Abstract
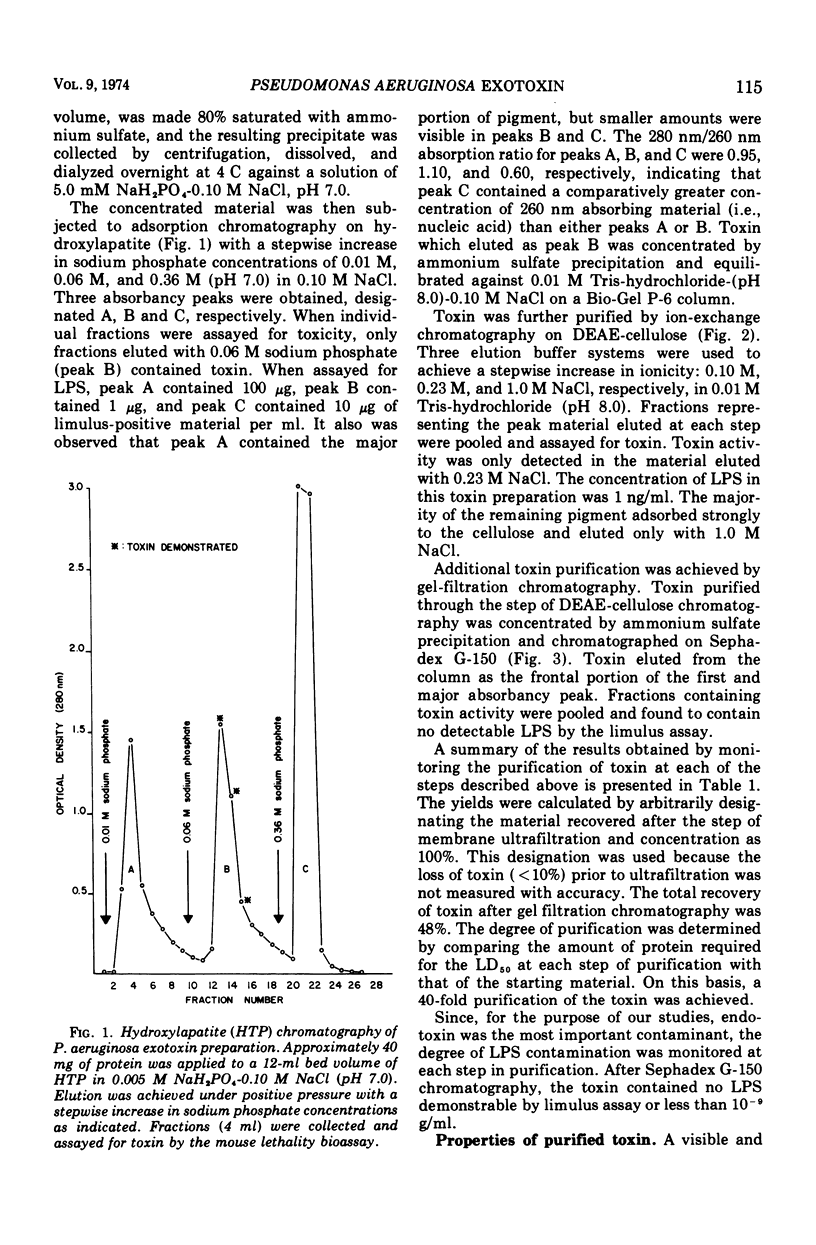
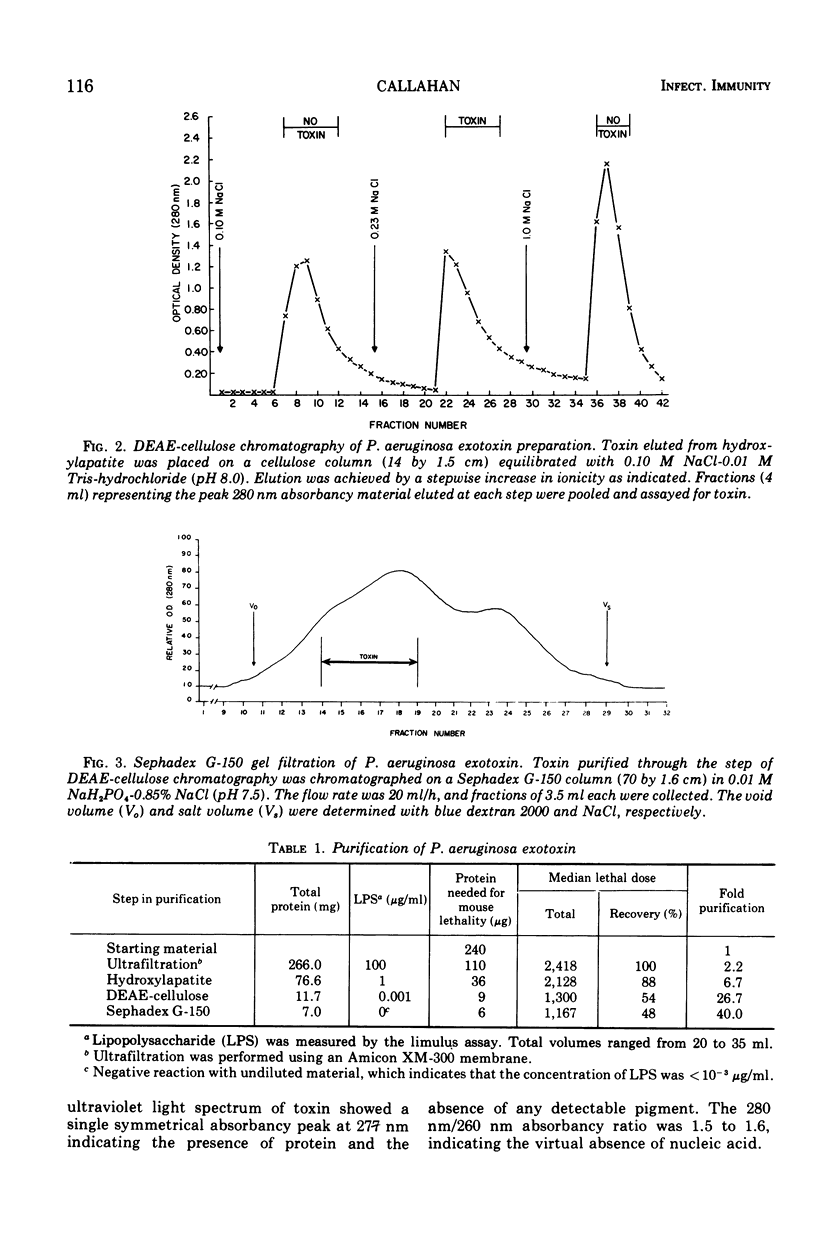
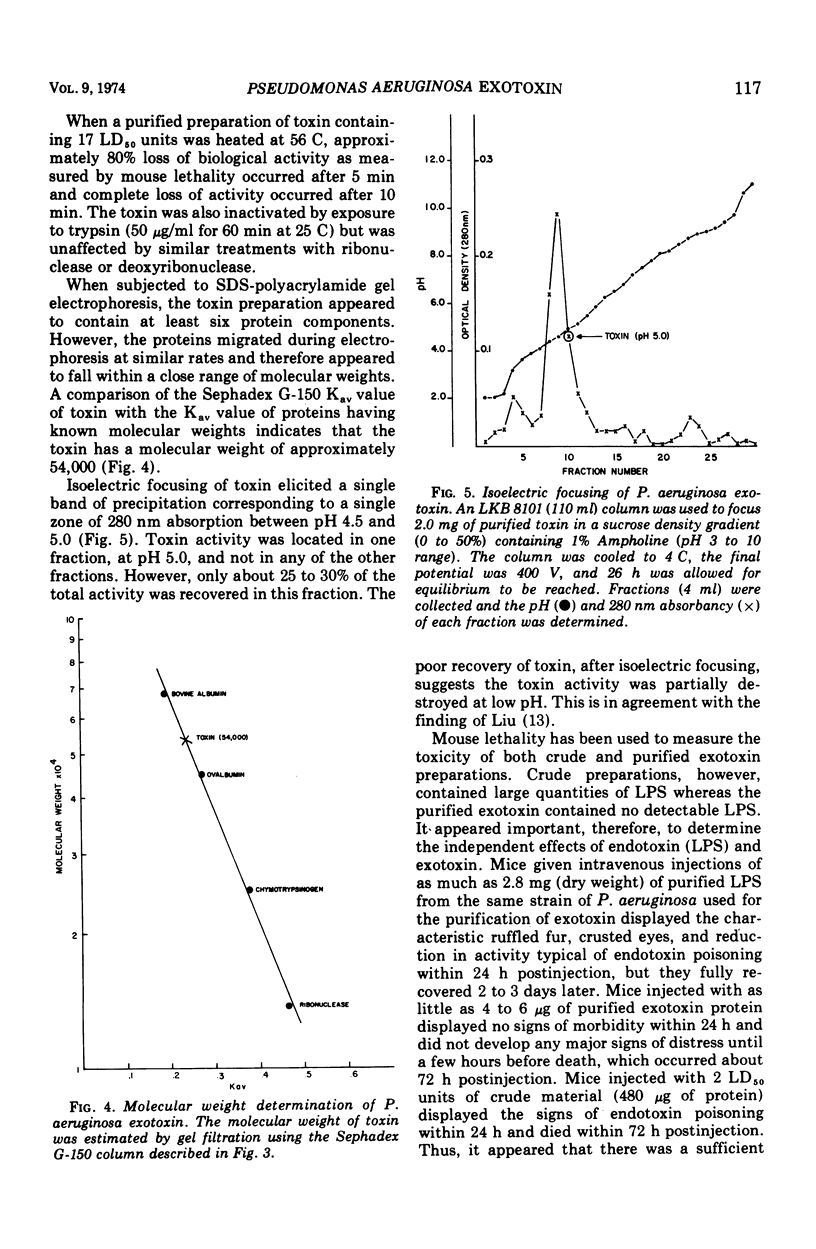
A trypsin-sensitive, heat-labile exotoxin of Pseudomonas aeruginosa strain P-A-103 has been purified by a procedure that includes membrane ultrafiltration, hydroxylapatite chromatography, ion-exchange cellulose chromatography, and gel filtration chromatography. The procedure resulted in the recovery of 48% of the exotoxin with a 40-fold increase in specific activity (micrograms of protein per median lethal dose [LD50]). The mean lethal dose of the purified toxin administered intravenously into mice weighing 20 g was approximately 6 μg of protein. The toxin contained virtually no nucleic acid, detectable pigment, or lipopolysaccharide. When subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, the toxin separated into at least six protein components which appeared to have similar molecular weights. The estimated molecular weight of the toxin is 54,000, and its isoelectric point is 5.0
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Young L. S., Meyer R. D., Blevins A. H. Infectious complications of neoplastic disease. Med Clin North Am. 1971 May;55(3):729–745. doi: 10.1016/s0025-7125(16)32514-7. [DOI] [PubMed] [Google Scholar]
- Atik M., Liu P. V., Hanson B. A., Amini S., Rosenberg C. F. Pseudomonas exotoxin shock. A preliminary report of studies in dogs. JAMA. 1968 Jul 15;205(3):134–140. doi: 10.1001/jama.205.3.134. [DOI] [PubMed] [Google Scholar]
- BALCH H. H. Resistance to infection in burned patients. Ann Surg. 1963 Jan;157:1–19. doi: 10.1097/00000658-196301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERK R. S. PARTIAL PURIFICATION OF THE EXTRACELLULAR HEMOLYSIN OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:559–565. doi: 10.1128/jb.88.3.559-565.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAHAN W. S., BEYERLEIN B., MULL J. D. TOXICITY OF PSEUDOMONAS AERUGINOSA SLIME. J Bacteriol. 1964 Sep;88:805–806. doi: 10.1128/jb.88.3.805-806.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINLAND M., JONES W. F., Jr, BARNES M. W. Occurrence of serious bacterial infections since introduction of antibacterial agents. J Am Med Assoc. 1959 Aug 29;170:2188–2197. doi: 10.1001/jama.1959.63010180008012. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Liu P. V. An enterotoxin of Pseudomonas aeruginosa. J Infect Dis. 1971 Jan;123(1):97–98. doi: 10.1093/infdis/123.1.97. [DOI] [PubMed] [Google Scholar]
- Kusama H., Suss R. H. Vascular permeability factor of Pseudomonas aeruginosa. Infect Immun. 1972 Mar;5(3):363–369. doi: 10.1128/iai.5.3.363-369.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966 Feb;116(1):112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- Meinke G., Barum J., Rosenberg B., Berk R. In Vivo Studies with the Partially Purified Protease (Elastase) from Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):583–589. doi: 10.1128/iai.2.5.583-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R. Pseudomonas aeruginosa exotoxin: effect on cellular and mitochondrial respiration. J Infect Dis. 1972 Jul;126(1):48–53. doi: 10.1093/infdis/126.1.48. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]



