., depressive symptoms, HRQoL mental components, HRQoL physical components). Planned univariate analyses were also conducted to examine the relationships between BSQ group and each of the six outcomes of interest utilizing incremental ANOVA/ANCOVA models (separately for each outcome). Unadjusted univariate analyses were performed, followed by adjustment for demographic variables (age, sex, race, educational attainment). Co-morbid medical conditions (diabetes, dyslipidemia) were included in a third and final model. Interactions between BSQ group and model covariates were evaluated and interaction plots were constructed for significantly interacting variables. In a sensitivity analysis, we replicated these models in a sample restricted to participants at highest OSA risk (BSQ positive in all three symptom categories) and lowest OSA risk (BSQ negative in all three symptom categories) (total sensitivity analysis n = 733). Analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
The mean age of the sample at the time of completion of the BSQ was 67.5 years, with a range of 47-93 years. Approximately 57% of the sample were women, and 65% were white. Over 90% of the sample had an education level of high school or above, with only 8% having less than a high school education. Participants with high OSA risk were younger on average, more likely to be male, and more likely to have diabetes and dyslipidemia than those at low risk (Table 1).
Table 1.
Demographic and co-morbid characteristics of study population by risk for Obstructive Sleep Apnea (OSA).
| OSA Risk | ||||
|---|---|---|---|---|
| Variable | Overall n=2925 | High Risk+ n=782 | Low Risk n=2143 | p-value |
| Mean (sd) Age at sleep questionnaire | 67.53 (8.73) | 65.30 (7.95) | 68.34 (8.86) | <0.0001 |
| N (%) male | 1246 (42.60) | 357 (45.65) | 889 (41.48) | 0.0436 |
| N (%) Education | ||||
| Less than high school | 234 (8.00) | 65 (8.31) | 169 (7.89) | |
| High school graduate | 755 (25.81) | 203 (25.96) | 552 (25.76) | 0.7691 |
| Some college | 812 (27.76) | 225 (28.77) | 587 (27.39) | |
| College graduate and above | 1124 (38.43) | 289 (36.96) | 835 (38.96) | |
| N (%) Black | 1016 (34.74) | 277 (35.42) | 739 (34.48) | 0.6374 |
| N (%) Diabetes* | 539 (18.97) | 199 (26.18) | 340 (16.34) | <0.0001 |
| N (%) Dyslipidemia** | 1656 (58.35) | 510 (67.11) | 1146 (55.15) | <0.0001 |
Diabetic if fasting glucose >=126 mg/dL / non-fasting glucose >=200 mg/dL or on diabetic medication or insulin
Dyslipidemia if Total cholesterol C >=240 mg/dL or LDL >=160 mg/dL or HDL <= 40 mg/dLor on lipid-lowering medication
Respondents were considered high risk for Obstructive Sleep Apnea (OSA) if they met criteria in 2 or 3 of the three symptom categories of the Berlin Sleep Questionnaire, while those with symptoms in none or only one of the symptom categories were considered low risk for OSA.
A previous diagnosis of OSA was self-reported by 11.6% of this sample, and 10.1% reported having been treated. Preliminary analyses of participants who reported an OSA diagnosis revealed no significant association between OSA treatment and OSA risk (p = 0.618). Additional analysis with OSA treatment as a predictor of outcome revealed little difference in cognitive outcome as compared with the use of OSA risk groups; therefore, treatment status was not included as a covariate in subsequent analyses. However, those who were not being treated and were at low risk for OSA reported significantly better mood and HRQoL at younger ages versus the treated or high risk untreated groups (at age 65 Wilks’ Lambda = 0.965, F(3, 2784)=33.97, p < .0001).
MANOVA results assessing the cognitive measures simultaneously showed a marginal association between OSA risk and cognitive function, which became significant in demographics-adjusted and co-morbidity adjusted MANCOVA models (Table 2).
Table 2.
Significance (p-value)* for associations of multivariable cognitive outcomes with OSA Risk, Demographics, and Comorbidities; and univariate parameter estimates from the demographic and comorbidity model for significant (p < 0.05) association.
| Multivariable Unadjusted p-value n=2925 | Multivariable Demographics Adjusted p-value n=2925 | Multivariable Demographics and Comorbidity Adjusted p-value n=2799 | Univariate Parameter Estimates for Significant Predictors (Demographics and Comorbidity Adjusted Model)
|
|||
|---|---|---|---|---|---|---|
| Word List Delayed Recall | Animal Fluency | Letter Fluency | ||||
| OSA risk (high vs. low) | 0.0547 | 0.025 | 0.0192 | n.s.+ | -4.85 | n.s. |
| Age at BSQ completion (per 1 year increment) | <.0001 | <.0001 | <.0001 | -0.07 | -0.21 | -.06 |
| Gender (men vs. women) | <.0001 | <.0001 | <.0001 | -0.92 | n.s. | -0.51 |
| Education (college grad vs. less than high school) | <.0001 | <.0001 | <.0001 | 1.57 | 4.12 | 4.12 |
| Race (black vs. white) | <.0001 | <.0001 | <.0001 | -0.77 | -3.06 | -0.57 |
| Diabetes (yes vs. no) | <.0001 | -- | <.0001 | -0.20 | -1.03 | -1.06 |
| Dyslipidemia (yes vs. no) | <.0001 | -- | 0.5256 | n.s. | n.s. | n.s. |
| Age × OSA risk interaction | -- | 0.0457 | 0.0272 | n.s. | 0.07 | n.s. |
Cells contain p-values of Wilks’ Lambda tests derived from MANOVA unadjusted models and MANCOVA adjusted models.
Demographics and Comorbidity Adjusted p-value for Word List =.052, no parameter generated.
Those at high risk for OSA performed more poorly (Table 3; Wilks’ Lambda = 0.996, F(3, 2786) = 3.31, p < .05). Simultaneous assessment of depressive symptoms and HRQoL indices also yielded a significant difference by OSA risk category, with those at high risk showing more depressive symptoms and worse HRQoL (Table 3; Wilks’ Lambda = 0.989, F(3, 2786) = 10.02, p < .0001).
Table 3.
Significance (p-value)* for multivariable associations of quality of life outcomes with OSA Risk, Demographics, and Comorbidities; and univariate parameter estimates from demographic and comorbidity model for significant (p < 0.05) associations.
| Multivariable Unadjusted p-value n=2925 | Multivariable Demographics Adjusted p-value n=2925 | Multivariable Demographics and Comorbidity Adjusted p-value n=2799 | Univariate Parameter Estimates for Significant Predictors** (Demographics and Comorbidity Adjusted Model)
|
|||
|---|---|---|---|---|---|---|
| CES-D-4 | PCS | MCS | ||||
| OSA risk (high vs. low) | <.0001 | <.0001 | <.0001 | 2.42 | -15.1 | -6.32 |
| Age at BSQ completion (per 1 year increment) | <.0001 | <.0001 | <.0001 | -0.003 | -0.04 | 0.12 |
| Gender (men vs. women) | <.0001 | <.0001 | <.0001 | -0.33 | 2.30 | 1.39 |
| Education (college grad vs. less than high school) | <.0001 | <.0001 | <.0001 | -0.99 | 5.49 | 2.85 |
| Race (black vs. white) | <.0001 | 0.1958 | 0.6869 | n.s. | n.s. | n.s. |
| Diabetes (yes vs. no) | <.0001 | -- | <.0001 | 0.34 | -4.15 | -0.91 |
| Dyslipidemia (yes vs. no) | <.0001 | -- | 0.005 | -1.34 | n.s. | |
| Age × OSA risk interaction | -- | 0.0002 | 0.0002 | -0.03 | 0.19 | n.s. |
Cells contain p-values of Wilks’ Lambda tests derived from MANOVA unadjusted model and MANCOVA adjusted models.
CES-D-4 (4-item Center for Epidemiological Studies-Depression Scale) depressive symptoms, SF-12 PCS (Medical Outcomes Study Short Form-12: Physical Component Summary), and SF-12 MCS (Medical Outcomes Study Short Form-12: Mental Component Summary).
Follow-up univariate analyses (ANOVA/ANCOVA) of each discrete cognitive and quality of life outcome (depressive symptoms, mental components, physical components), adjusted for demographics and co-morbid conditions, generally revealed OSA risk category to be a significant predictor of score differences. Specifically, the following indices were significantly worse among those at high risk for OSA: Animal Fluency (fully adjusted F(1, 2788) = 8.09, p = .0045), CESD-4 (fully adjusted F(1, 2788) = 13.21, p= .0003), HRQoL MCS-12 (fully adjusted F(1, 2788) = 5.47, p = .0194), and HRQoL PCS-12 (fully adjusted F(1, 2788) = 20.79, p < .0001). OSA risk did not predict Letter Fluency performance (fully adjusted F(1, 2788) = 1.62, p = .20) and marginally predicted Delayed Recall performance (fully adjusted F(1, 2788) = 3.77, p = .052), although adjustment for demographics only did result in significant differences in Delayed Recall by OSA risk (F(1, 2916) = 4.41, p = .036).
There was a significant OSA risk by age interaction affecting several of the outcome variables, specifically, Animal Fluency (fully adjusted F(1, 2788) for interaction term = 8.18, p = .004), CESD-4 (fully adjusted F(1, 2788) for interaction = 9.32, p = .002), and HRQoL PCS-12 (fully adjusted F(1, 2788) for interaction = 13.84, p = .0002). Individuals at high risk for OSA had lower Animal Fluency scores than their low risk peers at younger ages (Figure 1). However, the trend for lower animal fluency scores at older ages was steeper for those with low OSA risk than for those with high risk, resulting in a crossover at approximately age 70. Younger high risk individuals reported more depressive symptoms than their low risk counterparts (Figure 2); however, at older ages, high risk individuals’ report of depressive symptoms did not differ from those of low risk participants. A similar trend was found for physical well-being (Figure 3) and mental well-being, although for the latter index the age by OSA risk interaction was not statistically significant. Physical well-being was more negatively perceived among younger individuals at high risk for OSA versus older individuals at high risk.
Figure 1. Interaction of Age and Obstructive Sleep Apnea Risk on Animal Fluency Score.
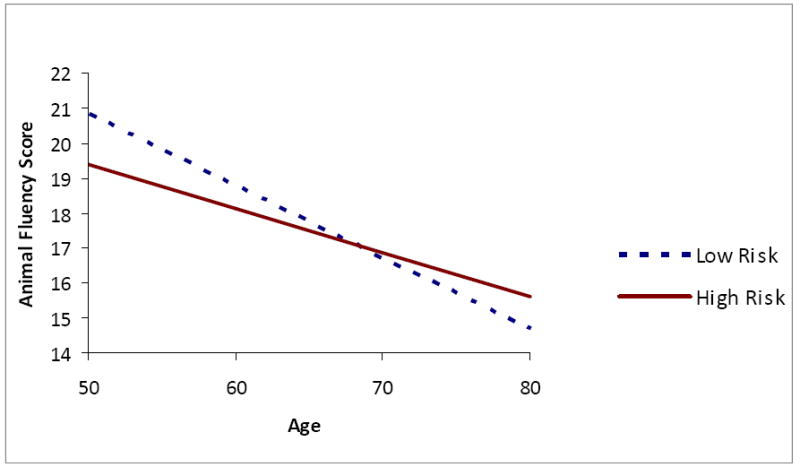
Figure 2. Interaction of Age and Obstructive Sleep Apnea Risk on Four-item Center for Epidemiological Studies Depression Score.
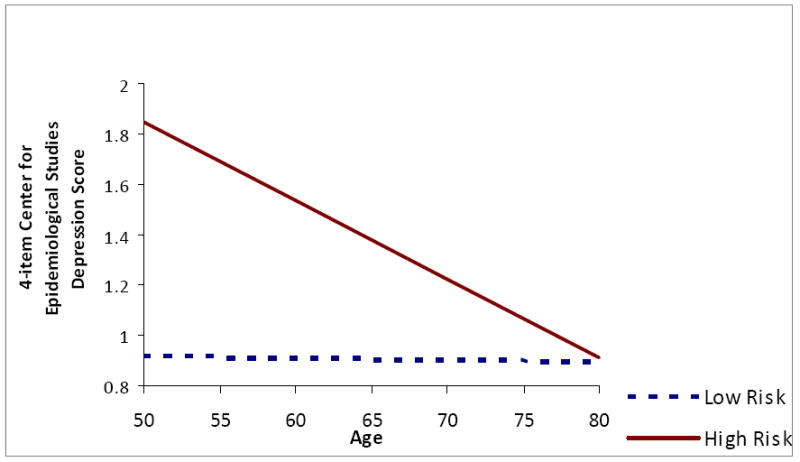
Figure 3. Interaction of Age and Obstructive Sleep Apnea Risk on Physical Component Score of Short Form - 12 Health-related Quality of Life.
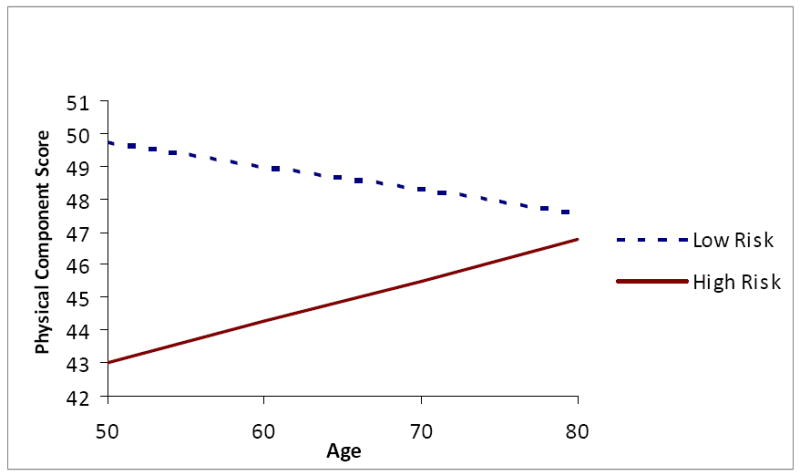
In sensitivity analyses restricted to those at highest and lowest OSA risk, the fully adjusted models including gender, education, race, diabetes, and dyslipidemia underscored the primary study results with respect to the main and interactive effects of OSA risk and age on both cognitive and quality of life outcomes (MANOVA p values for OSA risk = .007 and .0001 for cognition and quality of life, respectively; for age, p values = .0001 for both outcomes; and for the interaction of OSA risk and age, p values = .007 and .03 respectively). Univariate parameter estimates indicated that those at highest OSA risk scored on average 16.12 points lower than those at no risk on the animal fluency measure; 3.15 points higher on the CES-D-4, indicating more depressive symptoms; and 23.86 and 23.31 points lower on the SF-12 PCS and MCS scores respectively, indicating markedly lower HRQoL. These effects were pronounced at younger ages and were diminished to no difference or virtually no difference at older ages, with older age conferring modest but statistically significant protective effects in those at high OSA risk (i.e., less than 0.5 points better scores on animal fluency and MCS scores per year of age).
In post hoc analyses conducted to examine the possibility of healthy survivor effects, we found that older participants were less likely to be classified as high risk for OSA (p <0.0001). The percentage of participants classified as high risk OSA within age strata were: 27% of participants <50 years of age (13 of 47 participants), 36% aged 50-59 (157 of 441), 30% aged 60-69 (380 of 1,248), 22% age 70-79 (204 of 922), 11% aged 80-89 (28 of 255), and 0% aged 90+ (0 of 12).
Discussion
In this population-based sample, 27% of eligible participants were classified as high risk for OSA, consistent with previous studies utilizing the Berlin Sleep Questionnaire (Netzer et al., 2003). Although neuropsychological deficits, depressed mood, and decreased quality of life have been identified previously in individuals with OSA, demographic factors and co-morbid conditions have complicated interpretation of findings. The current study had ample power to control for age, sex, race, education, diabetes, and dyslipidemia. Compared to recent research controlling for similar factors (Yaffe et al., 2011), our study is unique in its balanced sampling of men and women, its substantial proportion of black participants, and its inclusion of middle aged adults . Our results suggest that OSA is associated with poorer cognition and quality of life even after adjusting for factors that have not been adequately investigated in prior studies.
Consistent with prior research, OSA significantly predicted differences in executive function as assessed with Animal Fluency (Incalzi et al., 2004), and marginally significant differences in memory as assessed with Delayed Recall (Boland et al., 2002) in fully adjusted models. However, unlike previous studies (Incalzi et al., 2004; Netzer et al., 1999), we did not find an association between OSA risk and Letter Fluency performance, perhaps due to differing OSA case finding strategies, differences in test administration (e.g., our administration of one letter versus three letters as in Incalzi et al., 2004), or selection factors including those associated with clinical versus epidemiological samples. On the other hand, some clinical studies of OSA (eg. Naismith et al 2004) have reported no association of either letter or semantic fluency performance with certain aspects of OSA, including sleepiness and hypoxemia. OSA may have differential effects on various aspects of cognitive performance depending on the primary symptoms involved, duration of symptoms, and symptom severity (Quan et al., 2006).
Age interacted with OSA risk in determining executive function performance as well as quality of life, with fewer depressive symptoms and better HRQoL among older participants. Our finding that OSA risk is less related to quality of life among older adults mirrors previous research (Martinez-Garcia, 2009), and this phenomenon likely is multiply determined. First, older adults who do not die or become lost to follow-up during studies represent “healthy survivors.” Consistent with this interpretation, our post hoc analyses confirmed that high risk OSA classification decreased with age. Another plausible explanation is differential misclassification due to self-report, with older adults tending to underreport subjective depressive symptoms (O’Hara et al., 2006). It is also possible that the symptoms represented by the Berlin Questionnaire (e.g., hypertension, snoring, daytime sleepiness) reflect different processes in older people than younger people, some of which may be unrelated to OSA. The mental and physical HRQoL variables used in this study may not be as salient once individuals exit the work force. Interestingly, some research suggests that functional capacity and health status are not requisites for quality of life in older adults. Rather, quality of life is evaluated relative to expectations, and expectations for mental and physical function may decline with advancing age (Dempster & Donnelly, 2002). Social comparison theory suggests that older adults use a “downward” comparison strategy, whereby they compare themselves with individuals in worse health, consequently perceiving their own situations more positively (Beaumont & Kenealy, 2004; DiLorenzo et al., 2009).
Limitations and Conclusions
The brevity of the cognitive evaluation in the present study is a limitation; however, our selection of measures was dictated by the scope of the population-based sample, affording a large sample that provided adequate data to control for key covariates. Another potential limitation is the administration of cognitive assessment measures by telephone. However, comparisons of in-person and telephone administration of measures similar to those used in this study suggest comparable results (Unverzagt et al., 2007), and the fluency assessments we used have been administered by telephone in other studies. Therefore, telephone administration appears to be a useful tool for gathering of large amounts of data without sacrificing accuracy.
The Berlin Sleep Questionnaire used to stratify participants by OSA risk category includes hypertension and body mass index in its criteria for group placement. We were therefore unable to use these comorbidities as covariates, even though both may be important contributors to cognitive function in particular. Future studies utilizing clinical diagnostic techniques will allow direct examination of these variables. Use of the BSQ is a convenient method for calculating OSA risk but lacks the precision of polysomnography, which likely diminished our estimates of the true relationship of OSA to cognitive function and quality of life. Even so, our less cumbersome OSA measure allowed for a large community-dwelling sample, which represents a significant contribution of the current study.
We found only minimal differences in outcomes based on CPAP treatment. Noncompliance, commonly reported in OSA literature (Weaver & Grustein, 2008), is one possible explanation for the absence of differences in cognition and HRQoL based on treatment. Previous studies examined individuals with moderate to severe OSA (Kawahara et al., 2005; Siccoli et al., 2008) and/or required formal diagnosis with polysomnography (Means et al, 2003). Such samples may benefit more from treatment compared to a community-dwelling sample drawn from an epidemiological sample such as ours, which likely includes individuals with less severe OSA. Duration of OSA also is likely an important marker of susceptibility to cognitive sequelae, and we did not have information regarding duration of symptoms or of treatment among those with self-reported diagnosis of OSA.
Finally, the current analyses are cross-sectional, limiting the extent to which conclusions can be drawn. Longitudinal approaches could be used to identify risk factors for OSA, establish the temporal relationship between markers for OSA and cognitive outcomes, and examine effects of OSA treatment. The REGARDS cohort lends itself to this type of approach in the future.
Despite these limitations, we were able to confirm OSA’s effect on cognition, depressive symptoms, and HRQoL while accounting for potentially confounding demographic and co-morbid factors. However, we also found differential effects based on age, with more detrimental correlates of OSA in younger versus older adults, particularly in terms of mood and HRQoL. The direction of this finding is consistent with a reported age threshold of 55 years as the peak after which the heightened OSA-associated risk of morbidity and mortality declines (Lurie, 2011). Therefore, accurate detection and treatment of OSA in younger adults may be particularly important. Finally, although it appears that older adults at high risk for OSA may perceive better quality of life than their younger peers despite similar symptoms, it is also possible that self-report symptom questionnaires may fail to capture symptoms indicative of distress among older adults. Age differences deserve further scrutiny in future studies of OSA and quality of life.
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views and positions of the National Institute of Neurological Disorders and Stroke, the National Institutes of Health. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Footnotes
Author Conflicts of Interest:
Addison-Brown, Letter, Yaggi, McClure, Unverzagt, Howard, Lichtman, Wadley: none
References
- Beaumont JG, Kenealy PM. Quality of life perceptions and social comparisons in healthy old age. Ageing Soc. 2004;24:755–769. [Google Scholar]
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Boland LL, Shahar E, Iber C, Knopman DS, Kuo TJ, Neito FJ. Sleep Heart Health Study Investigators. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11:265–272. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- Dempster M, Donnelly M. How well do elderly people complete individualised quality of life measures: an exploratory study. Qual Life Res. 2002;9:369–375. doi: 10.1023/a:1008959925664. [DOI] [PubMed] [Google Scholar]
- DiLorenzo TA, Halper J, Picone MA. Quality of life in MS: does aging enhance perceptions of mental health? Disabil Rehabil. 2009;31:1424–1431. doi: 10.1080/09638280802624543. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Feuerstein C, Naegele B, Pepin JL, Levy P. Frontal lobe-related cognitive functions in patients with sleep apnea syndrome before and after treatment. Acta Neurol Belg. 1997;97:96–107. [PubMed] [Google Scholar]
- Incalzi RA, Marra C, Salvigni BL, et al. Does cognitive dysfunction conform to a distinctive pattern in obstructive sleep apnea syndrome? J Sleep Res. 2004;13:79–86. doi: 10.1111/j.1365-2869.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Kawahara S, Akashiba T, Akahoshi T, Horie T. Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Int Med. 2005;44:422–427. doi: 10.2169/internalmedicine.44.422. [DOI] [PubMed] [Google Scholar]
- Lurie A. Obstructive sleep apnea in adults: epidemiology, clinical presentation, and treatment options. Adv Cardiol. 2011;46:1–42. doi: 10.1159/000327660. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia MA, Soler-Cataluna JJ, Roman-Sanchez P, Gonzalez V, Amoros C, Monserrat JM. Obstructive sleep apnea has little impact on quality of life in the elderly. Sleep Med. 2009;10:104–111. doi: 10.1016/j.sleep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Means MK, Lichstein KL, Edinger JD, et al. Changes in depressive symptoms after continuous positive airway pressure treatment for obstructive sleep apnea. Sleep Breath. 2003;7:31–42. doi: 10.1007/s11325-003-0031-x. [DOI] [PubMed] [Google Scholar]
- Melchior LA, Brown VB, Huba GJ, Reback CJ. A short depression index for women. Educ Psycho l Meas. 1993;53:1117–1125. [Google Scholar]
- Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Naismith S, Winter V, Gotsopoulos H, Hickie I, Cistulli P. Neurobehavioral functioning in obstructive sleep apnea: differential effects of sleep quality, hypoxemia and subjective sleepiness. J Clin Exp Neuropsychol. 2004;26:43–54. doi: 10.1076/jcen.26.1.43.23929. [DOI] [PubMed] [Google Scholar]
- Netzer NC, Hoegel JJ, Loube D, et al. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–1414. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- O’Hara R, Coman E, Butters MA. Late-Life Depression. In: Snyder P, Nussbaum PD, Robins DL, editors. Clinical Neuropsychology: A Pocket Handbook for Assessment. Second edition. American Psychological Association; Washington, D.C: 2006. pp. 183–209. [Google Scholar]
- Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Siccoli MM, Pepperell JCT, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–1558. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests. Third edition. Oxford University Press; New York: 2006. [Google Scholar]
- Unverzagt FW, Monahan PO, Moser LR, et al. The Indiana University Telephone-Based Assessment of Neuropsychological Status: A new method for large scale neuropsychological assessment. J Int Neuropsychol Soc. 2007;13:799–806. doi: 10.1017/S1355617707071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SW, Snow J, Muldoon MF, Katzel LI. Neuropsychological Consequences of Cardiovascular Disease. In: Tarter RE, Butters M, Beers SR, editors. Medical Neuropsychology. Second edition. Kluwer Academic Publishers; New York: 2001. pp. 51–83. [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Grustein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. J A M A. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2(4):461–471. [PubMed] [Google Scholar]


