Abstract
Adverse events because of medical errors are a leading cause of death in the United States (US) exceeding the mortality rates of motor vehicle accidents, breast cancer, and AIDS. Improvements can and should be made to reduce the rates of preventable surgical errors because they account for nearly half of all adverse events within hospitals. Although minimally invasive surgery (MIS) has proven patient benefits such as reduced postoperative pain and hospital stay, its operative environment imposes substantial physical and cognitive strain on the surgeon increasing the risk of error. To mitigate errors and protect patients, a multidisciplinary approach is needed to improve MIS. Clinical human factors, and biomedical engineering principles and methodologies can be used to develop and assess laparoscopic surgery instrumentation, practices, and procedures. First, the foundational understanding and the imperative to transform health care into a high-quality and safe system is discussed. Next, a generalized perspective is presented on the impact of the design and redesign of surgical technologies and processes on human performance. Finally, the future of this field and the research needed to further improve the quality and safety of MIS is discussed.
Keywords: human factors and ergonomics, minimally invasive surgery, patient safety, health care quality
Background
In 2012, the health care sector in the United States (US) accounted for about 2.8 trillion dollars,1 and yet insignificant resources have been devoted to improving its processes and productivity (National Academy of Engineering (NAE), Institute of Medicine (IOM)).2 Although work is now being completed, the lack of attention and resources focused on optimizing health care has resulted in a significant amount of medical injuries and monetary costs. Specifically, it was estimated that the total national costs from lost income, lost household production, disability, and health care because of preventable medical injuries were between $17 and $29 billion.3,4 Many of these preventable medical injuries lead to significant morbidity and mortality, with an estimated 44,000–98,000 Americans dying in hospitals each year.5 Additionally, the fragmented and disjointed health care system in the US breeds medical mismanagement. For instance, in 2000 for “every dollar spent on health care, thirty to forty cents was spent on costs associated with overuse, underuse, misuse, duplication, system failures, poor communication and inefficiency.” With health care costs rising at double-digit rates and 47 million Americans lacking health insurance,6 the US health care system must undergo a drastic transformation to minimize economic hardship, increase access to care, and increase the quality and safety of care. To mitigate and prevent future medical errors, a holistic approach to health care delivery reform must be taken to improve its safety, quality, efficiency, and overall performance.
While manufacturing, aviation, and nuclear industries have implemented the use of various systems engineering tools, health care has predominately focused on diagnostic and therapeutic technological development. This has created the so-called “quality gap,” which is the divergence between the progress in medical science and the quality of care patients receive.7 In 2000 and 2001, the Institute of Medicine recognized the deepening quality crises and issued the two reports: “To Err is Human” and “Crossing the Quality Chasm,” respectively. These landmark reports documented not only the system failures that resulted in as many as 100,000 deaths but also a call to action for all stakeholders to transform the health care industry. As a result, the National Academy of Engineering and the Institute of Medicine united and initiated a project in 2002 to “1) identify engineering applications that could contribute significantly to improvements in health care delivery; 2) assess factors that would facilitate or impede the deployment of these applications; and 3) identify areas of research in engineering and other fields that could contribute to rapid improvements in performance.” These objectives call for the engineering community to develop a cooperative relationship with health care professionals and to implement engineering tools to eliminate the fundamental shortcomings in the way care is organized.7 Although the uptake and progress in both the health care and engineering communities has been slow, improvements have been made toward creating a “twenty-first century system capable of delivering safe, effective, timely, patient-centered, efficient, [and] equitable health care.” Pursuant to the Institute of Medicines7 recommendations, these six dimensions of quality form the foundational framework for the analysis, design, and improvement of the US health care system.
Human Factors of Surgery
Many engineering principles and tools have begun to take hold in health care in areas such as electronic medical records, medication management, and patient handoffs.8–10 Yet in the early 1900s, Frank and Lillian Gilbreth were among the first pioneers to systematically study processes in health care. Both were advocates of scientific management and the study of motion.11,12 They revolutionized surgery by introducing the concept of a “surgical caddy,” now referred to as the scrub nurse, so that surgeons did not waste time searching for instruments.11,12 Poignant even now, they also observed that “surgical practices and instrumentation varied greatly throughout the country, leading to inefficiency and the lack of a best approach to each treatment modality.”13 Many of the Gilbreths’ ideas are still used in hospital quality assurance and health care delivery improvement programs. The Gilbreths’ efforts provided the initial groundwork for engineers and human factors professionals to examine and improve the quality and safety of surgical procedures.
Human factors and ergonomics (HFE) can be defined as “the scientific discipline concerned with the understanding of interactions among humans and other elements of a system, and the profession that applies theory, principles, data, and other methods to design in order to optimize human well-being and overall system performance.”14 HFE is uniquely constructed to assist surgeons in that it:15
focuses on the two closely related outcomes of performance and well-being,
is design driven, and
takes a systems approach.
These three fundamental characteristics of HFE enable it to contribute to the design and evaluation of a wide array of work and service systems. HFE also has great potential to impact inherently complex and risky systems, including health care, to shape the system around the capacities and aspirations of humans to optimize performance and the well-being of clinicians and patients. Specifically, the focus is to improve both performance (quality) and well-being (safety) by “designing the integrative whole better, and by integrating the human into the system better.”15 In all, HFE utilizes multidisciplinary tools and techniques to plan, design, evaluate, redesign, and continuously improve tasks, jobs, products, technologies, processes, organizations, environments, and systems to make them compatible with the needs, abilities, and limitations of people.14
Human error
For over 30 years, researchers have been studying the cause and effect of human error.16 Human errors can be defined as unintentional random events that are inherent in all human activities and professions. These events can be characterized as any type of error, mistake, incident, accident, or deviation, regardless of whether it results in patient harm. In an effort to increase accountability and consumer access to health care performance, the National Quality Forum (NQF) created a listing of critical errors, called serious reportable events (SREs). According to the NQF, the 29 SREs are “largely preventable, grave errors and events that are of concern to the public and health care providers, and that warrant careful investigation, and should be targeted for mandatory public reporting.”17 The list of SREs includes both injuries caused by care management (rather than the underlying disease) and errors that occur from the failure to follow standard care or institutional practices and policies.18 The 29 SREs are categorized into surgical or invasive procedure, product or device, patient protection, care management, and environmental, radiological, and potential criminal events. Of these medical errors, 18 SREs account for about 2.4 million extra hospital days and $9.3 billion in excess charges every year.19 Owing to the large variation among hospitals, there has been some debate about the magnitude of the impact of medical errors. However, the general consensus is that these serious yet preventable errors lead to a significant increase in mortality, length of stay, and cost.20
Surgery has received considerable attention because of its complexity, high risk, and financial impact. For over a decade, the operating room (OR) has been one of the main targets of health care quality and patient safety research. Owing to the fact that surgical errors account for about 50% of all adverse events and up to 13% of all hospital deaths,21–23 it is not surprising that the NQF has specifically targeted the OR for quality and safety improvement. The NQF surgical or invasive procedure SREs include (1) surgery or other invasive procedure performed on the wrong site, (2) surgery or other invasive procedure performed on the wrong patient, (3) wrong surgical or other invasive procedure performed on a patient, (4) unintended retention of a foreign object in a patient after surgery or other invasive procedure, and (5) intraoperative or immediately postoperative/post-procedure death in an American Society of Anesthesiologists (ASA) Class 1 patient.18
Although the NQF has made strides since 2002 to create visibility and accountability of the most critical and costly medical errors, there have not been substantial gains in patient safety or health care quality. This is in part because all of the SREs have a high severity or patient effect, high detectability, and yet a relatively low likelihood of occurrence. For example, the likelihood of amputating the wrong leg of a patient is decreased through several checks before and during a surgical procedure. However, this type of unfortunate event is highly detectable and typically well-publicized in the media. It also has a substantial impact fiscally and emotionally on all of the parties involved (eg patient, surgeon, family, hospital, etc.). Consequently, the overall impact of mitigating these types of errors within the health care system is minimal within the current reporting paradigm. Extensive change can only occur through systematic improvements across all elements of a system including the personnel, micro-environment such as the OR, and macro-environment such as the hospital, network, and region.
In surgery, there has been progress toward analyzing errors rather than complications, which allows personnel to more accurately anticipate, avoid, and identify adverse events.24 In an effort to prevent, mitigate, and identify errors, classifications of human error have been created to determine the underlying source(s) or root cause(s) that leads to errors. For instance, one categorization classifies errors as skill based (ie faulty execution of the task), rule based (ie misclassification or misdiagnosis leading to the action), or knowledge based (ie from incomplete or incorrect knowledge).25 An alternative categorization is that errors are either active (ie enacted by front-line operators and have an immediate effect) or latent (ie hidden within the system and may lie dormant and unnoticed without causing any adverse effect until they summate to create the necessary trajectory for a major catastrophe).26 Active errors tend to be apparent such as cutting the wrong vessel, whereas latent errors tend to occur in complex and high-technology activities at a later time.
Classifying and investigating errors allows policies, procedures, and processes to be put in place that aim for optimal performance by reducing errors such that the residual risk within the system is as low as reasonably possible. As portrayed by the two very different error classification schema, human errors can occur at different levels within a system, can occur immediately or with some delay, and can have multiple root causes. The inherent complexity of human error makes it critical to have prospective and prescriptive policies, procedures, and processes that reduce the risk of error in the system as a whole. These types of policies, procedures, and processes aim to identify what may go wrong, the probability of occurrence, the consequence of occurrence, and the necessary defensive measures to minimize or eliminate risk.
One way to create these transparent and accountable structures is to utilize HFE analyses, tools and techniques to improve the surgeon’s user experience and thereby improve patient safety and outcomes by implementing changes in the system to minimize risk and make the system more resilient to error. Many of the errors in complex systems can be attributed to the mismatch between the work system and the capabilities and limitations of the human operator.27 These poor surgeon–patient and surgeon–technology interfaces produce a significant level of physical and cognitive stress on the surgeon contributing to surgical errors.28 HFE utilizes scientific data-driven analyses such as observations, questionnaires, interviews, checklists, expert appraisals, workload analyses, accident/injury analyses, task analyses, safety analyses, root cause analyses, and/or critical incident techniques to understand and implement changes within complex systems.29–32
HFE analyses and techniques are unique because they focus on different stakeholders within the system and create an understanding of the systemic aspects that lead to both excellence and failure in complex systems.33 Until recently, efforts to implement HFE practices in the OR have been largely unsuccessful.34,35 Although there has been progress, there are still no true HFE standards of practice in the OR, and limited standards for the design and testing of medical equipment. As surgical technologies become more complicated, there is an even greater risk of active and latent operative errors because of technology misunderstanding and misuse. As such, it is vital that HFE professionals partner with medical professionals, hospital administrators, and medical device manufacturers to improve these interfaces and processes to protect both patients and surgeons from harm.
Human performance
Surgeons require a significant amount of intellectual and physical preparation to perform their highly specialized work tasks. Similar to occupations in the nuclear and aviation industries, surgeons must also be adept at performing these tasks in highly stressful and risky situations.22 The inherent demands of surgery therefore warrant attention on maximizing the surgeon’s performance to optimize outcomes. Using HFE principles, an overarching goal is to enable optimal performance even under adverse conditions through the design of improved surgical technologies and processes. HFE, following a systems-based perspective, can be used to analyze surgical technologies, performance, and workload toward the improvement of the quality and safety of surgery.
Surgeons have long been interested in the design of surgical technologies and processes to maximize their efficiency, effectiveness, and outcomes.36 Even today, many surgeons develop unconventional instruments and workarounds to overcome the inherent challenges in surgery and improve their performance.36 It appears that many surgeons’ design processes are subjective and personal, whereas HFE strives to generalize and operationalize any design/redesign to increase efficiency, effectiveness, and outcomes. To show improvement, it is critical to quantify these increases as related to human performance, which can be thought of as any type of user behavior that can be measured.37
Although human performance can be measured in many different ways, typical performance metrics include success (outcome), efficiency (time), and safety (errors).37 Following the landmark publication of “To Err is Human,” there was a surge to improve patient safety and mitigate medical errors by improving human performance in the complex health care system.5 The IOM report stated that all humans are fallible and make mistakes daily even during the most routine activities.5 Yet we have come to expect perfection from surgeons in a decentralized and fragmented health care system or “nonsystem.”5 As a result of the IOM’s efforts, there was a renewed interest and awareness of HFE and systems-based analysis.
Over the last decade, there has been considerable effort to improve health care through the development and widespread implementation of robust systems that maximize the safety and quality of health care delivery. As expected, the human’s performance is critical to the overall functioning of these systems. Within the system, the human(s) and the complex processes/technologies are interdependent for optimal performance. Accordingly, it is pivotal to understand the roots of human performance including its fallibility and variability to develop these robust systems that enable humans to deliver safe and high-quality health care.
Human fallibility
Currently there is no ubiquitous “error check” function in the OR; however, current research between clinicians and engineers is demonstrating the value of such error mitigation functions/practices.16,38 The outcomes of this joint research can change the status quo of poorly designed surgical technologies and processes that lead to a countless number of preventable errors.21,22 As we build the 21st century health care system, the antiquated view that safety and quality lie only with the individual surgeon’s abilities must be eliminated.39 This individualized “blame and shame” culture does not recognize that surgeons are operating in complex sociotechnical environments with a diverse amount of people, various technologies, and patient-specific variations.29 Viewing surgical error as a personal failure at only the individual level, or the person approach, will not enable the root cause of the error to be determined and guarded against.39
In contrast to the individual or person approach to reduce human error, the HFE systems-based approach recognizes that inherently humans are prone to error regardless of skill level and that the system must guard against adverse events by mitigating human error to be as low as reasonably possible. For this approach, a system is strengthened by implementing defenses at various levels (eg individual, organizational, etc.). Reason’s24 Swiss cheese model provides an excellent depiction of how “holes” in system defenses usually lead to small incidents or failures at each defense level, which can aggregate to form a catastrophic loss within the system (Fig. 1). This catastrophic loss occurs because each of the holes or failures aligned at every level magnifying the severity of the loss downstream. To decrease the probability of a loss, the systems approach seeks to minimize these “holes” by strengthening the system’s defenses.
Figure 1.
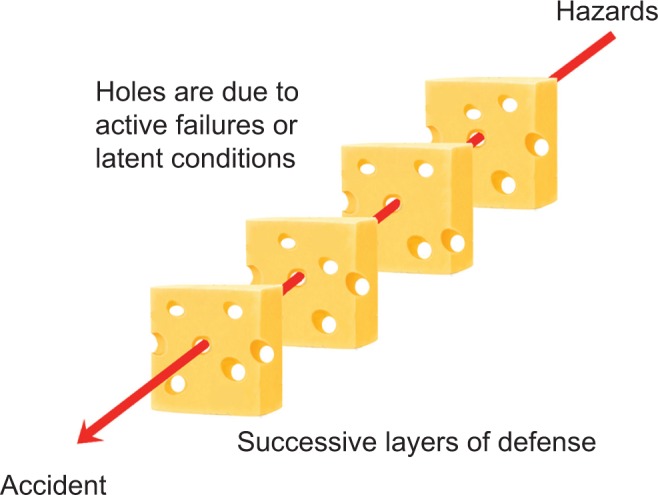
Accident path in the Swiss cheese model. Adapted from Reason.26
For minimally invasive surgery (MIS), Dankelman and Grimbergen39 identified the following five strategies to reduce errors using the systems approach: (1) reduce complexity, (2) standardize procedures, (3) implement checklists, (4) improve the quality and standardization of instruments and equipment, and (5) training. Each of the five strategies could be targeted at one or more levels portrayed in the hierarchical model of the interacting elements in a surgical system (Fig. 2). Within this “onion model” of a surgical system, surgeon–instrument interaction could be improved by reducing complexity, standardizing procedures, and improving the quality and standardization of instruments and equipment. Implementing these five strategies would enable the surgeon at the “sharp end” and the overall system to perform at a higher level by eliminating unnecessary and inefficient interactions and processes.40
Figure 2.
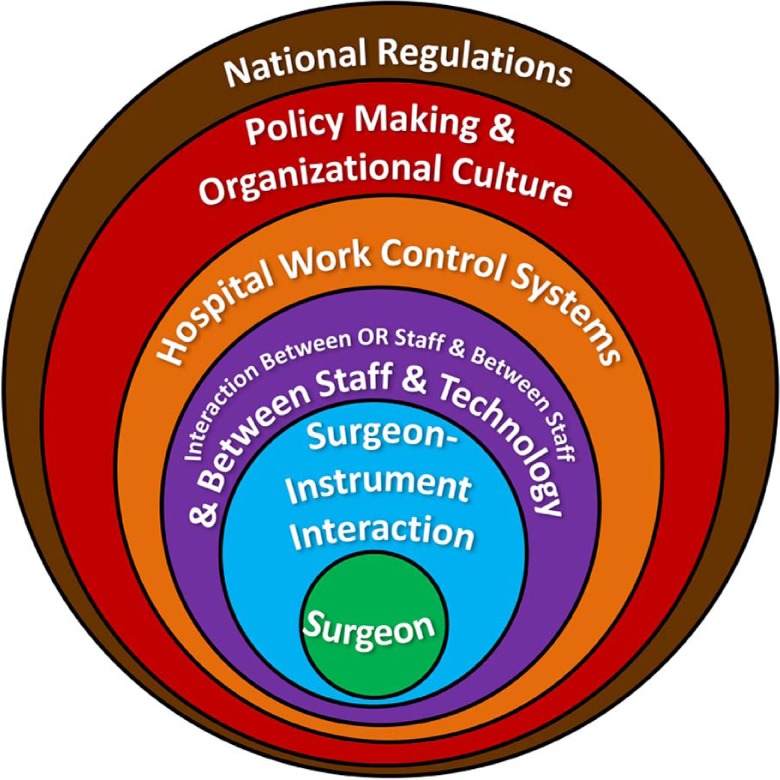
Surgical system onion model. Adapted from Dankelman and Grimbergen.39
To create a more resilient surgical system, errors or near-misses must be identified, studied, and mitigated. From the analysis of errors and near-misses, such as root cause analysis (RCA) for current systems or health care failure mode and effects analysis (HFMEA) for proposed systems, it is critical to identify the weak points or potential hazards in the system and intervene at one or more levels to reduce their risk. One systems-based method to accomplish this is to create forcing functions, which are purposely designed system elements that make it difficult or impossible for humans to perform the incorrect action and actually facilitate performance of the correct action. Although automation is one method to accomplish this, there are inherent problems with automation, and in health care, the goal is to maintain as much flexibility and adaptability as possible while minimizing technological complexity. As a result, surgical care requires a unique mix of human and technology-based operations that systematically design safety and error prevention into every system level. This more robust and error-resistant system will strengthen each defensive level, so that if a failure occurs at one level, the next defensive level will “catch” or mitigate the failure from becoming a more severe error, accident, or sentinel event downstream (Fig. 3). Overall, the systems-based approach can significantly reduce the number of preventable human errors in surgery, if errors and their causes are thoroughly studied and the overall system is strengthened through error-prevention strategies at multiple levels, including good systems design/redesign using HFE principles and practices.
Figure 3.
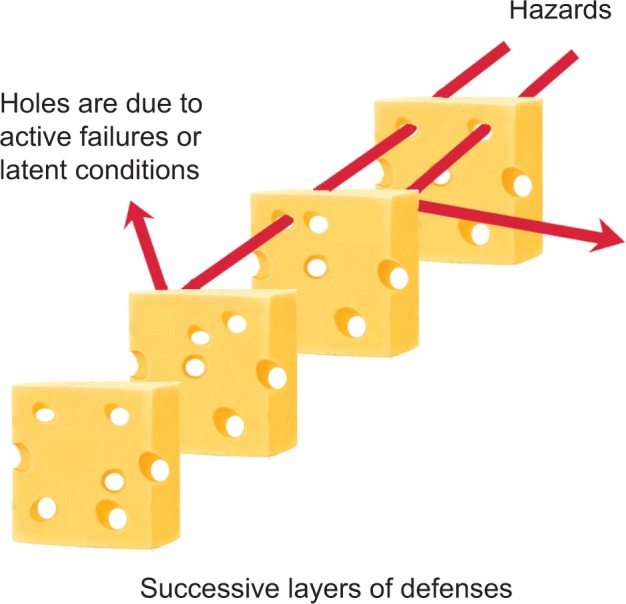
Accident mitigation in the Swiss cheese model. Adapted from Reason.26
Human variability
All types of work can be considered a process, and processes are the main source of defects or errors because of performance variability.41 Accordingly, understanding and minimizing variability in key processes are critical to improving the quality of the health care system. Health care quality is safe, effective, timely, patient centered, efficient, and equitable care. For engineers, quality is a broad term that encompasses quality assurance, quality control, and quality management. Dr. Joseph M. Juran, the “Father of Quality,” helped define the modern quality movement, and was the first to incorporate human aspects into quality management.41 Juran’s definition of quality was “fitness for intended use,” which can be translated into meeting or exceeding customer expectations.41 Per the International Organization for Standardization (ISO), the currently accepted definition of quality is “the degree to which a set of inherent characteristics fulfills requirements.”42 Other agencies within health care have begun to recognize the similarities between the quality efforts within industrial sectors and health care. For instance, the Institute for Health care Improvement (IHI) has defined quality as “turning into outcomes management, and involves minimizing unnecessary variation so that outcomes become more predictable and certain” (2012). Regardless of definition, it is widely accepted that “variation is the enemy of quality.”43 Reducing or eliminating variability within systems is the ultimate goal of all quality efforts, because it increases performance and well-being. The strikingly similar approaches to reduce variability and improve outcomes elegantly bridge the gap between the quality efforts in industrial and health care settings.
One of the main precepts from the Gilbreths’ work was standardization and best practices. “Traditionally, surgery has been taught by an apprentice model, where the learner imitates the actions of a skilled mentor.”44 Although this model has been effective, it leads to great variation within surgical practice because training and assessment are based heavily upon the mentors’ individual abilities of the task, teaching/mentoring, and their subjective assessment of the trainee. The traditional apprentice model is also time inefficient for both the trainee and mentor, because it requires residents to be “exposed to a large number of surgeries performed by a limited number of dedicated teaching faculty.”44
Surgeons understand the need to hone and refine their skills for optimal performance. The rigor of surgical training fundamentally pursues micro-level (individual) optimization and perfection by minimizing errors and variabilities. However, the proficiency-gain curve, sometimes referred to as the learning curve, is individualized and varies for each surgical procedure (Fig. 4).45 It therefore requires a significant amount of time, effort, money, and individualized training to reach proficiency using the apprentice model. During residency, each surgical trainee is assessed on his/her proficiency to demonstrate that he/she has the necessary skills and competencies to execute high-quality and safe operative procedures. This internal quality assurance program ensures that residents can cope with the demands of surgery and execute at an acceptable level of care. Although surgical proficiency underpins quality and safe surgical practice,16 the inherent variability in surgical skill acquisition time, new resident duty-hour restrictions, and patient safety concerns calls for a change in the fundamental way in which we train and assess surgeons.46–49
Figure 4.
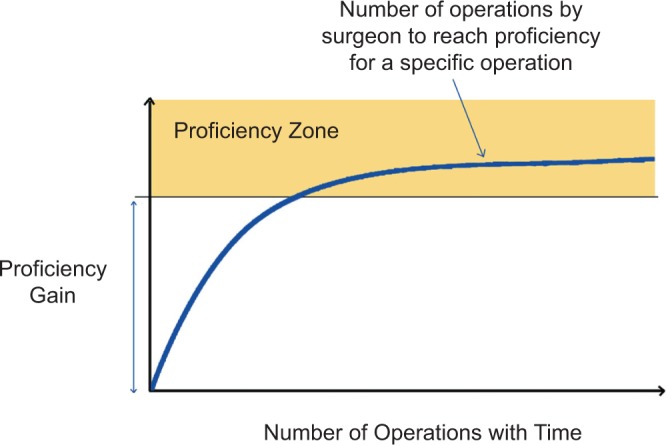
A surgeon’s idealized proficiency-gain curve. Adapted from Cuschieri and Tang.45
Returning to the Gilbreth’s precepts for standardization and best practice, it is evident that it contrasts the apprentice model, which inherently generates variability. However, the process of standardizing surgical training and assessment is complex and reducing variability is not as straightforward as minimizing product variation on a manufacturing line. Humans (clinicians and patients) are complex systems unto themselves. The physical, physiological, psychological (affective and cognitive), and social aspects of humans and the variability of human performance make standardization and optimization within the system difficult. Additionally, different levels within the system may or may not benefit from the same strategies. At the micro-level (eg humans using tools or performing single tasks), surgeons may benefit from standardized surgical instrumentation, but this strategy may not enhance human performance at the meso-level (eg humans as part of technical processes or organizations) or macro-level (eg humans as part of networks of organizations, regions, countries, or the world).15 Accordingly, it is imperative to take a holistic, integrative, and tailored approach to improve performance and decrease variability among the interacting and interdependent elements throughout a system to the extent possible. Finally, determining the appropriate processes to study and to reduce their variability is an important aspect to consider.
In all, HFE system-based approaches can assist in the improvement of health care quality through the reduction of variability because HFE principles and techniques are goal oriented and purposefully design systems around humans and their environment.15 This hierarchical approach of fitting humans within the system by focusing on the interactions within their physical, organizational, and social environments enables humans better able to contribute to performance.15
MIS: Past, Present, and Future
Conventional laparoscopic surgery (CLS)
CLS is a form of MIS where a surgeon makes several small incisions (0.5–1.2 cm) to insert long, slender instruments and a camera into the patient’s abdomen. Patient benefits from CLS include reduced trauma, postoperative pain, and recovery time.50–52 However, the disadvantages of CLS include a two-dimensional surgical field, awkward instruments with fulcrum effects, an unstable camera platform, and increased static postural stress compared to open surgery.53,54 Maneuvering laparoscopic instruments also increases muscle activity and requires the adoption of non-ergonomic positions of the upper limbs resulting in arm, shoulder, and spine discomfort compared to open surgical procedures.53,55 Finally, the physical workload of manual laparoscopic surgery compared to an open surgery has been shown to be significantly greater for an equivalent procedure.56,57 Despite the great strain on surgeons, CLS is still considered the gold-standard for many routine surgical procedures.
In the 1980s, there was a surge to perform the new technique of CLS in lieu of open surgeries.59,60 This quick adoption resulted in significant morbidity and mortality because of a lack of training, proper instrumentation, systematic evaluation, prospective comparative data, standardization, and oversight.61–63 Although prospective clinical trials did reveal improved patient outcomes for CLS compared to open surgery,64–66 the acceptance and implementation of CLS should have occurred in a more coordinated and responsible manner to protect patients from undue harm.
As expected, much can and should be learned about surgical error prevention and management from the early failures of CLS. The most poignant lessons learned were that novel techniques must be critically evaluated before widespread adoption;67,68 regardless of surgical specialty and expertise, there is a significant skill acquisition time for new techniques and instrumentation,69,70 and there is a need for training and certification of basic knowledge and technical skills outside of the OR.71–73 It was also shown that the CLS environment causes fatigue, physical discomfort, and cognitive over-loading for surgeons.74–77 In all, these risk factors further predisposed the pioneering CLS surgeons to preventable medical errors. To improve health care quality and patient safety, it is critical to learn from past mistakes and to develop a robust system that prevents, identifies, and mitigates medical errors. It is also vital to critically assess new techniques, processes, and technologies that may impact all or part of the health care system.
Laparoendoscopic single-site surgery (LESS)
As the next evolution of MIS, LESS is currently being performed without formal guidance or standardization. This seemingly “scarless” surgical technique is performed using a single, small incision (~2.0 cm) typically through the navel. The surgeon inserts several instruments and a laparoscopic camera into the single incision leaving virtually no scar. Although LESS represents the next logical step toward less invasive surgery, its patient benefits and best practices are currently unproven.78,79 At present, the only recognized benefit of LESS compared to conventional laparoscopy is improved cosmesis.80–83 Single-institution comparative case reports indicate that potential patient benefits include an increase in patient satisfaction and a decrease in postoperative pain and recovery time compared to CLS.81,82,84–86 These initial reports demonstrate that LESS is safe, effective, and feasible for noncomplex cases;87–89 however, a large-scale multicenter randomized control trial is needed to verify the reproducibility of these results.
As previously stated, the early adoption of CLS resulted in significant patient harm.61–63 Early complication and conversion to open surgery rates for conventional laparoscopic cholecystectomy were 4–8% and 4%, respectively.59,60 However, today the technique has been thoroughly studied, validated, and standardized with complication, and mortality rates are less than 1.5% and 0.1% for laparoscopic cholecystectomy, respectively.91 For LESS, the preliminary complication and conversion rates appear to much higher than the rates for conventional laparoscopy, which is still considered the gold-standard in MIS. From single-institution case reports, the complication and conversion rates for LESS cholecystectomy are as high as 24% and 52% (Table 1), respectively. Preliminary comparative studies of LESS and CLS cholecystectomies show more favorable results (Table 2); however, many of these studies were performed by expert laparoscopic surgeons on young, healthy patients. Although not a comprehensive review of the current literature, these data are staggering and are cause for concern. The threshold for complications and conversion should be low and should reflect the rates of the current standard of practice. As evidenced by these preliminary data, a critical evaluation of LESS is needed. In particular, a coordinated and systematic evaluation of LESS should occur to ensure that the widespread implementation of LESS occurs in a responsible manner that protects patient safety.
Table 1.
Intraoperative outcomes of LESS cholecystectomies.
| FIRST AUTHOR | YEAR | PATIENTS | CONVERSION TO | COMPLICATIONS | |
|---|---|---|---|---|---|
| CONV. LAP. | OPEN | ||||
| Chow, A. | 200992 | 14 | NR | NR | 7.14% |
| Edwards, C. | 201093 | 80 | 11.25% | None | 8.75% |
| Elsey, J.K. | 201094 | 238 | 2.50% | 0.42% | 2.10% |
| Erbella, J., Jr | 201095 | 100 | 2.00% | None | None |
| Ersin, S. | 201096 | 20 | 5.00% | None | None |
| Langwieler, T.E. | 200997 | 14 | None | None | None |
| Petrotos, A.C. | 200998 | 10 | None | None | None |
| Philipp, S.R. | 200999 | 29 | 52.0% | None | 24.1% |
| Podolsky, E.R. | 2009100 | 5 | None | None | None |
| Rivas, H. | 2010101 | 100 | None | None | NR |
| Roberts, K.E. | 2010102 | 56 | 1.79% | 1.79% | 5.36% |
| Romanelli, J.R. | 2010103 | 22 | 4.55% | None | 4.55% |
| Solomon, D. | 2010104 | 56 | 1.79% | 1.79% | 5.5% |
| Tacchino, R. | 2009105 | 12 | None | None | 16.7% |
| Tsimoyiannis, E.C. | 2010106 | 20 | None | None | 5.26% |
Abbreviations: Conv. Lap., conventional laparoscopy; NR, not reported.
Table 2.
Cholecystectomy comparative studies.
| FIRST AUTHOR | PHILIPP, S.R. | TSIMOYIANNIS, E.C. | ||
|---|---|---|---|---|
| YEAR | 2009 | 2010 | ||
| INTERVENTION | CLS | LESS | CLS | LESS |
| Patients | 22 | 29 | 20 | 20 |
| Operative time (min) | 67a | 85a | 37.2 ± 9.16 | 49.65 ± 9.02 |
| Length of stay (days) | 0a | 0a | 1.10 ± 0.44 | 1.25 ± 0.44 |
| Complications | 13.6% | 24.1% | 11.1% | 5.26% |
| Estimated blood loss (mL) | 15a | 15a | 8.50 ± 6.30 | 9.90 ± 14.38 |
| Postoperative pain VAS | 2a | 4a | 0.85 ± 0.67 | 0.05* ± 0.22 |
Note: Mean ± standard deviation.
Median.
LESS has become more prevalent not only primarily because of the recent development of advanced access port (Table 3) and hand instrument technologies (Table 4) but also because of the technical performance difficulty in natural orifice transluminal endoscopic surgery (NOTES). On a continuum from more to less invasive, LESS lies somewhere between conventional laparoscopy and NOTES. While NOTES was conceived first, its widespread uptake has been severely hindered because of a lack of patient acceptance, enabling surgical technology, training opportunities, and safety concerns.107–111
Table 3.
LESS multi-channel access devices.
| PRODUCT | DESCRIPTION |
|---|---|
| Triport+ (Olympus America Inc, Center Valley, PA, USA) | A multi-instrument disposable access port that allows up to three instruments to be used simultaneously through a single incision. |
| Gelpoint (Applied Medical Corp, Rancho Santa Margarita, CA, USA) | A multi-instrument disposable port that facilitates triangulation of standard instruments through the gel cap. Maximizes internal working diameter and offers greater freedom of movement. |
| SILS port (Covidien, Mansfield, MA, USA) | A flexible laparoscopic port that can accommodate up to three instruments through a single incision. This product is designed to use multiple instruments with maximal maneuverability. |
| SSL access system (Ethicon Endo-Surgery, Inc, Cincinnati, Ohio USA) | Enables the insertion of multiple surgical instruments through the seal cap. Seal cap rotates 360° for quick reorientation. Eliminates need for trocars. |
| OCTO port (dalimSurgNet Corp, Seoul, South Korea) | Detachable port cap with soft silicon cover and different port heights. Includes four ports for introducing instruments via one incision. |
| AirSeal for single port surgery (SurgiQuest, Inc, Orange, CT, USA) | Insert multiple instruments using a single cannula. Possible to use unique size and shape instruments for triangulation. |
| X-cone (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) | Reusable access for transumbilical laparoscopy. The design offers high instrument mobility, stable instrument guidance and comfortable introduction technique. |
| Cuschieri endocone (KARL STORZ GmbH & Co KG, Tuttlingen, Germany) | Reusable system was developed as a holistic solution (port-instruments-retraction system) to facilitate the execution of LESS. |
| InnoPort (Innovia LLC, Miami, FL, USA) | Simple, cone-shape design grants physicians unrestricted access to the abdominal cavity with up to three rigid, curved, and/or articulating 5 mm instruments. |
Table 4.
Hand instruments used for LESS.
| PRODUCT | DESCRIPTION |
|---|---|
| Autonomy laparo-angle articulating instruments (Cambridge Endoscopic Devices, Inc., Framingham, MA, USA) | Seven degrees of freedom, allowing unprecedented access to the most difficult to reach areas. Full articulation that maps the surgeon’s hand motions. A tip that can rotate 360° around its axis for precise positioning. The capability of performing simultaneous actions such as articulating downward while rotating. Handle locks at any angle and rotates. |
| Roticulator endo-instruments (Covidien, Mansfield, MA, USA) | Single use instruments with a grooved collar that articulates the jaws and the last 2 cm of the shaft from 0 to 80 degrees. The scalloped dial located on the handle rotates the shaft and jaws 360 degrees. |
| SILS hand instruments (Covidien, Mansfield, MA, USA) | All four new instruments have been designed to enhance the surgeon’s flexibility and visualization when performing SILS™ procedures. While the new line has the potential to revolutionize surgical instrumentation, the design is intuitive enough to allow surgeons and nurses to quickly master the operation of the instruments. |
| Diamond-flex articulating dissectors (Cardinal Health, Dublin, OH, USA) | These instruments can articulate once placed in the peritoneal space for access around anatomical structures. |
| DAPRI curved instruments (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) | The first-generation curved coaxial instruments to increase the operative space between the surgeon’s hands. Special curved instruments permit adequate triangulation, a good overview of the site and exact manipulation both inside and outside of the body. |
| Pre-bent HiQ LS hand Instruments (Olympus Corp, Tokyo, Japan) | These reusable instruments have a double-curved shaft to allow for independent jaw rotation and excellent maneuverability. |
Although LESS has been well accepted by both patients and surgeons, it has similar technical challenges to NOTES.78,79,108 Specifically, all of the instrumentation is inserted through a single incision, which results in intracorporeal and extracorporeal instrument collisions, an in-line view of the instruments, transposed instrument viewing (ie right instrument operates on the left side of a monitor), altered instrument pivot point above the skin incision, and the surgeon’s close proximity to assistants.87–89 As in conventional laparoscopy and NOTES, the surgeon must also still contend with a non-neutral posture because of the instruments, monitor position, foot pedals, table height, and static body position.112–114
Owing to the multitude of challenges facing LESS, a rigorous assessment of the technique and its technologies is needed to optimize surgical performance and mitigate preventable errors. For LESS to become the gold-standard in MIS, it is also imperative that the lessons learned from the uptake of conventional laparoscopy two decades ago be integrated into the assessment, refinement, and standardization of LESS.
The development and testing of new techniques and technologies can be harmful to patients and health care providers. Accordingly, robust and impactful analyses of MIS are needed to continually improve its quality and safety. The variability of human performance and the design and redesign of surgical technologies and processes are critical considerations for this research. Multifunctional assessments conducted in high-fidelity simulators to assess the performance, functionality, risk of error, workload, and joint kinematics of laparoscopic surgery instrumentation, practices, and procedures will aid in the determination of the variability of human performance and how to improve the design of the entire surgical system to optimize surgical performance and patient outcomes.
As the next frontier of MIS, the technical challenges and safety concerns of LESS must be overcome. Although medical device manufacturers have quickly embraced LESS and rapidly produced novel, repurposed, and redesigned surgical equipment, there have been limited published studies on the HFE of these devices and their potential effects on the surgeon, surgical performance, and patient safety.115–117 Additionally, the influx of these highly complex technologies may be increasing the risk of operative error because of misunderstanding and misuse. In the near future, it will be critical to develop, assess, and validate LESS-specific practices and technologies that improve operative performance, mitigate potential errors, and enable all laparoscopic surgeons to safely perform this pioneering technique.
To systematically assess LESS techniques and technologies, and to develop tailored instrumentation and training programs that enable a safe and quick transition to LESS, the following major research areas should be attended next:
the development of LESS-specific technologies (eg access devices, hand instruments, cameras, etc.) that optimize performance and enable current laparoscopic surgeons to transition to LESS in a safe and responsible manner and
the development and validation of a LESS-specific training program tailored to varying levels of surgical experience (ie resident to expert surgeon) and multiple surgical disciplines (eg general, urological, gynecological surgery).
Integral in both of these two research areas is the omnipresent need to standardize LESS by validating its best practices based on scientific evaluation and objective data. The expected outcomes of this future research are the development of enabling LESS technologies and a simulation-based LESS training model. Gains toward both of these goals will disseminate evidence-based information for training and procedural standardization, which will minimize threats to patients and surgeons.
Acknowledgments
Nebraska Research Initiative provided partial support of this study. The authors would also like to thank the University of Nebraska Medical Center’s Center for Advanced Surgical Technology (CAST) and Innovative Design and Ergonomic Analysis (IDEA) Laboratory for their work with this study.
Footnotes
Author Contributions
Conceived and designed the experiments: BM, CL, MSH. Analyzed the data: BM. Wrote the first draft of the manuscript: BM. Contributed to the writing of the manuscript: BM, CL, MSH. Agree with manuscript results and conclusions: BM, CL, MSH. Jointly developed the structure and arguments for the paper: BM, CL, MSH. Made critical revisions and approved final version: BM, CL, MSH. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
ACADEMIC EDITOR: Kayvan Najarian, Editor in Chief
FUNDING: The Nebraska Research Initiative provided partial support of this study.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
REFERENCES
- 1.Centers for Medicare and Medicaid Services (CMS) National Health Expenditure Data: Highlights. 2014. Retrieved March 12, 2014, Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf.
- 2.National Academy of Engineering (NAE) and Institute of Medicine (IOM) Committee on Engineering and the Health Care System . Building a better delivery system: A new engineering/health care partnership. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 3.Johnson WG, Brennan TA, Newhouse JP, et al. The economic consequences of medical injuries. Implications for a no-fault insurance plan. JAMA. 1992;267(18):2487–2492. [PubMed] [Google Scholar]
- 4.Thomas EJ, Studdert DM, Newhouse JP, et al. Costs of medical injuries in Utah and Colorado. Inquiry. 1999;36(3):255–264. [PubMed] [Google Scholar]
- 5.Institute of Medicine (IOM) Committee on Quality of Health Care in America . To err is human: building a safer health system. Washington, DC: The National Academies Press; 2000. [PubMed] [Google Scholar]
- 6.DeNavas-Walt C, Proctor BD, Smith J. Income, Poverty, and Health Insurance Coverage in the United States: 2006. Washington, DC: US Census Bureau, Government Printing Office; 2007. [Google Scholar]
- 7.Institute of Medicine Committee (IOM) on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001. [Google Scholar]
- 8.Holden RJ. Cognitive performance-altering effects of electronic medical records: an application of the human factors paradigm for patient safety. Cogn Technol Work. 2011;13(1):11–29. doi: 10.1007/s10111-010-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348(25):2526–2534. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 10.Wayne JD, Tyagi R, Reinhardt G, et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485. doi: 10.1016/j.jsurg.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Baumgart A, Neuhauser D. Frank and Lillian Gilbreth: scientific management in the operating room. Qual Saf Health Care. 2009;18(5):413–415. doi: 10.1136/qshc.2009.032409. [DOI] [PubMed] [Google Scholar]
- 12.Towill DR. Frank Gilbreth and health care delivery method study driven learning. Int J Health Care Qual Assur. 2009;22(4):417–440. doi: 10.1108/09526860910964861. [DOI] [PubMed] [Google Scholar]
- 13.Berguer R. Surgery and ergonomics. Arch Surg. 1999;134(9):1011–1016. doi: 10.1001/archsurg.134.9.1011. [DOI] [PubMed] [Google Scholar]
- 14.International Ergonomics Association (IEA) The Discipline of Ergonomics. 2000. Retrieved March 28, 2012, Available at http://www.iea.cc/01_what/What%20is%20Ergonomics.html.
- 15.Dul J, Bruder R, Buckle P, et al. A strategy for human factors/ergonomics: developing the discipline and profession. Ergonomics. 2012;55(4):377–395. doi: 10.1080/00140139.2012.661087. [DOI] [PubMed] [Google Scholar]
- 16.Cuschieri A. Reducing errors in the operating room. Surg Endosc. 2005;19(8):1022–1027. doi: 10.1007/s00464-005-8110-7. [DOI] [PubMed] [Google Scholar]
- 17.National Quality Forum (NQF) Serious Reportable Events in Healthcare: A Consensus Report. Washington, DC: NQF; 2002. [Google Scholar]
- 18.National Quality Forum (NQF) Serious Reportable Events in Healthcare—2011 Update: A Consensus Report. Washington, DC: NQF; 2011. [Google Scholar]
- 19.Weingart SN, Iezzoni LI. Looking for medical injuries where the light is bright. JAMA. 2003;290(14):1917–1919. doi: 10.1001/jama.290.14.1917. [DOI] [PubMed] [Google Scholar]
- 20.Reilly AF, Reilly PM. Medical injuries can increase mortality risk and incur additional costs. Evidence Based Healthcare. 2004;8(2):60–62. [Google Scholar]
- 21.Cuschieri A. Nature of human error: implications for surgical practice. Ann Surg. 2006;244(5):642–648. doi: 10.1097/01.sla.0000243601.36582.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Addessi A, Bongiovanni L, Volpe A, Pinto F, Bassi P. Human factors in surgery: from Three Mile Island to the operating room. Urol Int. 2009;83(3):249–257. doi: 10.1159/000241662. [DOI] [PubMed] [Google Scholar]
- 23.Tang B, Hanna GB, Joice P, Cuschieri A. Identification and categorization of technical errors by observational clinical human reliability assessment (OCHRA) during laparoscopic cholecystectomy. Arch Surg. 2004;139(11):1215–1220. doi: 10.1001/archsurg.139.11.1215. [DOI] [PubMed] [Google Scholar]
- 24.Cox A, Dolan L, Macewen CJ. Human reliability analysis: a new method to quantify errors in cataract surgery. Eye (Lond) 2008;22(3):394–397. doi: 10.1038/sj.eye.6702648. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen J. Skills, rules, and knowledge; signals, signs, and symbols, and other distinctions in human performance models. Book System design for human interaction. IEEE Press; Piscataway, NJ, USA ©: 1987. pp. 291–300. [Google Scholar]
- 26.Reason JT. Human Error. Cambridge England/New York: Cambridge University Press; 1990. [Google Scholar]
- 27.Parker WH. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437–449. doi: 10.1016/j.ogc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 28.van Det M, Meijerink WJ, Hoff C, Totté ER, Pierie JP. Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: a review and guidelines. Surg Endosc. 2009;23(6):1279–1285. doi: 10.1007/s00464-008-0148-x. [DOI] [PubMed] [Google Scholar]
- 29.Carayon P, Bass EJ, Bellandi T, Gurses AP, Hallbeck MS, Mollo V. Sociotechnical systems analysis in health care: a research agenda. IIE Trans Healthc Syst Eng. 2011;1(3):145–160. doi: 10.1080/19488300.2011.619158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapanis A. Ergonomics in product development: a personal view. Ergonomics. 1995;38(8):1625–1638. [Google Scholar]
- 31.Karwowski P. International encyclopedia of ergonomics and human factors. In: Hedge A, editor. Consumer Product Design. Boca Raton, FL: CRC Press; 2006. pp. 1555–1558. [Google Scholar]
- 32.Stanton N, Young M. Is utility in the mind of the beholder? A study of ergonomics methods. Appl Ergon. 1998;29(1):41–54. doi: 10.1016/s0003-6870(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 33.Carthey J, de Leval MR, Reason JT. Understanding excellence in complex, dynamic medical systems. Ergonomics for the new millennium. Proceedings of the Human Factors and Ergonomics Society Annual Meeting; Proceedings of the XIVth Triennial Congress of the IEA; San Diego, CA, USA. 2000. pp. 136–139. http://pro.sagepub.com/content/44/26/136.abstract. [DOI] [Google Scholar]
- 34.Matern U, Koneczny S. Safety, hazards and ergonomics in the operating room. Surg Endosc. 2007;21(11):1965–1969. doi: 10.1007/s00464-007-9396-4. [DOI] [PubMed] [Google Scholar]
- 35.Wong SW, Smith R, Crowe P. Optimizing the operating theatre environment. ANZ J Surg. 2010;80(12):917–924. doi: 10.1111/j.1445-2197.2010.05526.x. [DOI] [PubMed] [Google Scholar]
- 36.Riskin DJ, Longaker MT, Gertner M, Krummel TM. Innovation in surgery: a historical perspective. Ann Surg. 2006;244(5):686–693. doi: 10.1097/01.sla.0000242706.91771.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tullis T, Albert W. Measuring the User Experience: Collecting, Analyzing, and Presenting Usability Metrics (Interactive Technologies) Burlington, MA: Morgan Kaufmann; 2008. [Google Scholar]
- 38.Rosenfield LK, Chang DS. The error of omission: a simple checklist approach for improving operating room safety. Plast Reconstr Surg. 2009;123(1):399–402. doi: 10.1097/PRS.0b013e318193472f. [DOI] [PubMed] [Google Scholar]
- 39.Dankelman J, Grimbergen C. Systems approach to reduce errors in surgery. Surg Endosc. 2005;19(8):1017–1021. doi: 10.1007/s00464-005-8109-0. [DOI] [PubMed] [Google Scholar]
- 40.Flin RH. Safety at the Sharp End: A Guide to Non-Technical Skills. Aldershot, England/Burlington, VT: Ashgate Publishing Company; 2008. [Google Scholar]
- 41.Aft LS. Fundamentals of Industrial Quality Control. 3rd ed. Boca Raton, FL: CRC Press; 1998. [Google Scholar]
- 42.International Organization for Standardization (ISO) 9000:2000 . Quality Management Systems—Fundamentals and Vocabulary. 2000. [Google Scholar]
- 43.Petersen PB. Total quality management and the Deming approach to quality management. J Manage Hist. 1999;5(8):468–488. [Google Scholar]
- 44.Walter AJ. Surgical education for the twenty-first century: beyond the apprentice model. Obstet Gynecol Clin North Am. 2006;33(2):233–236. doi: 10.1016/j.ogc.2006.01.003. vii. [DOI] [PubMed] [Google Scholar]
- 45.Cuschieri A, Tang B. Human reliability analysis (HRA) techniques and observational clinical HRA. Minim Invasive Ther Allied Technol. 2010;19(1):12–7. doi: 10.3109/13645700903492944. [DOI] [PubMed] [Google Scholar]
- 46.Buschemeyer WC, III, Cunningham DK, Edwards MJ. Surgical training and implementation of emerging surgical technologies. Am J Surg. 2005;190(2):166–172. doi: 10.1016/j.amjsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Herring SR, Hallbeck MS. Evaluation of a two cursor control device for development of a powered laparoscopic surgical tool. Ergonomics. 2009;52(8):891–906. doi: 10.1080/00140130802645198. [DOI] [PubMed] [Google Scholar]
- 48.Schneider JR, Coyle JJ, Ryan ER, Bell RH, Jr, DaRosa DA. Implementation and evaluation of a new surgical residency model. J Am Coll Surg. 2007;205(3):393–404. doi: 10.1016/j.jamcollsurg.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Trejo A, Jung MC, Oleynikov D, Hallbeck MS. Effect of handle design and target location on insertion and aim with a laparoscopic surgical tool. Appl Ergon. 2007;38(6):745–753. doi: 10.1016/j.apergo.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Laurence JM, Tran PD, Richardson AJ, Pleass HC, Lam VW. Laparoscopic or open cholecystectomy in cirrhosis: a systematic review of outcomes and meta-analysis of randomized trials. HPB (Oxford) 2012;14(3):153–161. doi: 10.1111/j.1477-2574.2011.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Zhang J, Sang L, et al. Laparoscopic versus conventional appendectomy—a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2010;10:129. doi: 10.1186/1471-230X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49–57. doi: 10.7150/jca.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berguer R, Rab GT, Abu-Ghaida H, Alarcon A, Chung J. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11(2):139–142. doi: 10.1007/s004649900316. [DOI] [PubMed] [Google Scholar]
- 54.Berguer R, Forkey DL, Smith WD. Ergonomic problems associated with laparoscopic surgery. Surg Endosc. 1999;13(5):466–468. doi: 10.1007/pl00009635. [DOI] [PubMed] [Google Scholar]
- 55.Person J, Hodgson A, Nagy A. Automated high-frequency posture sampling for ergonomic assessment of laparoscopic surgery. Surgical Endoscopy. 2001;15(9):997–1003. doi: 10.1007/s004640080155. [DOI] [PubMed] [Google Scholar]
- 56.Berguer R, Gerber S, Kilpatrick G, Remler M, Beckley D. A comparison of forearm and thumb muscle electromyographic responses to the use of laparoscopic instruments with either a finger grasp or a palm grasp. Ergonomics. 1999;42(12):1634–1645. doi: 10.1080/001401399184721. [DOI] [PubMed] [Google Scholar]
- 57.Emam TA, Hanna GB, Kimber C, Cuschieri A. Differences between experts and trainees in the motion pattern of the dominant upper limb during intracorporeal endoscopic knotting. Dig Surg. 2000;17(2):120–123. doi: 10.1159/000018813. discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 58.Protocol Snow. Medical School 3rd Year Rotation: Surgery. 2012. Retrieved March 28, 2010, Available at http://www.protocolsnow.com/2010/10/13/medical-school-3rd-year-rotation-surgery/trackback/
- 59.Peters JH, Ellison EC, Innes JT, et al. Safety and efficacy of laparoscopic cholecystectomy. A prospective analysis of 100 initial patients. Ann Surg. 1991;213(1):3–12. doi: 10.1097/00000658-199101000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott TR, Zucker KA, Bailey RW. Laparoscopic cholecystectomy: a review of 12,397 patients. Surg Laparosc Endosc. 1992;2(3):191–198. [PubMed] [Google Scholar]
- 61.Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165(1):9–14. doi: 10.1016/s0002-9610(05)80397-6. [DOI] [PubMed] [Google Scholar]
- 62.Green FL. New York state health department ruling—a “wake-up call” for all. Surg Endosc. 1992;6(6):271. doi: 10.1007/BF02498856. [DOI] [PubMed] [Google Scholar]
- 63.Wherry DC, Rob CG, Marohn MR, Rich NM. An external audit of laparoscopic cholecystectomy performed in medical treatment facilities of the department of defense. Ann Surg. 1994;220(5):626–634. doi: 10.1097/00000658-199411000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kane RL, Lurie N, Borbas C, et al. The outcomes of elective laparoscopic and open cholecystectomies. J Am Coll Surg. 1995;180(2):136–145. [PubMed] [Google Scholar]
- 65.Majeed AW, Troy G, Nicholl JP, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347(9007):989–994. doi: 10.1016/s0140-6736(96)90143-9. [DOI] [PubMed] [Google Scholar]
- 66.Trondsen E, Reiertsen O, Andersen OK, Kjaersgaard P. Laparoscopic and open cholecystectomy. A prospective, randomized study. Eur J Surg. 1993;159(4):217–221. [PubMed] [Google Scholar]
- 67.Gilchrist BF, Vlessis AA, Kay GA, Swartz K, Dennis D. Open versus laparoscopic cholecystectomy: an initial analysis. J Laparoendosc Surg. 1991;1(4):193–196. doi: 10.1089/lps.1991.1.193. [DOI] [PubMed] [Google Scholar]
- 68.Hodgson WJ, Byrne DW, Savino JA, Liberis G. Laparoscopic cholecystectomy. The early experience of surgical attendings compared with that of residents trained by apprenticeship. Surg Endosc. 1994;8(9):1058–1062. doi: 10.1007/BF00705719. [DOI] [PubMed] [Google Scholar]
- 69.Lekawa M, Shapiro SJ, Gordon LA, Rothbart J, Hiatt JR. The laparoscopic learning curve. Surg Laparosc Endosc. 1995;5(6):455–458. [PubMed] [Google Scholar]
- 70.Sariego J, Spitzer L, Matsumoto T. The “learning” curve in the performance of laparoscopic cholecystectomy. Int Surg. 1993;78(1):1–3. [PubMed] [Google Scholar]
- 71.Dent TL. Training and privileging for new procedures. Surg Clin North Am. 1996;76(3):615–621. doi: 10.1016/s0039-6109(05)70467-9. [DOI] [PubMed] [Google Scholar]
- 72.Grundfest WS. Credentialing in an era of change. JAMA. 1993;270(22):2725. [PubMed] [Google Scholar]
- 73.Parsa CJ, Organ CH, Jr, Barkan H. Changing patterns of resident operative experience from 1990 to 1997. Arch Surg. 2000;135(5):570–573. doi: 10.1001/archsurg.135.5.570. discussion 573–575. [DOI] [PubMed] [Google Scholar]
- 74.Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg. 2010;210(3):306–313. doi: 10.1016/j.jamcollsurg.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Sari V, Nieboer TE, Vierhout ME, Stegeman DF, Kluivers KB. The operation room as a hostile environment for surgeons: physical complaints during and after laparoscopy. Minim Invasive Ther Allied Technol. 2010;19(2):105–109. doi: 10.3109/13645701003643972. [DOI] [PubMed] [Google Scholar]
- 76.Szeto GP, Ho P, Ting AC, Poon JT, Cheng SW, Tsang RC. Work-related musculoskeletal symptoms in surgeons. J Occup Rehabil. 2009;19(2):175–184. doi: 10.1007/s10926-009-9176-1. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Patel VL, Johnson TR, Shortliffe EH. A cognitive taxonomy of medical errors. J Biomed Inform. 2004;37(3):193–204. doi: 10.1016/j.jbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Gettman MT, White WM, Aron M, Autorino R, Averch T, Box G, European Society of Urotechnology NOTES and LESS Working Group Where do we really stand with LESS and NOTES? Eur Urol. 2011;59(2):231–234. doi: 10.1016/j.eururo.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Gill IS, Advincula AP, Aron M, et al. Consensus statement of the consortium for laparoendoscopic single-site surgery. Surg Endosc. 2010;24(4):762–768. doi: 10.1007/s00464-009-0688-8. [DOI] [PubMed] [Google Scholar]
- 80.Lee J, Baek J, Kim W. Laparoscopic transumbilical single-port appendectomy: initial experience and comparison with three-port appendectomy. Surg Laparosc Endosc Percutan Techn. 2010;20(2):100–103. doi: 10.1097/SLE.0b013e3181d84922. [DOI] [PubMed] [Google Scholar]
- 81.Raman JD, Bagrodia A, Cadeddu JA. Single-incision, umbilical laparoscopic versus conventional laparoscopic nephrectomy: a comparison of perioperative outcomes and short-term measures of convalescence. Eur Urol. 2009;55(5):1198–1204. doi: 10.1016/j.eururo.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Tsimoyiannis EC, Tsimogiannis KE, Pappas-Gogos G, et al. Different pain scores in single transumbilical incision laparoscopic cholecystectomy versus classic laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2010;24(8):1842–1848. doi: 10.1007/s00464-010-0887-3. [DOI] [PubMed] [Google Scholar]
- 83.Vidal O, Valentini M, Ginesta C, et al. Laparoendoscopic single-site surgery appendectomy. Surg Endosc. 2010;24(3):686–691. doi: 10.1007/s00464-009-0661-6. [DOI] [PubMed] [Google Scholar]
- 84.Canes D, Berger A, Aron M, et al. Laparo-endoscopic single site (LESS) versus standard laparoscopic left donor nephrectomy: matched-pair comparison. Eur Urol. 2010;57(1):95–101. doi: 10.1016/j.eururo.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 85.Chow A, Purkayastha S, Nehme J, Darzi LA, Paraskeva P. Single incision laparoscopic surgery for appendicectomy: a retrospective comparative analysis. Surg Endosc. 2010;24(10):2567–2574. doi: 10.1007/s00464-010-1004-3. [DOI] [PubMed] [Google Scholar]
- 86.Philipp SR, Miedema BW, Thaler K. Single-incision laparoscopic cholecystectomy using conventional instruments: early experience in comparison with the gold standard. J Am Coll Surg. 2009;209(5):632–637. doi: 10.1016/j.jamcollsurg.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Rivas H, Varela E, Scott D. Single-incision laparoscopic cholecystectomy: initial evaluation of a large series of patients. Surg Endosc. 2010;24(6):1403–1412. doi: 10.1007/s00464-009-0786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saber AA, Elgamal MH, El-Ghazaly TH, Dewoolkar AV, Akl A. Simple technique for single incision transumbilical laparoscopic appendectomy. Int J Surg. 2010;8(2):128–130. doi: 10.1016/j.ijsu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Teixeira J, McGill K, Koshy N, McGinty J, Todd G. Laparoscopic single-site surgery for placement of adjustable gastric band—a series of 22 cases. Surg Obes Relat Dis. 2010;6(1):41–45. doi: 10.1016/j.soard.2009.03.220. [DOI] [PubMed] [Google Scholar]
- 90.Wong KH, Chiu WY. Laparo-Endoscopic Single-Site Surgery (LESS) 2010. Retrieved February 27, 2012, Available at http://www.esurg.net/less-main.htm.
- 91.Osborne DA, Alexander G, Boe B, Zervos EE. Laparoscopic cholecystectomy: past, present, and future. Surg Technol Int. 2006;15:81–85. [PubMed] [Google Scholar]
- 92.Chow A, Purkayastha S, Paraskeva P. Appendicectomy and cholecystectomy using single-incision laparoscopic surgery (SILS): the first UK experience. Surg Innov. 2009;16(3):211–217. doi: 10.1177/1553350609344413. [DOI] [PubMed] [Google Scholar]
- 93.Edwards C, Bradshaw A, Ahearne P, et al. Single-incision laparoscopic cholecystectomy is feasible: initial experience with 80 cases. Surg Endosc. 2010;24(9):2241–2247. doi: 10.1007/s00464-010-0943-z. [DOI] [PubMed] [Google Scholar]
- 94.Elsey JK, Feliciano DV. Initial experience with single-incision laparoscopic cholecystectomy. J Am Coll Surg. 2010;210(5):620–624. 624–626. doi: 10.1016/j.jamcollsurg.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 95.Erbella J, Jr, Bunch GM. Single-incision laparoscopic cholecystectomy: The first 100 outpatients. Surgical Endoscopy. 2010;24(8):1958–1961. doi: 10.1007/s00464-010-0886-4. [DOI] [PubMed] [Google Scholar]
- 96.Ersin S, Firat O, Sozbilen M. Single-incision laparoscopic cholecystectomy: Is it more than a challenge? Surgical Endoscopy. 2010;24(1):68–71. doi: 10.1007/s00464-009-0543-y. [DOI] [PubMed] [Google Scholar]
- 97.Langwieler TE, Nimmesgern T, Back M. Single-port access in laparoscopic cholecystectomy. Surgical Endoscopy. 2009;23(5):1138–1141. doi: 10.1007/s00464-009-0389-3. [DOI] [PubMed] [Google Scholar]
- 98.Petrotos AC, Molinelli BM. Single-incision multiport laparoendoscopic (SIMPLE) surgery: Early evaluation of SIMPLE cholecystectomy in a community setting. Surgical Endoscopy. 2009;23(11):2631–2634. doi: 10.1007/s00464-009-0369-7. [DOI] [PubMed] [Google Scholar]
- 99.Philipp SR, Miedema BW, Thaler K. Single-incision laparoscopic cholecystectomy using conventional instruments: Early experience in comparison with the gold standard. Journal of the American College of Surgeons. 2009;209(5):632–637. doi: 10.1016/j.jamcollsurg.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Podolsky ER, Rottman SJ, Poblete H, King SA, Curcillo PG. Single port access (SPA) cholecystectomy: A completely transumbilical approach. Journal of Laparoendoscopic & Advanced Surgical TechniquesPart A. 2009;19(2):219–222. doi: 10.1089/lap.2008.0275. [DOI] [PubMed] [Google Scholar]
- 101.Rivas H, Varela E, Scott D. Single-incision laparoscopic cholecystectomy: Initial evaluation of a large series of patients. Surgical Endoscopy. 2010;24(6):1403–1412. doi: 10.1007/s00464-009-0786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts KE, Solomon D, Duffy AJ, Bell RL. Single-incision laparoscopic cholecystectomy: A surgeon’s initial experience with 56 consecutive cases and a review of the literature. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract. 2010;14(3):506–510. doi: 10.1007/s11605-009-1116-z. [DOI] [PubMed] [Google Scholar]
- 103.Romanelli JR, Roshek TB, Lynn DC, Earle DB. Single-port laparoscopic cholecystectomy: Initial experience. Surgical Endoscopy. 2010;24(6):1374–1379. doi: 10.1007/s00464-009-0781-z. [DOI] [PubMed] [Google Scholar]
- 104.Solomon D, Bell RL, Duffy AJ, Roberts KE. Single-port cholecystectomy: Small scar, short learning curve. Surgical Endoscopy. 2010;24(12):2954–2957. doi: 10.1007/s00464-010-1070-6. [DOI] [PubMed] [Google Scholar]
- 105.Tacchino R, Greco F, Matera D. Single-incision laparoscopic cholecystectomy: Surgery without a visible scar. Surgical Endoscopy. 2009;23(4):896–899. doi: 10.1007/s00464-008-0147-y. [DOI] [PubMed] [Google Scholar]
- 106.Tsimoyiannis EC, Tsimogiannis KE, Pappas-Gogos G, Farantos C, Benetatos N, Mavridou P, et al. Different pain scores in single transumbilical incision laparoscopic cholecystectomy versus classic laparoscopic cholecystectomy: A randomized controlled trial. Surgical Endoscopy. 2010;24(8):1842–1848. doi: 10.1007/s00464-010-0887-3. [DOI] [PubMed] [Google Scholar]
- 107.Auyang ED, Santos BF, Enter DH, Hungness ES, Soper NJ. Natural orifice translumenal endoscopic surgery (NOTES(R)): a technical review. Surg Endosc. 2011;25(10):3135–3148. doi: 10.1007/s00464-011-1718-x. [DOI] [PubMed] [Google Scholar]
- 108.Bucher P, Ostermann S, Pugin F, Morel P. Female population perception of conventional laparoscopy, transumbilical LESS, and transvaginal NOTES for cholecystectomy. Surg Endosc. 2011;25(7):2308–2315. doi: 10.1007/s00464-010-1554-4. [DOI] [PubMed] [Google Scholar]
- 109.Slim K, Launay-Savary MV. NOTES, the debate continues. Surg Endosc. 2008;22(10):2326. doi: 10.1007/s00464-008-0062-2. author reply 2327. [DOI] [PubMed] [Google Scholar]
- 110.Sodergren MH, Clark J, Athanasiou T, Teare J, Yang GZ, Darzi A. Natural orifice translumenal endoscopic surgery: critical appraisal of applications in clinical practice. Surg Endosc. 2009;23(4):680–687. doi: 10.1007/s00464-008-0278-1. [DOI] [PubMed] [Google Scholar]
- 111.Vettoretto N, Arezzo A. Human natural orifice translumenal endoscopic surgery: on the way to two different philosophies? Surg Endosc. 2010;24(2):490–492. doi: 10.1007/s00464-009-0600-6. [DOI] [PubMed] [Google Scholar]
- 112.Mallett J. Studying man-machine interfaces in the operating room. Minimally Invasive Therapy & Allied Technologies. 2001;10(3):133–137. doi: 10.1080/136457001753192240. [DOI] [PubMed] [Google Scholar]
- 113.Stassen HG, Dankelman J, Grimbergen KA, Meijer DW. Man-machine aspects of minimally invasive surgery. Annu Rev Control. 2001;25:111–122. [Google Scholar]
- 114.van Veelen MA, Nederlof EA, Goossens RH, Schot CJ, Jakimowicz JJ. Ergonomic problems encountered by the medical team related to products used for minimally invasive surgery. Surgical Endoscopy. 2003;17(7):1077–1081. doi: 10.1007/s00464-002-9105-2. [DOI] [PubMed] [Google Scholar]
- 115.Brown-Clerk B, de Laveaga AE, LaGrange CA, Wirth LM, Lowndes BR, Hallbeck MS. Laparoendoscopic single-site (LESS) surgery versus conventional laparoscopic surgery: comparison of surgical port performance in a surgical simulator with novices. Surg Endosc. 2011;25(7):2210–2218. doi: 10.1007/s00464-010-1524-x. [DOI] [PubMed] [Google Scholar]
- 116.de Laveaga AE, McCrory B, LaGrange CA, Hallbeck MS. Evaluation of instrument dexterity and static resistance of laparoendoscopic single-site (LESS) surgical ports. J Med Devices. 2012;6(2):7. [Google Scholar]
- 117.McCrory B, Lowndes BR, Wirth LM, de Laveaga AE, LaGrange CA, Hallbeck MS. Ergonomic evaluation of laparoendoscopic single-site surgery ports in a validated laparoscopic training model. Work. 2012;41(0):1884–1890. doi: 10.3233/WOR-2012-0402-1884. [DOI] [PubMed] [Google Scholar]


