Abstract
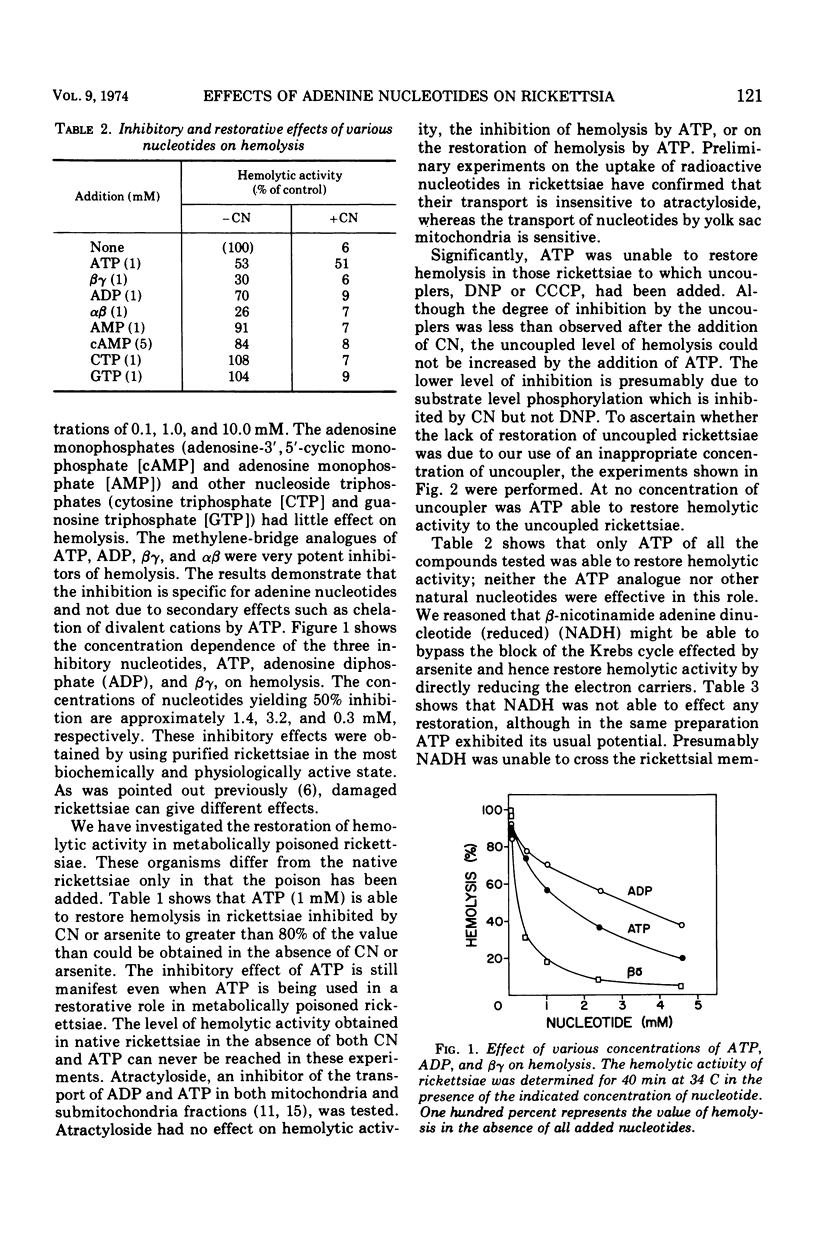
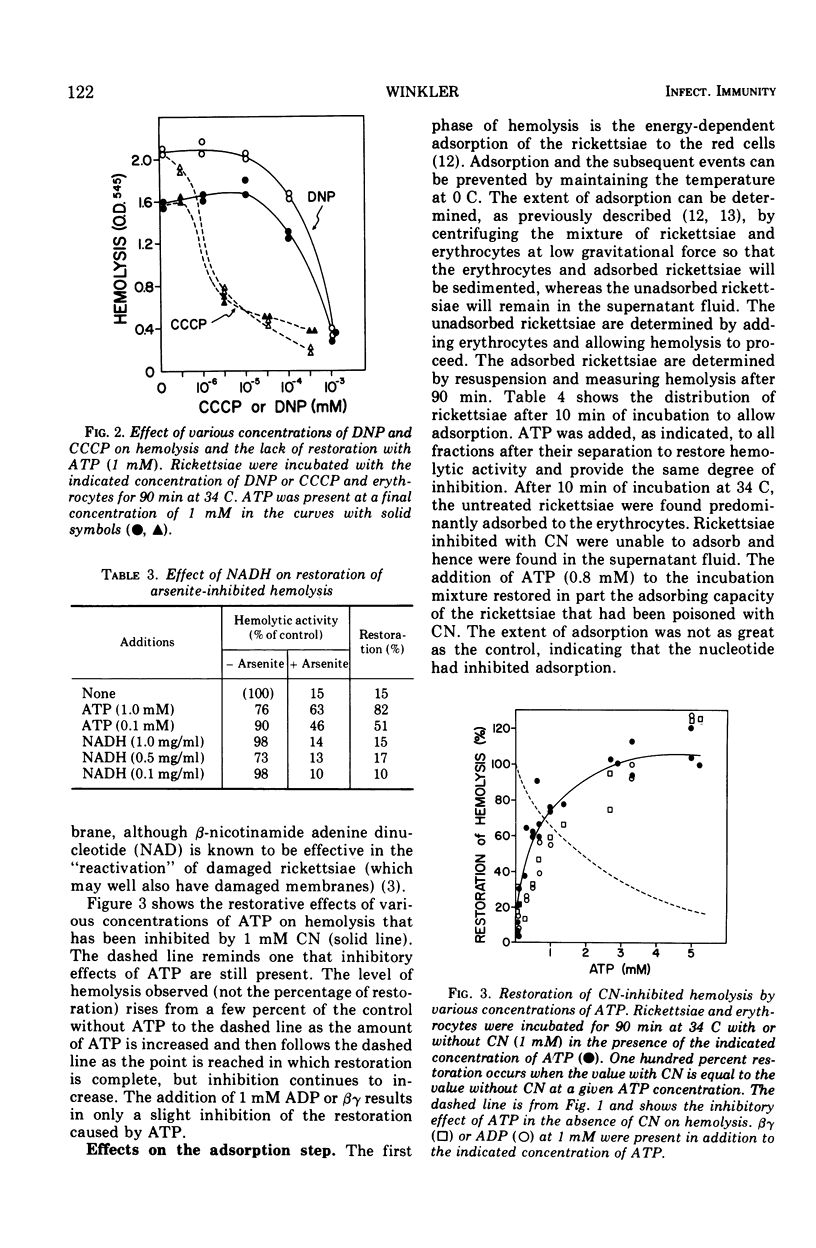
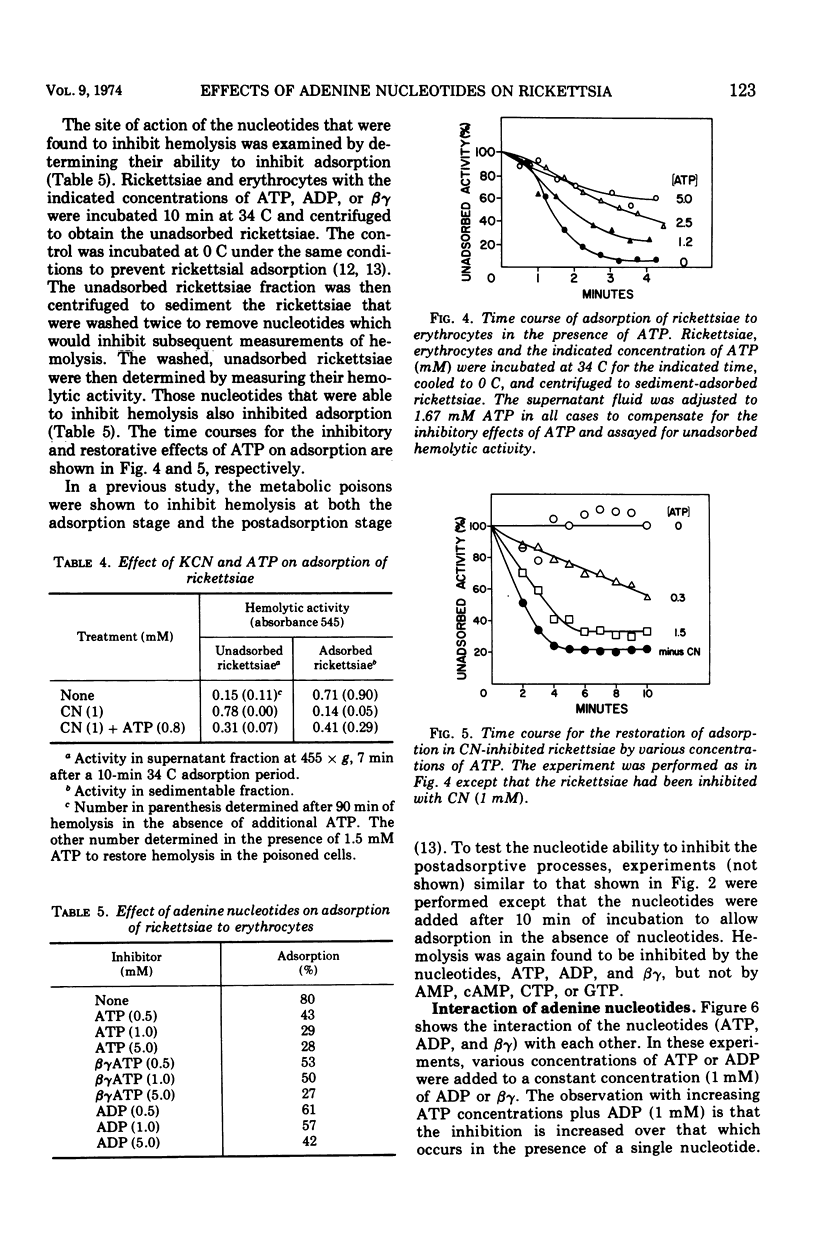
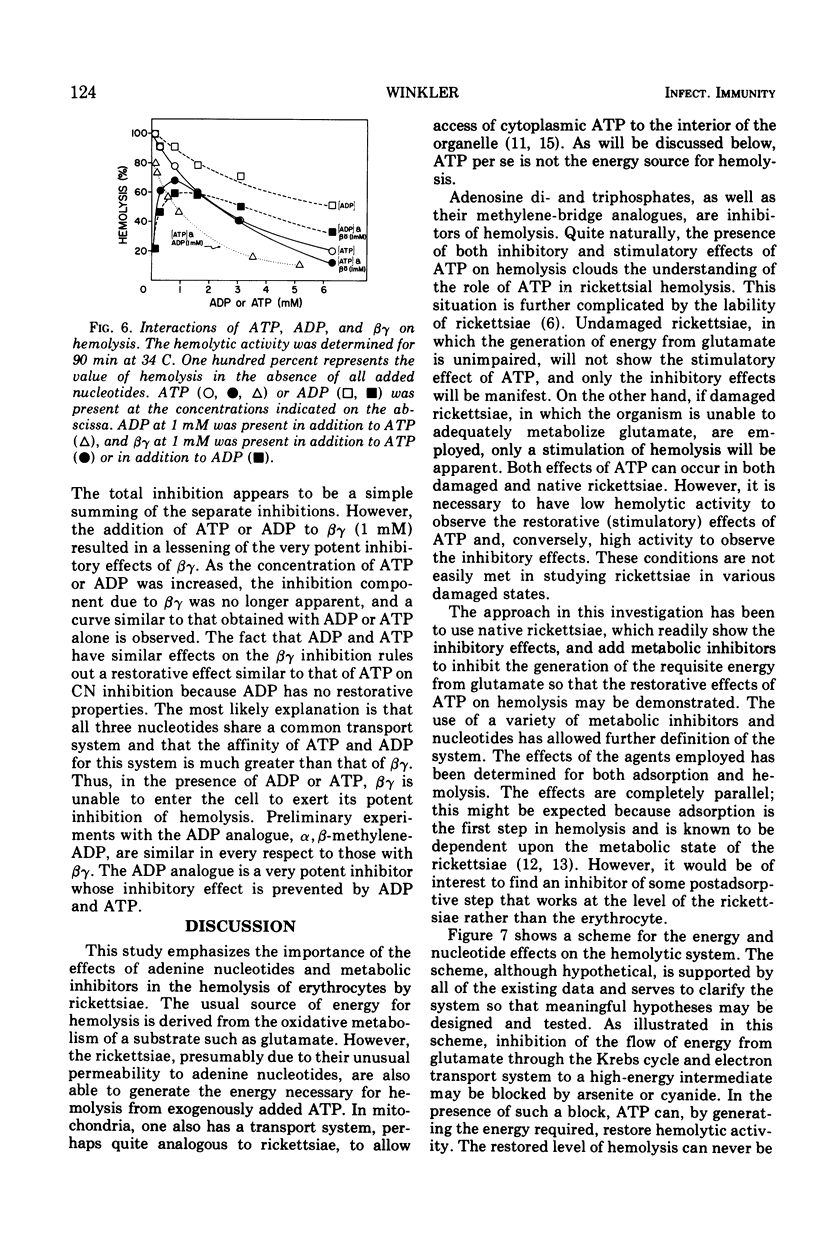
The adenine nucleotides, adenosine diphosphate, adenosine triphosphate, (ATP), and the methylene-bridge analogues are inhibitors of rickettsial adsorption to and the hemolysis of sheep erythrocytes. Other nucleotides, adenosine monophosphate, cyclic adenosine monophosphate, cytosine triphosphate, and guanosine triphosphate, are without effect. Adsorption and hemolysis require the generation of energy by the rickettsiae which is usually derived from glutamate. When the generation of energy from the metabolism of glutamate is inhibited by arsenite or cyanide, the addition of ATP can supply the energy to restore hemolysis. However, in the presence of the uncouplers, ATP can not restore hemolysis. Even when functioning in a restorative role, ATP still has its inhibitory properties. These results suggest that a high-energy intermediate (X ∼ I), rather than ATP itself, is the energy source. The interactions of inhibitory nucleotides suggest that these compounds share a common transport system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of the toxicity and hemolytic activity of typhus rickettsiae by starvation. J Bacteriol. 1957 Nov;74(5):637–645. doi: 10.1128/jb.74.5.637-645.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus rickettsiae at O C. J Bacteriol. 1957 Jan;73(1):56–62. doi: 10.1128/jb.73.1.56-62.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- Bovarnick M. R., Schneider L. ROLE OF ADENOSINE TRIPHOSPHATE IN THE HEMOLYSIS OF SHEEP ERYTHROCYTES BY TYPHUS RICKETTSIAE. J Bacteriol. 1960 Sep;80(3):344–354. doi: 10.1128/jb.80.3.344-354.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: adsorption of rickettsiae to erythrocytes. Infect Immun. 1973 Jan;7(1):93–99. doi: 10.1128/iai.7.1.93-99.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: effect of metabolic inhibitors upon hemolysis and adsorption. Infect Immun. 1973 Apr;7(4):550–555. doi: 10.1128/iai.7.4.550-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Winkler H. H., Bygrave F. L., Lehninger A. L. Characterization of the atractyloside-sensitive adenine nucleotide transport system in rat liver mitochondria. J Biol Chem. 1968 Jan 10;243(1):20–28. [PubMed] [Google Scholar]



