Abstract
Objectives
The objective of this study was to compare sex differences among referrals for evaluation of poor growth.
Study design
This study was based on chart reviews of all new-patient encounters at Children’s Hospital of Philadelphia Diagnostic and Research Growth Center for short stature or poor growth evaluations during 2001. Outcome measures were patient growth characteristics, frequency of underlying pathology, and frequency of laboratory and radiologic investigations before referral.
Results
One hundred eighty-two boys and 96 girls were referred (P < .0001). Girls were shorter, relative to the general population (median height z score, −2.4 vs −1.9 for boys, P = .02) and mid-parental target heights (median deficit, 1.9 vs 1.3 SD, P < .01). Differences were more pronounced starting at age 9 years. Median time to referral from initial fall-off on the growth curve was 35 months in girls and 24 months in boys (not significant). The percentage of girls (41%) with organic disease significantly exceeded that of boys (15%). Conversely, more boys (72%) than girls (48%) were of normal height or short but healthy (P < .0001). Sex was not associated with frequency of tests before referral; neither was severity of short stature.
Conclusions
Sex differences in short stature referrals may delay diagnosis of diseases in girls while promoting overzealous evaluations of healthy boys who do not appear to be tall enough.
Growth is perhaps the most sensitive indicator of a child’s overall health; growth failure may be the first and only sign of underlying disease in a child. For example, growth failure may precede abdominal symptoms by months or years in children with inflammatory bowel disease.1 Other diseases that can present with growth failure alone include celiac disease,2 cystic fibrosis,3 renal tubular acidosis,4 and HIV infection.5 Hence, growth should be carefully monitored, and when an abnormality is identified, it should be investigated to differentiate normal variants in healthy children from underlying pathology that requires treatment.6 The American Academy of Pediatrics acknowledged the importance of growth in its March 2000 ‘‘Recommendations for Preventative Pediatric Health Care,’’ stating that a child’s height and weight should be measured at least at birth, age 2 to 4 days, 1, 2, 4, 6, 9, 12, 15, 18, and 24 months, and every year thereafter through age 21 years.7
Because growth can serve as a marker for potential underlying disorders, growth failure should be given equal import when evaluating children of either sex. On the contrary, social pressures focus more on growth in boys than girls. Studies have linked height to elementary school teachers’ perceptions of competence in the male students,8 male success in mating selection,9 occupational success,10 achievement as an academic,11 perceptions of presidential candidates,12 and perceived performance and leadership in military service.13 Growth hormone registries indicate preferential treatment of boys with poor growth; boys receive growth hormone therapy by ratios of approximately 2:1, depending on the diagnosis, with the highest for idiopathic short stature.14,15 This study proposed to determine whether there are sex differences among children referred to a pediatric endocrine center for evaluation of short stature or poor growth.
METHODS
All encounters at The Children’s Hospital of Philadelphia Diagnostic and Research Growth Center for short stature or poor growth evaluations from January 1, 2001, through December 31, 2001, were reviewed. Only new patients with the chief complaint of short stature were included in this study (n = 278). Patients with prior evaluation by an endocrinologist or previous treatment with growth hormone (n = 34), children referred for pituitary evaluation (secondary to brain tumor, meningitis, septo-optic dysplasia, and other brain malformations) in whom growth was not the primary concern (n = 15), and girls with known Turner syndrome (n = 6) were excluded.
Sex, age, patient height, parent height, final diagnosis, and laboratory and radiologic evaluations before referral were extracted from patient charts and entered into a database. The patient’s height at the first clinic visit was the arithmetic mean of three sequential measurements, using a wall-mounted Holtain stadiometer for older children and a recumbent stadiometer for infants and toddlers. Parents’ heights were self-reported. For 115 children, a copy of their growth curves was available for estimating the age at first deviation, that is, the age at which the plotted heights first fell across major percentiles.
The patient’s height measurement was converted into a height z score, standardized for age and sex, using the Growth Calculator electronic program (developed by Phillip Cheng MD, 2000; referenced to the National Center for Health Statistics percentiles16). Mid-parental target height was calculated by the method of Tanner17 and then transformed into mid-parental target height z score using Growth Calculator. The patient’s height deficit was calculated by subtracting the patient’s height z score from the mid-parental height z score, and the time to referral was calculated by subtracting the age at first deviation across major percentiles from the age at the first visit. Normal height was defined as healthy children whose height z score was within 2 SD of both the population mean and their mid-parental target. Diagnoses were tabulated for children whose heights did not meet the definition of normal.
Descriptive statistics and tests of hypotheses were calculated through the use of JMP Software (SAS Institute; Cary, NC) and StatXact (Cytel Software Corp, Cambridge, MA). Differences between male and female means were evaluated by Wilcoxon rank sum test for continuous outcomes (due to skewed data sets) and by χ2 tests for categoric ones. Linear trend analysis of male proportions as a function of age was performed using the Statcalc program in EpiInfo (Centers for Disease Control and Prevention, Atlanta, GA). Logistic regression analyses were applied to investigate if laboratory or radiologic testing, both the total number of tests and frequency of each individual test, were related to sex, height z score, or height deficit as explanatory variables in main effect, second-order or third-order interactions.
Approval of the Children’s Hospital of Philadelphia Institutional Review Board was obtained before commencing this study.
RESULTS
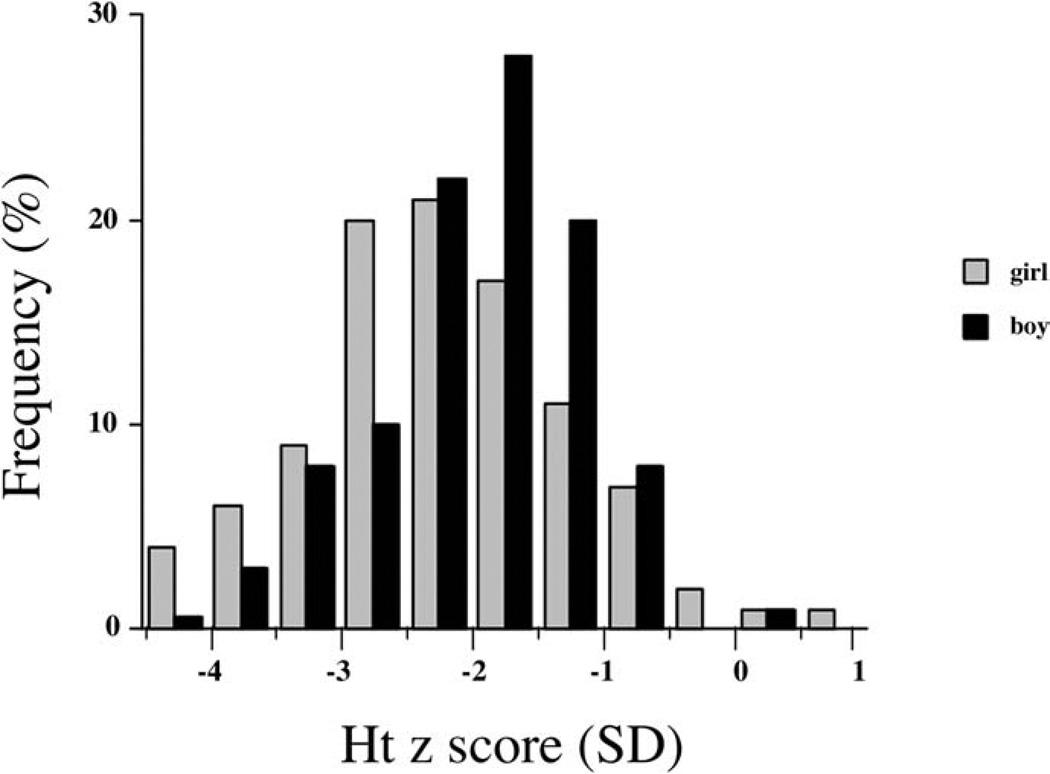
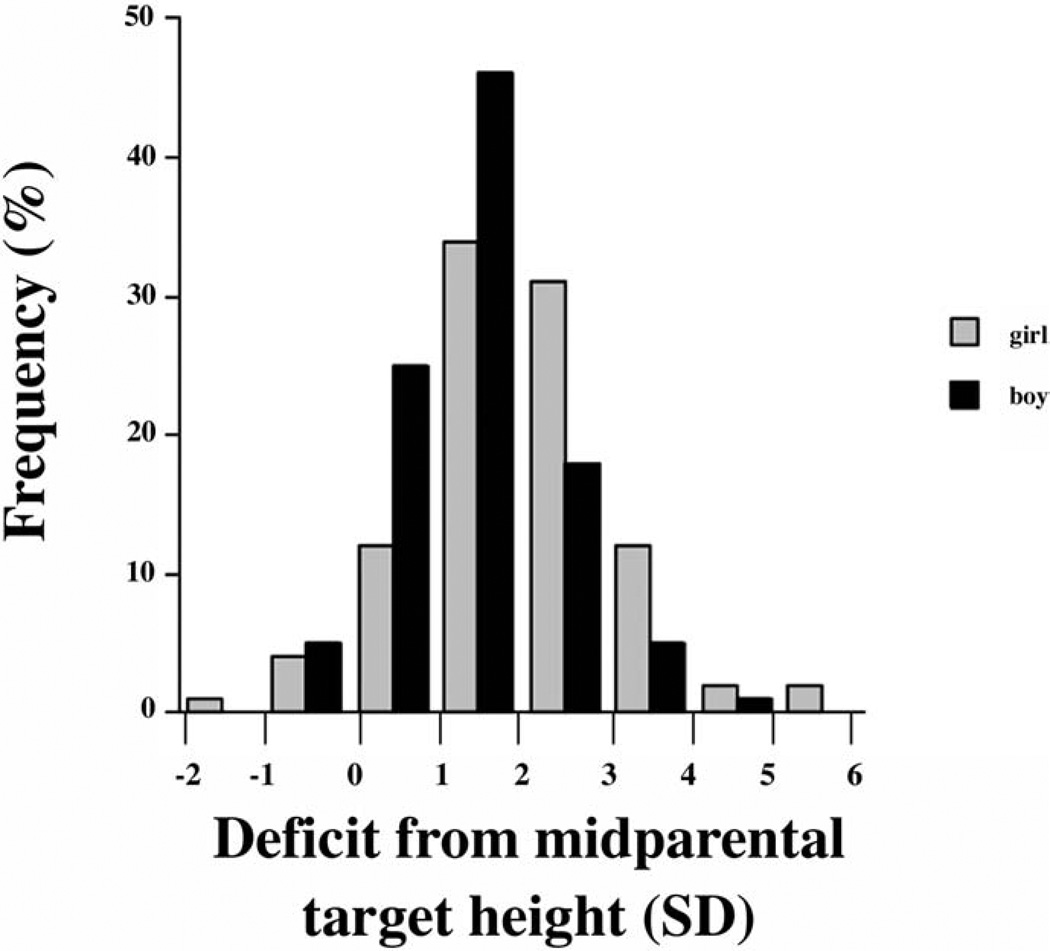
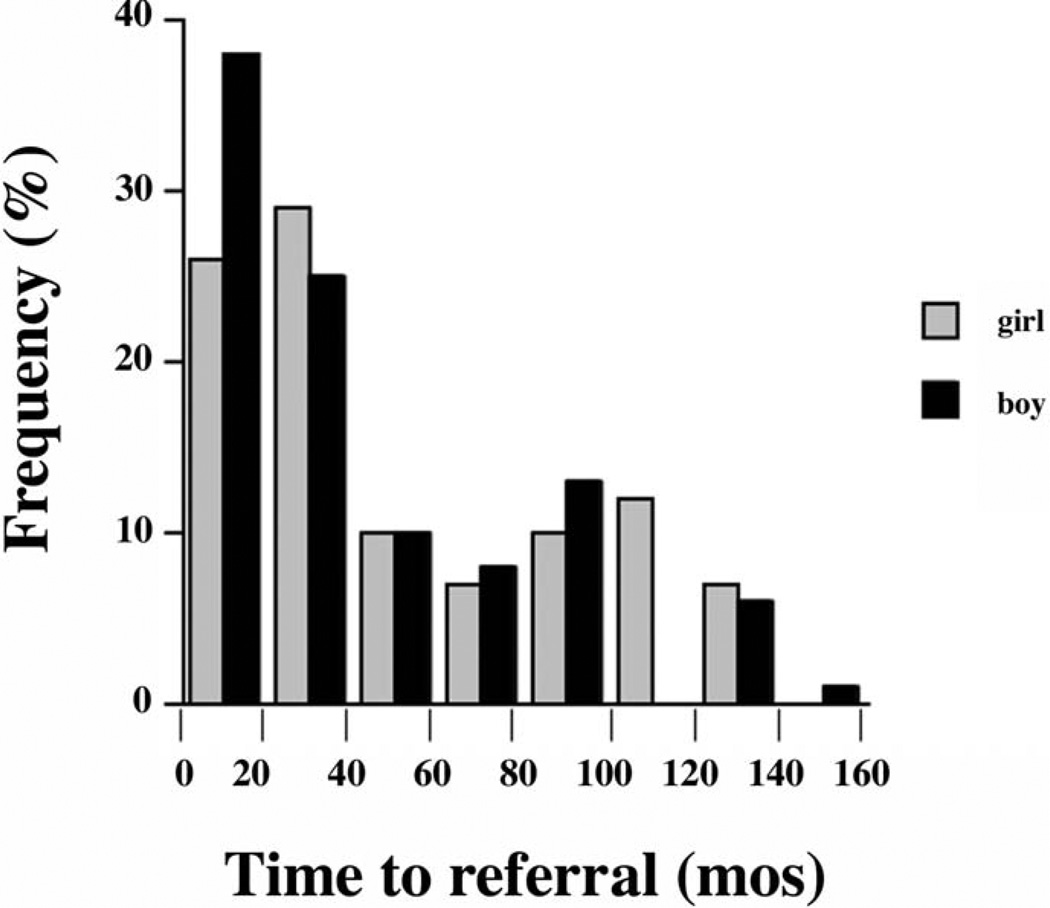
Referrals to our Growth Center included 182 boys and 96 girls, a significant (P < .0001) departure from the null hypothesis of equal proportions. At the time of referral, the height deficit was greater in girls than in boys, both relative to the general population (Figure 1) and relative to their midparental target heights (Figure 2). Median height z score for girls was −2.4 versus −1.9 for boys (P < .01), and median deficit from mid-parental target height z score was 1.9 for girls versus 1.3 for boys (P < .001). Mid-parental height z score could not be determined for 6 girls and 13 boys because of unknown height of one or both parents. Although the median time to referral was longer in girls (35 months) than boys (24 months), the difference was not statistically significant (P = .30) (Figure 3). The percentage of girls (44%) versus boys (40%) for whom prior growth curves were provided was not statistically different (P = .55).
Figure 1.
Histogram of height z scores at time of referral. Percentage of girls (gray) and boys (black) whose height measured within each 0.5 SD interval is depicted.
Figure 2.
Histogram of deficits from mid-parental target heights at time of referral. Percentage of girls (gray) and boys (black) whose height deficit from their mid-parental target fell within each 1 SD interval is depicted.
Figure 3.
Histogram of time to referral. Percentage of girls (gray) and boys (black) whose time to referral lasted within each 20-month interval is depicted.
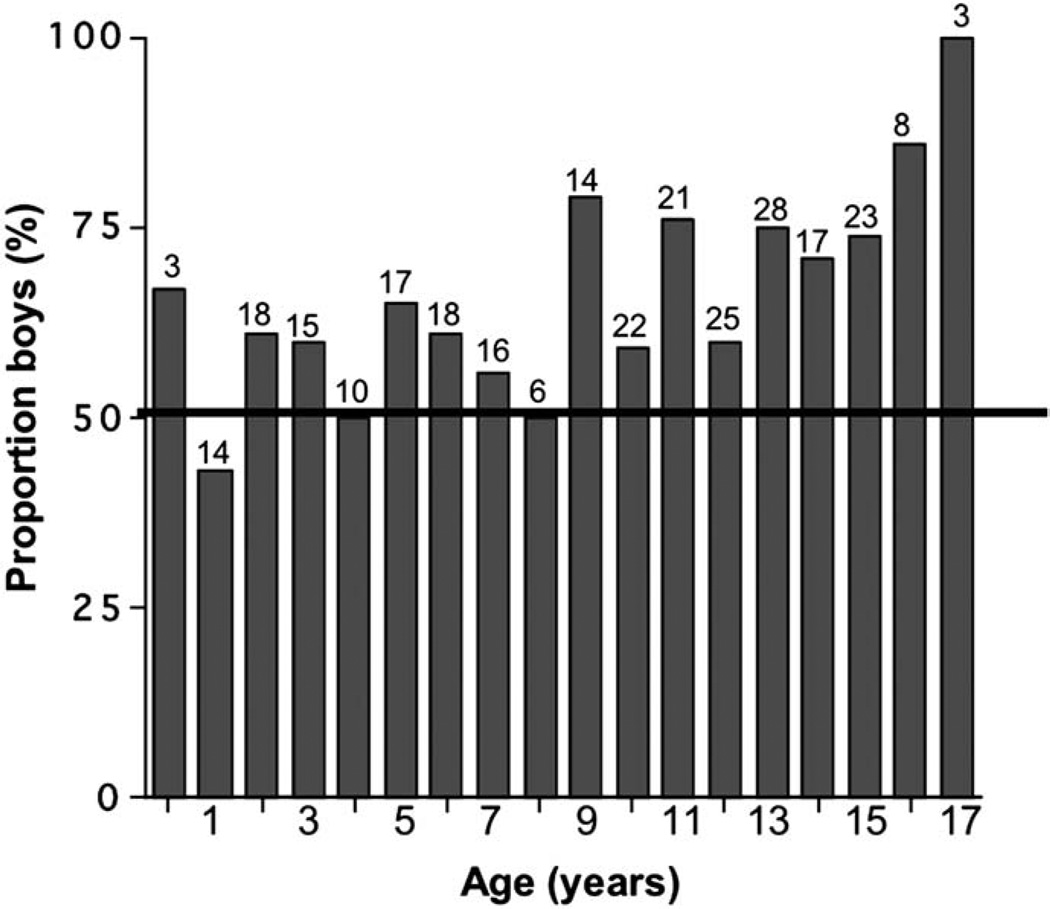
The proportion of male referrals by age is shown in Figure 4; the linear trend was positively associated with age across the entire group (P < .01). From Figure 4, it appears that the male predominance was particularly evident starting at age 9 years. Indeed, boys comprised 57% of the 117 referrals under the age of 9 years and 71% of the 161 children age 9 years and older (P < .05). Under age 9 years, neither height z scores (medians −2.4 for girls and −2.1 for boys; P = .48) nor height deficits from the mid-parental target heights (medians, 1.8 for girls and 1.5 for boys; P = .34) were significantly different between sexes. However, for children age 9 years and older, the median height z score for girls was −2.4 versus −1.9 for boys (P < .01), and median deficit from mid-parental target height z score was 2.1 for girls versus 1.2 for boys (P < .001).
Figure 4.
Histogram of male percentages by age. Number of children referred at each age is shown above each column; column heights depict percentage of each annual grouping that is male (50% is indicated by horizontal line).
Organic disease was more common (P < .0001) among girls (39/96) than boys (27/182). The risk ratio was 2.7, with 95% confidence interval of [1.8 to 4.2]. Even if the 9 girls found to have Turner syndrome were excluded, more girls (31%) than boys (15%) had organic disease. Conversely, the percentage of boys referred at normal height (38%) exceeded (P < .01) that of girls (20%). Of the boys, 25% had constitutional growth delay, 7% had familial short stature, and 2% had both; for girls, the proportions were 15%, 7%, and 6%, respectively (P = .31 for all three diagnoses combined). Nonorganic diseases (failure to thrive/nutritional dwarfing and psychosocial dwarfing) were similar (9% of boys and 8% of girls).
Sex was not significantly associated with the number of laboratory and/or radiologic studies obtained before referral (P = .90) (Table). Logistic regression analysis identified one statistically significant association of sex and laboratory testing: chromosomes (P < .001). A trend existed in prior measurement of celiac antibodies, more often obtained in boys than girls (P = .053), yet the one case of celiac disease found in our cohort was a girl. Prior evaluation by the primary physician was not found to be statistically associated with the severity of short stature, indicated by either height z score (P = .14) or deficit from mid-parental height z score (P = .85).
Table.
Frequency of prereferral investigations, distributed by sex
| Boys (%) | Girls (%) | |
|---|---|---|
| Chemistries/LFTs | 24 | 25 |
| CBC with differential | 24 | 26 |
| Sedimentation rate | 9 | 10 |
| Celiac antibodies | 4 | 0 |
| Sweat test | 2 | 0 |
| Urinalysis | 3 | 1 |
| Karyotype | 0.5 | 8* |
| Thyroid functions | 33 | 28 |
| IGF-I | 19 | 17 |
| IGFBP-3 | 13 | 16 |
| Growth hormone (random) | 2 | 1 |
| ACTH/cortisol | 1 | 0 |
| Gonadotropins | 2 | 0 |
| Testosterone | 2 | 0 |
| Estradiol | 0 | 0 |
| Other laboratory values† | 4 | 5 |
| Bone age | 54 | 45 |
| MRI of brain/pituitary | 2 | 1 |
| Skeletal radiography (R/O hypochondroplasia) | 0.5 | 0 |
Significance is expressed as P < .05.
Other laboratory values for boys: HIV (1), lead level (1), insulin level (0.5), ferritin/iron/total iron binding capacity (0.5), homocysteine (0.5), plasma amino acids (0.5); for girls: lead level (4), hepatitis serologies (1).
DISCUSSION
Our data demonstrate a sex bias in referrals for poor growth evaluation. It is unclear if this discrepancy results from dilution of the frequency of referrals for organic disease in boys by all the healthy boys of normal or short height. More worrisome is the possibility that underappreciation of growth problems in girls results in missed diagnoses in girls who are not referred. Additional studies with access to the total primary care population are needed to determine the characteristics of the children who are not referred. For example, in a Utah school-based screening study, 50% of children found to have growth hormone deficiency (2.7 male-to-female ratio) and 17% of the girls found to have Turner syndrome had been previously advised to see an endocrinologist; only 25% of the former and none of the latter had done so.18 It is also unclear what portion of the observed differences in referrals was due to bias by the primary physicians or to selection bias by the patients and their families. In response to the greater social pressures for tall stature in men, boys and their families may be more likely to request specialist referrals from their primary physicians or else to seek specialist care directly. Our study suggests that age plays a role, with the greatest pressures felt in the peripubertal years. It would be interesting to explore other potential factors in referral patterns, such as parental height, parental socioeconomic background, cultural background, and insurance status.
Comparison with other studies confirms the sex bias in referrals. In our center, 72% of boys and 48% of girls were of normal height or short with familial short stature, constitutional growth delay, or both. These same categories comprised 63% of short stature referrals to the Pediatric Endocrine Ambulatory Center at North Shore University Hospital during 1973 to 1991; the sex distribution was not reported.19 However, such marked differences were not seen in the Utah school-based screening program; of the 555 children identified as growing abnormally, 83% of boys and 77% of girls had familial short stature, constitutional growth delay, or both.18 In a prospective study of 220 pediatric endocrine centers regarding children referred for short stature whom the endocrinologists thought would be seen at least once more for follow-up, 69% of the 21,736 children enrolled during 1981 through 1999 were male (personal communication, Genentech). Although this population differs slightly from our study of all new short stature referrals, the 2:1 ratio was also found.
Either scenario portends a disservice to children. There may be significant medical consequences to delayed or missed diagnoses in girls whose growth problems are underappreciated. For example, long-standing unrecognized celiac disease can predispose to other autoimmune conditions.20 Conversely, early diagnosis and treatment are associated with better final height outcomes in growth hormone deficiency and Turner syndrome21 and can obviate the need for delaying puberty.22 Chart reviews of the University of North Carolina Turner Syndrome Clinic found an average 5.2-year delay in diagnosis from the time the height had fallen below the 5th percentile.23 There may also be significant consequences of overzealous evaluation and treatment of healthy boys. It reinforces to the boys and their families that their height is a bona fide problem that requires medical intervention, which may serve to exacerbate rather than alleviate the self-esteem issues with which the boys may be grappling. Despite the social stresses demonstrated in multiple studies, other studies have countered that short stature does not preclude normal psychosocial adjustment in children or adults.24 On a societal level, it raises questions about resource allocation and ethical debates about medical versus cosmetic treatment, a subject already extensively argued in the literature.24–31 Our study demonstrates a sex bias in referrals to a pediatric endocrine center for poor growth evaluations. In a survey of pediatric endocrinologists, the specialists recommended growth hormone treatment 1.3 times as often for identical hypothetical case scenarios that described male rather than female patients.32 The growth hormone registries already document a male predominance among treated children.14,15 With the new FDA-approved indication for growth hormone therapy to treat idiopathic short stature, these multilayered gender biases have the potential to translate into even more significant therapeutic discrepancies.
This study adds to a worrisome trend in the recent literature that indicates suboptimal use of growth monitoring. Despite the explicit American Academy of Pediatrics recommendations, 35% of well-child encounters at an academic pediatric clinic failed to plot growth measurements and/or document a growth abnormality.33 Often, recommendations for measuring and plotting length rather than height in younger children are not followed, or improper equipment is used.34 Our chart reviews found cases exemplifying these previously published errors, but most troublesome was the lack of prior growth curves for 163 of the 278 children referred. Failure to forward the growth curves does not necessarily mean that the primary physicians did not construct such curves for use in their practices. Rather, it highlights an underappreciation of the importance of growth curves as a tool for assessing growth. Growth velocity cannot be determined from a solitary height measurement, so 59% of the children referred specifically for the evaluation of poor growth did not have the data necessary to include growth velocity in that evaluation. Although incomplete, the percentage of growth curves provided for each sex did not differ statistically. The median time to referral for girls was nearly 1 year longer than for boys, but the difference was not statistically significant. This may be due to overlap of the two groups or to insufficient power, associated with the reduced sample size involving only 41% of the subjects in this study. A World Health Organization survey revealed suboptimal growth curve use to be a global issue, including North America,35 one that we hope will be addressed for all children.
Acknowledgments
We are grateful to Serono, Inc, for providing the Growth Calculator and the University of Pennsylvania General Clinical Research Center for biostatistical guidance.
Supported by a University of Pennsylvania Trustee’s Council Summer Faculty Research Fellowship (A.G.), the National Institute of Diabetes and Digestive and Kidney Diseases (K08-DK64352, A.G.), and the University of Pennsylvania General Clinical Research Center (NIH Grant M01-RR00040). Serono, Inc, provided the Growth Calculator.
REFERENCES
- 1.Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of Crohn’s disease. Gastroenterology. 1988;95:1523–1527. doi: 10.1016/s0016-5085(88)80072-6. [DOI] [PubMed] [Google Scholar]
- 2.Bonamico M, Scire G, Mariani P, Pasquino AM, Triglione P, Scaccia S, et al. Short stature as the primary manifestation of monosymptomatic celiac disease. J Pediatr Gastroenterol Nutr. 1992;14:12–16. doi: 10.1097/00005176-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Giglio L, Candusso M, D’Orazio C, Mastella G, Faraguna D. Failure to thrive: the earliest feature of cystic fibrosis in infants diagnosed by neonatal screening. Acta Paediatr. 1997;86:1162–1165. doi: 10.1111/j.1651-2227.1997.tb14836.x. [DOI] [PubMed] [Google Scholar]
- 4.Roth KS, Chan JC. Renal tubular acidosis: a new look at an old problem. Clin Pediatr. 2001;40:533–543. doi: 10.1177/000992280104001001. [DOI] [PubMed] [Google Scholar]
- 5.Arpadi SM. Growth failure in children with HIV infection. JAIDS. 2000;25(Suppl 1):S37–S42. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 6.Grimberg A, DeLeon D. Disorders of growth. In: Moshang T, editor. Pediatric Endocrinology: The Requisites in Pediatrics. Philadelphia: Elsevier, Inc; 2004. pp. 127–167. [Google Scholar]
- 7.American Academy of Pediatrics Policy Statement. Committee on Practice and Ambulatory Medicine. Recommendations for Preventive Pediatric Health Care. Pediatrics. 2000;105:645. [Google Scholar]
- 8.Villimez C, Eisenberg N, Carroll JL. Sex differences in the relation of children’s height and weight to academic performance and others’ attributions of competence. Sex Roles. 1986;15:667–681. [Google Scholar]
- 9.Hensley WE. Height as a basis for interpersonal attraction. Adolescence. 1994;29:469–474. [PubMed] [Google Scholar]
- 10.Hensley WE, Cooper R. Height and occupational success: a review and critique. Psychol Rep. 1987;60:843–849. doi: 10.2466/pr0.1987.60.3.843. [DOI] [PubMed] [Google Scholar]
- 11.Hensley WE. Height as a measure of success in academe. Psychol J Hum Behav. 1993;30:40–46. [Google Scholar]
- 12.Kassarjian HH. Voting intentions and political perceptions. J Psychol. 1963;56:85–88. [Google Scholar]
- 13.Tuvemo T, Jonsson B, Persson I. Intellectual and physical performance and morbidity in relation to height in a cohort of 18-year-old Swedish conscripts. Horm Res. 1999;52:186–191. doi: 10.1159/000023459. [DOI] [PubMed] [Google Scholar]
- 14.August GP, Lippe BM, Blethen SL, Rosenfeld RG, Seelig SA, Johanson AJ, et al. National Cooperative Growth Study (NCGS) Advisory Group, ed. Growth Hormone: Science, Research, and the NCGS: 10 Years of Research. Califon, NJ: Gardiner-Caldwell SynerMed; 1996. Growth hormone treatment in the United States: demographic and diagnostic features of 2331 children; pp. 179–183. [Google Scholar]
- 15.Chatelain P. Trends in the diagnosis and treatment of short stature as revealed by KIGS. In: Ranke MB, Wilton P, editors. Growth Hormone Therapy in KIGS: 10 Years’ Experience. Heidelberg, Germany: Johann Ambrosius Barth Verlag; 1999. pp. 11–20. [Google Scholar]
- 16.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–629. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 17.Tanner JM, Goldstein H, Whitehouse RH. Standards for children’s height at ages 2–9 years allowing for heights of parents. Arch Dis Child. 1970;45:755–762. doi: 10.1136/adc.45.244.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 19.Lifshitz F, Cervantes CD. Short stature. In: Lifshitz F, editor. Pediatric Endocrinology. 3rd edition. New York: Marcel Dekker; 1996. pp. 1–18. [Google Scholar]
- 20.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–242. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 21.Grimberg A, Cohen P. Optimizing growth hormone therapy in children. Horm Res. 1997;48(Suppl 5):11–15. doi: 10.1159/000191323. [DOI] [PubMed] [Google Scholar]
- 22.Reiter EO, Blethen SL, Baptista J, Price L. Early initiation of growth hormone treatment allows age-appropriate estrogen use in Turner’s syndrome. J Clin Endocrinol Metab. 2001;86:1936–1941. doi: 10.1210/jcem.86.5.7466. [DOI] [PubMed] [Google Scholar]
- 23.Savendahl L, Davenport ML. Delayed diagnoses of Turner’s syndrome: proposed guidelines for change. J Pediatr. 2000;137:455–459. doi: 10.1067/mpd.2000.107390. [DOI] [PubMed] [Google Scholar]
- 24.Sandberg DE, Colsman M, Voss LD. Short stature and quality of life: a review of assumptions and evidence. In: Pescovitz OH, Eugster E, editors. Pediatric Endocrinology: Mechanisms, Manifestations, and Management. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 191–202. [Google Scholar]
- 25.Henwood M, Grimberg A, Moshang T., Jr The expanded spectrum of pediatric growth hormone use: evidence for growth benefits. Curr Opin Pediatr. 2002;14:437–442. doi: 10.1097/00008480-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Bolt LL, Mul D. Growth hormone in short children: beyond medicine? Acta Paediatr. 2001;90:69–73. doi: 10.1080/080352501750064905. [DOI] [PubMed] [Google Scholar]
- 27.Kelnar CJ, Albertsson-Wikland K, Hintz RL, Ranke MB, Rosenfeld RG. Should we treat children with idiopathic short stature? Horm Res. 1999;52:150–157. doi: 10.1159/000023452. [DOI] [PubMed] [Google Scholar]
- 28.Haverkamp F, Ranke MB. The ethical dilemma of growth hormone treatment of short stature: a scientific theoretical approach. Horm Res. 1999;51:301–304. doi: 10.1159/000023417. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Pediatrics Committee on Drugs and Committee on Bioethics. Considerations related to the use of recombinant human growth hormone in children. Pediatrics. 1997;99:122–129. doi: 10.1542/peds.99.1.122. [DOI] [PubMed] [Google Scholar]
- 30.White GB. Human growth hormone: the dilemma of expanded use in children. Kennedy Inst Ethics J. 1993;3:401–409. doi: 10.1353/ken.0.0170. [DOI] [PubMed] [Google Scholar]
- 31.Lantos J, Siegler M, Cuttler L. Ethical issues in growth hormone therapy. JAMA. 1989;261:1020–1024. [PubMed] [Google Scholar]
- 32.Cuttler L, Silvers JB, Singh J, Marrero U, Finkelstein B, Tannin G, et al. Short stature and growth hormone therapy: a national study of physician recommendation patterns. JAMA. 1996;276:531–537. [PubMed] [Google Scholar]
- 33.Chen RS, Shiffman RN. Assessing growth patterns: routine but sometimes overlooked. Clin Pediatr. 2000;39:97–102. doi: 10.1177/000992280003900204. [DOI] [PubMed] [Google Scholar]
- 34.Lipman TH, Hench K, Logan JD, DiFazio DA, Hale PM, Singer-Granick C. Assessment of growth by primary health care providers. J Pediatr Health Care. 2000;14:166–171. doi: 10.1067/mph.2000.104538. [DOI] [PubMed] [Google Scholar]
- 35.de Onis M, Wijnhoven TM, Onyango AW. Worldwide practices in child growth monitoring. J Pediatr. 2004;144:461–465. doi: 10.1016/j.jpeds.2003.12.034. [DOI] [PubMed] [Google Scholar]