Summary
Identification of biologically relevant genes from genomic assays is limited by the complexity of changes present in most solid tumors. Assessing for coactivation of functionally related genes resulted in the identification of SENP2, DCUN1D1, and DVL3—consensus candidates that drive selection for 3q amplification in lung squamous cell carcinomas.
In this issue of Clinical Cancer Research, Wang and colleagues report the results of a novel integrative computational analysis that identified SENP2, DCUN1D1, and DVL3 as consensus candidate drivers in the 3q26–29 amplicon in squamous cell carcinoma (SCC) of the lung (1).
Theodor Boveri’s assertion that cancer is a heritable genetic malady took almost 70 years to validate and a century to become a universally accepted doctrine.(2) Seminal research in the 1980s and 1990s identified abnormalities in chromosomes and genes that can promote cancer development and predict clinical behavior. The realization that targeting genetic abnormalities in cancers can confer considerable therapeutic advantages made the identification of genetic events driving cancer pathogenesis a major priority. The development of genome-wide screening tools, such as comparative genomic hybridization (CGH) and spectral karyotyping, allowed the characterization of chromosomal abnormalities present in a wide range of tumors. However, the low resolution (10–20 Mb) of these molecular-cytogenetic assays limited the identification of individual gene targets. Technological advancements have resulted in the development of genome-wide assays that can detect abnormalities at the gene and nucleotide level, as well as identify epigenetic abnormalities (e.g., methylation). Analysis of results from the different screening tools has led to the development of novel prognostic assays and the identification of therapeutic targets (i.e., EGFR mutation in lung adenocarcinomas, BRAF in melanomas)(3).
The remarkable success of the initial genetic and epigenetic screening studies led to the initiation of The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium, with the goal of comprehensively mapping cancer genomes. However, several factors have limited the translation of results from TCGA and other genome-wide mapping studies, including (1) the heterogeneity of genetic changes in cancers, (2) the lack of targetable driver events in the majority of tumors, and perhaps most significantly, (3) the complexity of abnormalities present in individual cancers. Novel bioinformatic approaches have been developed to allow integration of data from multiple analytic platforms. While these assays have led to the identification of many new cancer genes (3), the biological significance of many of the identified genes has been difficult to validate.
Recurrent amplification at 3q was originally identified by CGH in SCCs of mucosal origin, including those of the lung, head and neck, esophagus, and cervix (4). Owing to its high prevalence, association with disease progression, and clinical outcome, there has been considerable interest in identifying genes that drive selection for 3q amplification in SCC (4). In the pregenomic era, this involved candidate gene and positional cloning approaches and led to the identification of several putative driver genes in the amplified locus (i.e., SCCRO [DCUN1D1], PIK3CA, PKCl, LAMP3, SnoN, SOX2, and eIF-5A2)—the clinical significance of many of these was corroborated by analysis of results from genome-wide assays (5). However, a strong tendency toward co-occurrence (confirmed by analysis of TCGA data sets for lung, cervical, and head and neck SCC; odds ratio, >10) of copy number and expression changes in genes within the 3q amplification complicates identification of biologically relevant drivers. For example, an analysis of results from a genome-wide assay led to the identification of SOX2 as the main driver for 3q amplification in lung SCC.(5) Although subsequent analyses supported its clinical relevance, transgenic expression of SOX2 gene in mice resulted in the development of adenocarcinoma-like rather than squamous cell carcinoma-like cancers (6).
So how then can we identify clinically and biologically relevant genes in the results from genome-wide assays? By using coexpression of functionally related genes in the 3q amplicon (using random walk analysis based on the integrated human protein-protein interaction networks) to guide analysis of copy number and expression data from multiple data sets, Wang and colleagues added a biological dimension to the identification of driver genes. Using this approach, they identified three genes (SENP2, DCUN1D1, and DVL3) in the 3q amplicon in lung cancers that function in pathways involved in the conjugation of ubiquitin and ubiquitin-like proteins. They validated the biological significance of these genes through in vitro experiments and their clinical significance by correlating the expression of these genes with responses to adjuvant chemotherapy and survival.
Although two of the genes identified by Wang et al. have not been analyzed previously, prior work corroborates the significance of DCUN1D1 in lung SCC. Moreover, subset analysis (see Fig. S4 in Wang, et. al. (1)) suggests that, of the three genes identified by Wang et al., DCUN1D1 is the largest contributor to the observed differences in outcomes of lung SCC. Using positional cloning analysis, our group initially identified DCUN1D1 as a driver for selection of the 3q amplification in lung and head and neck SCC. We established the clinical and biological significance of DCUN1D1 by showing (1) a correlation between copy number, mRNA, and protein expression; (2) an independent association with clinical outcome; (3) the presence of oncogene addiction in cancer cell lines; (4) transforming activity in vitro and in vivo; and (5) function in neddylation, a pathway highly relevant to cancer pathogenesis (7–9). Corroborating its clinical relevance, others have demonstrated an association between DCUN1D1 expression and regional nodal metastasis, brain metastasis, and survival in patients with lung SCC(10).
Functionally, DCUN1D1 is an essential component of the E3 for neddylation, a tripartite enzymatic process analogous to ubiquitination that culminates with the covalent modification of cullins by the ubiquitin-like protein Nedd8 (7, 8, 11). Neddylation is a key regulator of cullin-RING-ligase–type ubiquitination E3 activity and is an emerging anticancer target. MLN4924—a small molecule inhibitor of E1 in neddylation (Nedd8-activating enzyme [NAE])—has shown promising results in initial clinical trials. The success of MLN4924 highlights the benefits of targeting neddylation-pathway components in anticancer treatment (12). Several findings suggest that inhibiting DCUN1D1 activity may be an effective way to target the neddylation pathway activity in human cancers: (1) In contrast to NAE, which is rarely dysregulated in human cancers, DCUN1D1 dysregulation is common and is associated with an oncogene addiction phenotype, which was confirmed by results from Wang et al. (2) Whereas knocking out other core components of neddylation is associated with lethality in mice, DCUN1D1 knockout mice are viable and have no appreciable defects in essential functions (7). This suggests that inhibiting DCUN1D1 may have limited detrimental effect on normal cells. (3) DCUN1D1’s molecular interactions and crystal structure are suitable for inhibition by small molecules (7, 8, 11). And (4) DCUN1D1 is one of five paralogues in the human genome, all of which function in neddylation. Aberrations of one or more DCUN1D1 paralogues (copy number, mRNA expression, mutation) are present in more than 50% of head and neck cancers, 70% of lung SCCs, 55% of ovarian serous cystadenocarcinomas, 34% of cervical and endometrial carcinomas, 26% of lung adenocarcinomas, and 15% of glioblastomas (on the basis of analysis of interim data from respective TCGA projects [Fig. 1]). Thus, targeting the activity of DCUN1D1 and its paralogues may prove beneficial in the treatment of a significant proportion of patients with lung SCC, as well as many other cancers.
FIGURE.
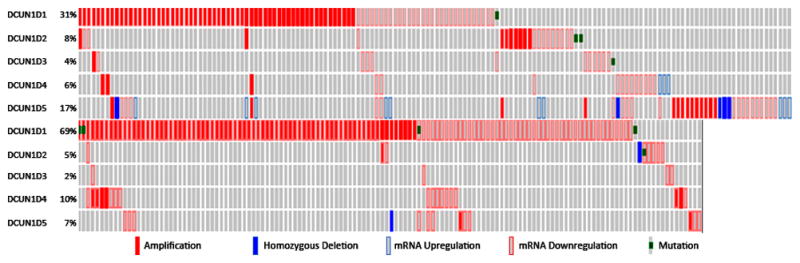
Analysis of results from TCGA showing abnormalities in DCUN1D1 family of genes in head and neck (154 of 295 cases; top panel) and lung (136 of 177 cases; bottom panel) squamous cell carcinomas showing both activating and inactivating alterations (data analysis was through the MSKCC cBio Portal for TCGA at http://cbio.mskcc.org/gdac-portal/).
Footnotes
Conflict of interest statement: The authors have no conflicts of interest.
Bibliography
- 1.Wang J, Qian J, Hoeksema MD, Zou Y, Espinosa AV, Rahman JS, et al. Integrative genomics analysis identifies candidate drivers at 3q26–29 amplicon in squamous cell carcinoma of the lung. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-13-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowell PC, Hungerford DA. MINUTE CHROMOSOME IN HUMAN CHRONIC GRANULOCYTIC LEUKEMIA. Science. 1960;132:1497. doi: 10.1126/science.144.3623.1229. [DOI] [PubMed] [Google Scholar]
- 3.McDermott U, Downing JR, Stratton MR. Genomics and the continuum of cancer care. The New England journal of medicine. 2011;364:340–50. doi: 10.1056/NEJMra0907178. [DOI] [PubMed] [Google Scholar]
- 4.Singh B, Gogineni SK, Sacks PG, Shaha AR, Shah JP, Stoffel A, et al. Molecular cytogenetic characterization of head and neck squamous cell carcinoma and refinement of 3q amplification. Cancer research. 2001;61:4506–13. [PubMed] [Google Scholar]
- 5.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature genetics. 2009;41:1238–42. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PloS one. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang G, Kaufman AJ, Ramanathan Y, Singh B. SCCRO (DCUN1D1) promotes nuclear translocation and assembly of the neddylation E3 complex. The Journal of biological chemistry. 2011;286:10297–304. doi: 10.1074/jbc.M110.203729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim AY, Bommelje CC, Lee BE, Yonekawa Y, Choi L, Morris LG, et al. SCCRO (DCUN1D1) is an essential component of the E3 complex for neddylation. The Journal of biological chemistry. 2008;283:33211–20. doi: 10.1074/jbc.M804440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkaria I, OcP, Talbot SG, Reddy PG, Ngai I, Maghami E, et al. Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer research. 2006;66:9437–44. doi: 10.1158/0008-5472.CAN-06-2074. [DOI] [PubMed] [Google Scholar]
- 10.Yoo J, Lee SH, Lym KI, Park SY, Yang SH, Yoo CY, et al. Immunohistochemical Expression of DCUN1D1 in Non-small Cell Lung Carcinoma: Its Relation to Brain Metastasis. Cancer research and treatment: official journal of Korean Cancer Association. 2012;44:57–62. doi: 10.4143/crt.2012.44.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott DC, Monda JK, Bennett EJ, Harper JW, Schulman BA. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science. 2011;334:674–8. doi: 10.1126/science.1209307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–6. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]


