Abstract
From examination of some 40 patient strains of Mycobacterium tuberculosis, a statistically very significant correlation (Spearman's rho) can be drawn between the root index of virulence for the guinea pig (D. A. Mitchison) and the ability of the individual strains to elaborate strongly acidic lipids (SAL) in culture. These include both sulfatides (SL) and phospholipids (PL). Since essentially all, if indeed not all, of the virulent and only few attenuated strains are prolific in elaborating SAL, this criterion may be a necessary requirement for the expression of virulence in M. tuberculosis. Tested by chi-square, this premise is seen to be statistically and pragmatically highly significant. We speculate that SL may contribute to the pathogenesis of tuberculosis because of a demonstrable activity directed against host liver mitochondrial membranes (manuscript in preparation) and its synergistic potentiation of the specific toxicity of trehalose dimycolate (cord factor). The activity may also be expressed against phagosomal and lysosomal membranes within macrophages. Because of their strongly anionic character, SL and PL may interact with cationic sites on lysosomal hydrolases with resultant immobilization and/or inactivation of the enzymes. By a similar mechanism, these ionic lipids may alter the activity of bactericidal basic proteins, previously recognized in the lysosomal armamentarium. Since a minor but significant fraction of demonstrably attenuated strains is nevertheless prolific in SL or SAL elaboration, this facility alone is evidently not a sufficient criterion for expression of virulence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZUMA I., NAGASUGA T., YAMAMURA Y. Studies on the toxic substances isolated from mycobacteria. II. Toxic glycolipids of Mycobacterium smegmatis, Mycobacterium phlei and atypical mycobacteria strain No. 6 and No. 22. J Chromatogr. 1962 Aug;8:92–98. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN C. M., MIDDLEBROOK G. The effects of some sulfhydryl compounds on growth of catalase-positive and catalase-negative tubercle bacilli. Am Rev Tuberc. 1956 Jul;74(1):42–49. doi: 10.1164/artpd.1956.74.1.42. [DOI] [PubMed] [Google Scholar]
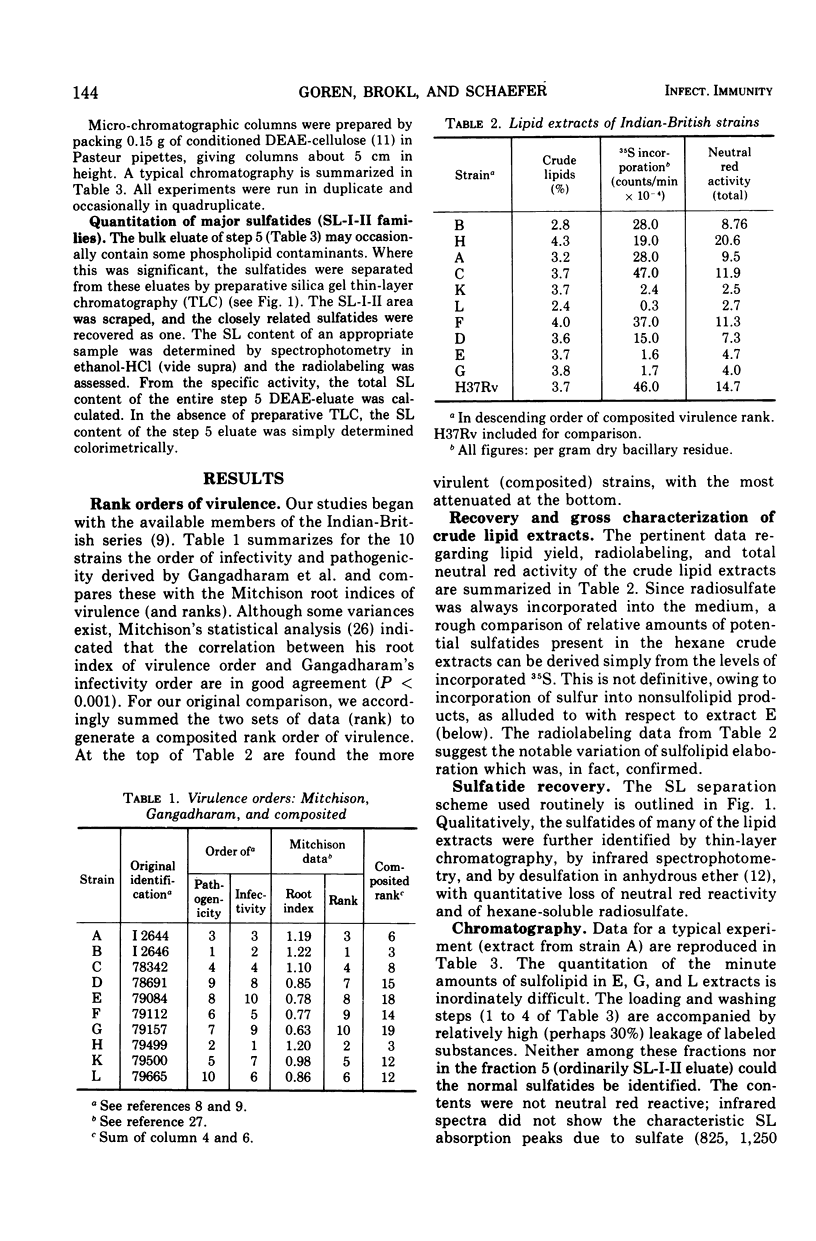
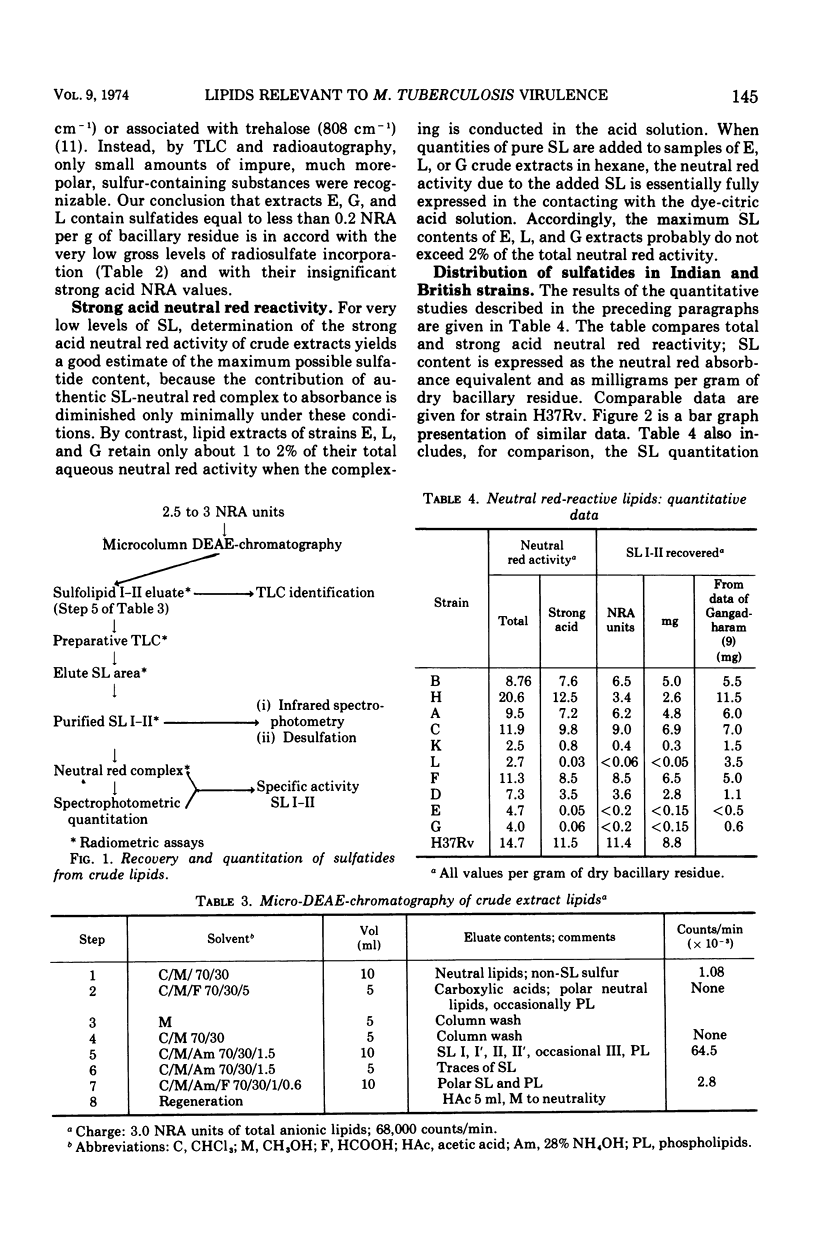
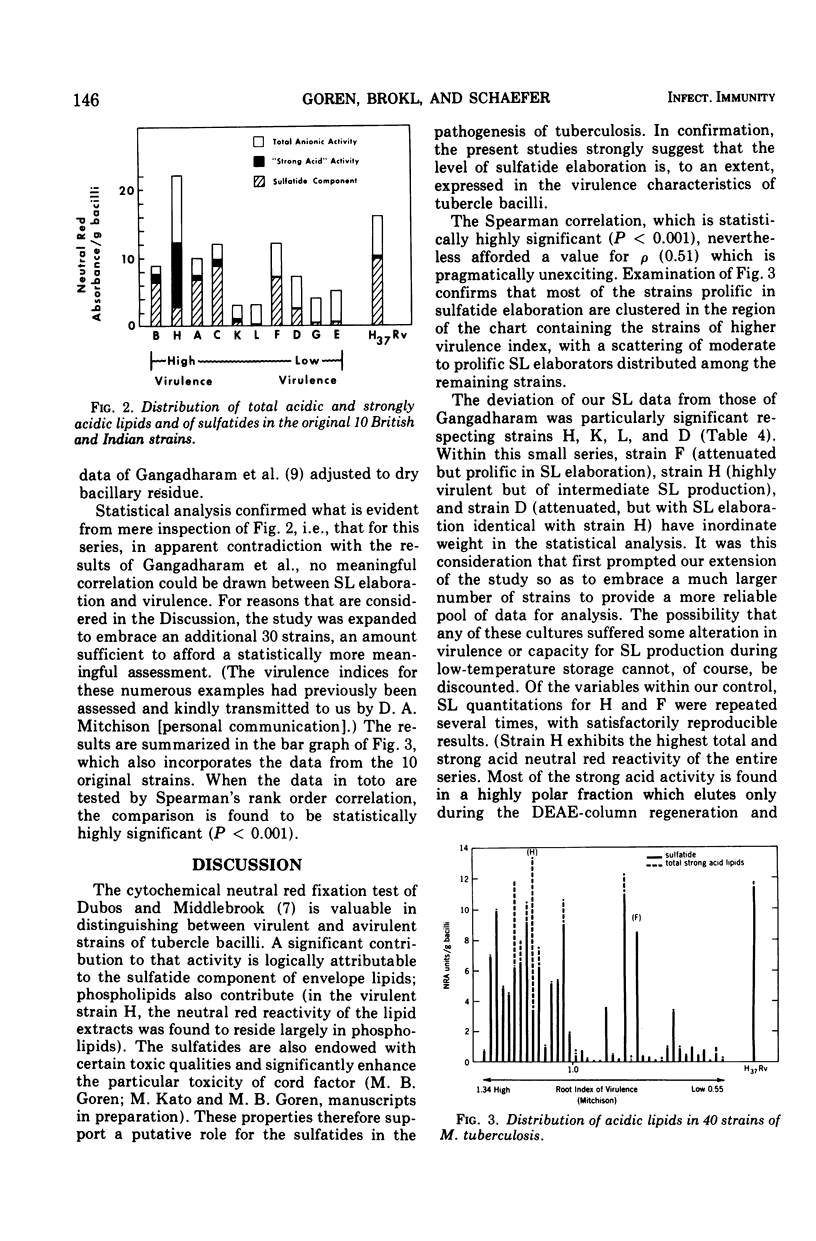
- GANGADHARAM P. R., COHN M. L., DAVIS C. L., MIDDLEBROOK G. Infectivity and pathogenicity of Indian and British strains of tubercle bacilli studied by aerogenic infection of guinea pigs. Am Rev Respir Dis. 1963 Feb;87:200–205. doi: 10.1164/arrd.1963.87.2.200. [DOI] [PubMed] [Google Scholar]
- GANGADHARAM P. R., COHN M. L., MIDDLEBROOK G. INFECTIVITY, PATHOGENICITY AND SULPHOLIPID FRACTION OF SOME INDIAN AND BRITISH STRAINS OF TUBERCLE BACILLI. Tubercle. 1963 Dec;44:452–455. doi: 10.1016/s0041-3879(63)80087-2. [DOI] [PubMed] [Google Scholar]
- GERSHON Z., OLITZKI A. L. MONOCYTIN, A PROTECTING SUBSTANCE PRODUCED BY MURINE MONOCYTES. Proc Soc Exp Biol Med. 1965 May;119:32–36. doi: 10.3181/00379727-119-30090. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Das B. C., Lederer E. Sulfolipid I of Mycobacterium tuberculosis, strain H37RV. Nature of the acyl substituents. Biochemistry. 1971 Jan 5;10(1):72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Schaefer W. B. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: phthiocerol dimycocerosate and the attenuation indicator lipid. Infect Immun. 1974 Jan;9(1):150–158. doi: 10.1128/iai.9.1.150-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Mycobacterial sulfolipids: spontaneous desulfation. Lipids. 1971 Jan;6(1):40–46. doi: 10.1007/BF02536373. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 9;210(1):116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- HUSSEINI H., ELBERG S. Cellular reactions to phthienoic acid and related branch ed-chain acids. Am Rev Tuberc. 1952 Jun;65(6):655–672. doi: 10.1164/art.1952.65.6.655. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K. Biology of the mycobacterioses. Some aspects of the lysosome-bacillus interaction in experimental mouse tuberculosis. Ann N Y Acad Sci. 1968 Sep 5;154(1):177–193. doi: 10.1111/j.1749-6632.1968.tb16708.x. [DOI] [PubMed] [Google Scholar]
- Kato M. Site II-specific inhibition of mitochondria oxidative phosphorylation by trehalose-6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis. Arch Biochem Biophys. 1970 Oct;140(2):379–390. doi: 10.1016/0003-9861(70)90079-2. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. VI. Effect of toxic bacterial constituents of tubercle bacilli on oxidative phosphorylation in host cell. Am Rev Respir Dis. 1967 Nov;96(5):998–1008. doi: 10.1164/arrd.1967.96.5.998. [DOI] [PubMed] [Google Scholar]
- Kochan I., Cahall D. L., Golden C. A. Employment of tuberculostasis in serum-agar medium for the study of production and activity of Mycobactin. Infect Immun. 1971 Aug;4(2):130–137. doi: 10.1128/iai.4.2.130-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERER E. Glycolipids of acid-fast bacteria. Adv Carbohydr Chem. 1961;16:207–238. doi: 10.1016/s0096-5332(08)60263-5. [DOI] [PubMed] [Google Scholar]
- MITCHISON D. A., SELKON J. B., LLOYD J. VIRULENCE IN THE GUINEA-PIG, SUSCEPTIBILITY TO HYDROGEN PEROXIDE, AND CATALASE ACTIVITY OF ISONIAZID-SENSITIVE TUBERCLE BACILLI FROM SOUTH INDIAN AND BRITISH PATIENTS. J Pathol Bacteriol. 1963 Oct;86:377–386. doi: 10.1002/path.1700860213. [DOI] [PubMed] [Google Scholar]
- MITCHISON D. A. THE VIRULENCE OF TUBERCLE BACILLI FROM PATIENTS WITH PULMONARY TUBERCULOSIS IN INDIA AND OTHER COUNTRIES. Bull Int Union Tuberc. 1964 Sep;35:287–306. [PubMed] [Google Scholar]
- Middlebrook G., Coleman C. M., Schaefer W. B. SULFOLIPID FROM VIRULENT TUBERCLE BACILLI. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1801–1804. doi: 10.1073/pnas.45.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLL H. The chemistry of cord factor, a toxic glycolipid of M. tuberculosis. Bibl Tuberc. 1956;(10):149–183. [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



