Abstract
Marijuana is the most widely used illicit drug by pregnant women in the world. In utero exposure to Δ9-tetrahydrocannabinol (Δ9-THC), a major psychoactive component of marijuana, is associated with an increased risk for anencephaly and neurobehavioural deficiencies in the offspring, including attention deficit hyperactivity disorder (ADHD), learning disabilities, and memory impairment. Recent studies demonstrate that the developing central nervous system (CNS) is susceptible to the effects of Δ9-THC and other cannabimimetics, including the psychoactive ingredients of the branded product ‘Spice’ branded products. These exocannabinoids interfere with the function of an endocannabinoid (eCB) system, present in the developing CNS from E12.5 (week 5 of gestation in humans), and required for proliferation, migration, and differentiation of neurons. Until recently, it was not known whether the eCB system is also present in the developing CNS during the initial stages of its ontogeny, i.e. from E7.0 onwards (week 2 of gestation in humans), and if so, whether this system is also susceptible to the action of exocannabinoids. Here, we review current data, in which the presence of an eCB system during the initial stage of development of the CNS is demonstrated. Furthermore, we focus on recent advances on the effect of canabimimetics on early gestation. The relevance of these findings and potential adverse developmental consequences of in utero exposure to ‘high potency’ marijuana, Spice branded products and/or cannabinoid research chemicals during this period is discussed. Finally, we address the implication of these findings in terms of the potential dangers of synthetic cannabinoid use during pregnancy, and the ongoing debate over legalization of marijuana.
Keywords: marijuana, synthetic cannabinoids, Spice, neural development, animal model, pregnancy, legalization, rescheduling
Introduction
Marijuana, the crude drug derived from Cannabis sativa L. pistillate inflorescence, is the most prevalent illicit drug consumed by pregnant women, with a usage rate of 11%, as measured by serum metabolites.[1] Classic animal studies have shown that during gestation, marijuana's predominant psychoactive constituent, Δ9-THC,[2] crosses the fetoplacental barrier and accumulates in fetal tissues, particularly in the developing brain, with the potential to harm the development of the central nervous system (CNS).[3–5] Human studies demonstrate that gestational exposure to Δ9-THC is associated with an increased risk for anencephaly (a non-sustaining life condition in which the brain fails to form; Figure 1A[6]), and neurobehavioural deficiencies in the offspring.[7,8] In addition to traditional marijuana, new markets of synthetic marijuana have recently emerged[9–12] (Figure 1): these markets are distributed mainly under the branded name ‘Spice’, and include products such as Spice gold, Kronic Pineapple Express, Marley Extra Strength, etc.; in addition to the brand Spice, there are other herbal blends for which the claim is made that they have a composition similar to that of Spice: these include K2, Genie, Yucatan Fire, Skunk, Sence, Smoke, ChillX, Highdi's Almdröhner, Earth Impact, Gorillaz, Galaxy Gold, Space Truckin, Solar Flare, Moon Rocks, Blue Lotus, Aroma, Scope, Sky, OG Potpourri etc.[9–12] Finally a market of “cannabinoid research chemicals”, which fall under the category of “designer drugs”, also termed ‘legal high’ and which claim to have marijuana-like effects, is now surfacing on various websites.[9–12]
Figure 1.

A, A typical case of anencephaly in human. Source: Wikimedia.org; B, Various forms of synthetic marijuana containing synthetic cannabinoids: Popular brands include Spice (of which Spice Tropical Synergy and Spice Diamond are shown here); other brand names include Yucatan Fire, Smoke, Chill X and Space (of which some blends are shown here) as well as others. Because there are no current FDA or DEA regulations, these products can be sold as “proprietary blend” and not list ingredients on the package. Source UNODC;[9]C, Synthesis of Δ1-THC using transgenic tobacco hairy roots expressing THCAS[17] (reproduced with permission from Wikimedia.org (A),[6] UNODC (B)[9] and the American Society for Biochemistry and Molecular Biology (C).[17]
The risk of human gestational exposure to these novel drugs is so far completely unknown. In this review, we attempt to discuss the potentially adverse effects of exocannabinoids, including Δ9-THC, synthetic cannabinoids found in Spice branded products, and other cannabinoid research chemicals, on CNS development during pregnancy in both human and animal models. First, we review what is currently known in terms of the potency of marijuana; second, we review the mechanisms, by which Δ9-THC interacts with the eCB system in the adult CNS. We also describe the emergence of the Spice brand of synthetic marijuana, and discuss potential new markets for cannabinoid research chemicals. Third, we review the literature indicating the presence of eCB system in the embryo/fetus during CNS development and the potential effects of gestational exposure to Δ9-THC, to psychoactive components detected in Spice branded products, and to cannabinoid research chemicals on CNS development.
Prevalence of ‘high potency’ marijuana
The potentially adverse effects of marijuana abuse during pregnancy are aggravated by the fact that the mean potency of marijuana, in terms of its Δ9-THC content, has increased substantially in the past 30 years. Data collected from seized samples between 1985 and 2008 show a steadily increasing trend in the mean potency of marijuana, with a Δ9-THC content of 2.8% in 1985, 3.4% in 1993, and 5.8 to 9.3% in 2008 in marijuana, and a Δ9-THC content of 7.3% in 1985, 5.8% in 1993, and 11.7% in 2008 in Sinsemilla (i.e. the flowering tops of unfertilized female plants with no seeds),[13,14] with individual 2008 Sinsemilla samples reaching up to 37.2% Δ9-THC content. Similarly, Δ9-THC content in hashish (dried cannabis resin and compressed flowering buds) averages 28.2% in 2008 samples, compared to 2.3% in the 1970s, with some hashish samples composed of up to 66% Δ9-THC.[13] In the last 25 years, there has been an alarmingly steady increase in the availability of “high potency” marijuana, i.e. marijuana consisting of high Δ9-THC content (i.e. 9% or higher) versus “low potency” marijuana, i.e. marijuana with low Δ9-THC content (less than 3%): In 1989, only 1.8% of seized marijuana samples were composed of high Δ9-THC content, compared to 52.6% of seized marijuana samples composed of low Δ9-THC content; by contrast, in 2007, 37% of seized marijuana samples were composed of high Δ9-THC content.[13] From the studies above, we can infer that, since pregnant women are now exposed to the availability of high potency marijuana, the developing CNS of embryo/fetus is potentially exposed to high levels of Δ9-THC. Furthermore, marijuana is becoming the focus of intense biotechnological research.[15] Initial steps in the production of Δ9-THC have been undertaken with the synthesis of Δ[1]-THC through the use of yeast-based expression systems[16] and transgenic tobacco hairy roots[17] (Figure 1C). Δ[1]-THC can then be readily transformed into psychoactive Δ9-THC through heat decarboxylation within a laboratory setting[18] with the potential of opening a new era of extensive marijuana production.
9Δ-THC interferes with the eCB system in the adult CNS
Overview of the eCB system in adult CNS
In the adult CNS, Δ9-THC exerts its effects by interfering with the eCB signalling system. This system is responsible for modulating synaptic neurotransmitter release to regulate motor control, memory, and other brain functions, reviewed in Pertwee,[19] Mackie,[20] and Kano et al.,[21] and consists primarily of endogenous ligands (eCBs), such 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamide (AEA; anandamide).[22–24] These eCBs are synthesized and released upon demand from postsynaptic neuronal compartments, where they act as retrograde messengers at presynaptic terminals to stimulate cannabinoid (CB) receptor 1 and 2 (CB1 and CB2).[23–25] Activation of CB1 receptor elicits changes in transcription, translation, cell motility, shape, proliferation, cell fate, and differentiation of neurons, as well as modulation of neurotransmitter release in many excitatory and inhibitory synapses, within the basal ganglia, hippocampus, amygdala, cerebral cortex, tectum, substantia nigra, nucleus accumbens, ventral tegmentum area (VTA) and cerebellum.[19–21,26,27] These changes are shown to be mainly mediated through CB1 receptor-coupled Gi/o protein activation, which results in inhibition of adenylate cyclase activity, resulting in inhibition of cAMP and decreased activity of cAMP-dependent protein kinase (PKA), and activation of MAPK (ERK1/2, JNK and p38) and other signalling pathways.[28–31] These events in turn result in phosphorylation of nuclear transcription factors and other cytosolic targets, as well as modulation of Ca2+ and K+ channels, events which together lead to the changes mentioned above including modulation of glutamatergic, GABAergic, opioidergic, serotonergic and dopaminergic neurotransmission, as well as regulation of cell fate.[19–21,26–28,32–34]
The eCB system also comprises endocannabinoid ligands (eCBs) 2-AG and AEA, which act as full agonist and partial agonist at the CB1 receptor, respectively.[19,20] Several other eCBs have been identified lately and their physiological roles are yet to be delineated. The eCB system is also composed of enzymes, which are responsible for the metabolism of eCBs. The synthesis of 2-AG mainly occurs via sn-1-selective diacylglycerol lipases α and β (DAGL α/β) and it is degraded by monoglyceride lipase (MAGL) and α/β-hydrolase domain-containing serine hydrolases (ABHD6/12).[35–38] The synthesis of AEA occurs mainly by NAPE-specific phospholipase D (NAPE-PLD) and it is degraded via fatty acid amide hydrolase (FAAH).[39,40]
Δ9-THC interferes with the eCB system in the CNS
Δ9-THC, a major psychoactive component of marijuana, exerts it action mainly through activation of CB1 receptor in the CNS, and mimics many effects of 2-AG and AEA. It acts as a partial agonist or a full agonist depending on the area of the CNS under study.[19,20] For instance, Δ9-THC acts as a full agonist at CB1 receptors on GABAergic and glutamatergic axonal terminals in hippocampal neurons and inhibiting GABA and glutamate release.[41–44] The distribution of CB1 receptor in the adult CNS accounts for the psychoactive properties of Δ9-THC, since it encompasses regions implicated in the actions of Δ9-THC[45] (Table 1). Due to its psychoactive effects on the CNS, Δ9-THC is currently classified as a Schedule I drug under the Controlled Substances Act.[46]
Table 1.
CB1 receptor containing regions in the adult CNS, associated functions and embryonic origin of these regions in the forebrain, midbrain and hindbrain primordia of the CNS at the pre-neuronal stages of embryonic CNS development
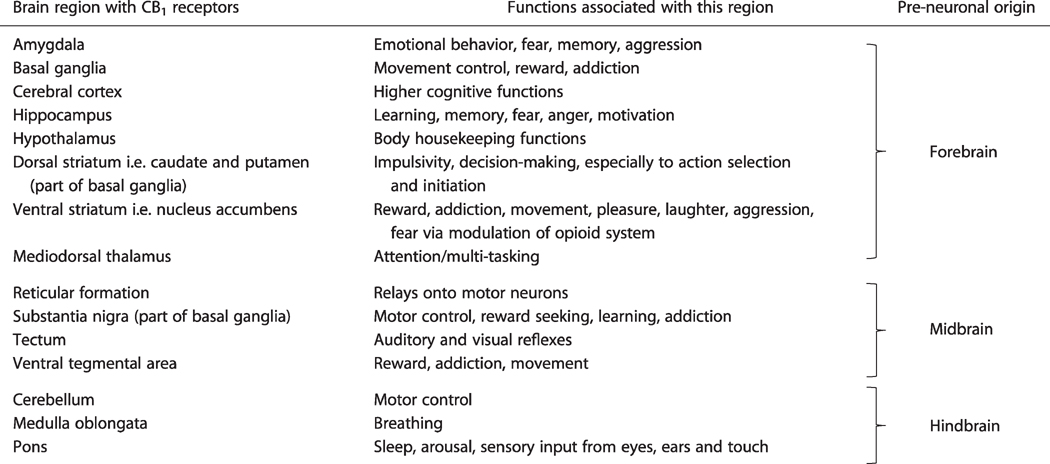
|
Synthetic marijuana and Δ9-THC analogues found in the brand Spice branded products
Potency of cannabimimetics found in Spice branded products
Blends falling under the brand Spice, such as Spice Gold, 2Spice, Mojo, etc, are composed of herbal mixes sprayed with potent Δ9-THC analogues (or cannabimimetics). The blends are then inhaled via smoke, producing psychoactive effects similar to the ones observed with high-potency marijuana. Since these compounds are extremely potent Δ9-THC analogues, they have the potential to cause deleterious effects on the CNS.[9–12,47–62] Cannabimimetics found in Spice branded products are CB1 receptor agonists, originally manufactured for laboratory research use, with varying degrees of potency.
Some of these cannabimimetics have extremely high potency, with potency ranging from 40 to 660 fold higher than Δ9-THC, and include AM694 (500x more potent than Δ9-THC),[51] AM-2201,[[51]] (±)-CP-47,497-C8,[52–55,57,58]] (—)-CP-55,940,[49,50,55,57]] HU-210 (660× more potent than Δ9-THC), [24,54,56,59]–[61] and others[62] (Table 2). The potential cytotoxicity of these compounds was recently investigated in neuronal cell line NG 108–15. These potent cannabimimetics are found to induce apoptosis via CB1 activation, through caspase-3 mediated mechanisms.[57] Furthermore, (±)-CP-47,497-C8 (as well as JWH-018 and JWH-073) is able to inhibit neurotransmission in cultured hippocampal neurons.[53,58] Some cannabimimetics in this group, such as HU-210, have neuroprotective effects in primary neurons of the adult cortex. These effects are mediated via PI3K/Akt signalling.[59] In addition, HU-210 has neurogenic properties, i.e. proliferative effects, in neural stem/progenitor cells (NS/PCs) derived from E17 and adult hippocampus.[60] These neuroproliferative effects are mediated via activation of PI3K/Akt and ERK1/2 pathways.[60,61]
Table 2.
Potency of cannabimimetics found in major Spice branded products: Major ‘Spice’ (or ‘herbal high’) psychoactive constituents are listed, together with their alternative names: a, Cannabicyclohexanol, CCH; b, AM678; c, RCS-04 butyl analogue, SR-19, BTM-4, E-4; d, SR-18, BTM-8. The major Spice branded products mentioned herein are (1) Tai High Afghan Kush, (2) Lazy J, (3) Puff The Philosophers Stone, (4) Puff Super Strength, (5) Kronic Pineapple Express, (6) Marley Extra Strength 1.5, (7) Euphoric Blends White Rhino, (8) Euphoric Blends Big Bang, (9) Euphoric Blends Bubble Gum, (10) High Dro, (11) Kronic Tropical Explosion, (12) Kronic Purple Haze, (13) Electric Puha Ganja Guru Delta, (14) Kronic Skunk, (15) Space V2 Herbal Incense, (16) Spice Diamond. (18) Spice Gold. (19) Blueberry Posh, (20) Black Mamba, (21) Magic Gold; data compiled from.[9,48,68,69] Fold potency indicates potency relative to Δ9-THC in terms of affinity to CB1 receptor, as measured by Ki activity (the lower the Ki value, the higher the affinity for CB1 receptor)
| Compound | Specificity | Ki CB1 (nM) | Fold potency | Spice branded product | Cannabimimetic activity in adult CNS | Refs |
|---|---|---|---|---|---|---|
| Extremely high potency compared to Δ9-THC: (40–660 fold): | ||||||
| AM-694 | Potent selective CB1 agonist (also weaker CB2 agonist) | 0.08 | 500x | 7-9 | ? | 51 |
| AM-2201 | Potent, non-selective CB1/CB2 agonist | 1.0 | 40x | 1, 10 | ? | 51 |
| (±)-CP-47497-C8a | Potent selective CB1 agonist | 0.83 | 48x | 18 | Induces cell death in neuronal cell line NG108-15; inhibits neurotransmission in cultured hippocampal neurons | 52-55,57,58 |
| (−)-CP-55,940 | Potent, non-selective CB1/CB2 agonist | 0.58 | 70x | 18 | Induces cell death in neuronal cell line NG108-15 | 49,50,55,57 |
| HU-210 | Full non-selective agonist at CB1/CB2 | 0.06 | 660x | 18 | Promotes adult hippocampus neurogenesis (NS/PCs neurosphere proliferation via PI3K signaling); neuroprotective effect in primary CNS neurons via PI3K signaling | 24,54,56,59-61 |
| JWH 210 | Potent, non-selective CB1/CB2 agonist | 0.46 | 87x | ? | 62 | |
| High potency, compared to Δ9-THC: (4 to 20 fold): | ||||||
| (±)-CP-47,497 | Potent selective CB1 agonist | 2.2 | 18x | Induces cell death in neuronal cell line NG108-15 | 52,54,55,57,63-65 | |
| JWH-018b | Very potent selective CB2 agonist (also potent CB1 agonist) | 9.0 | 5x | 1-21 | Induces ERK1/2 MAPK phosphorylation in CB1 receptor expressing HEK and CHO cells; inhibits postsynaptic currents via CB1 receptor in cultured hippocampal neurons | 53,58,62,66-69 |
| JWH-073 | Potent selective CB1 agonist (also weaker CB2 agonist) | 8.9 | 5x | 2-9, 14–16, 21 | Induces cell death in neuronal cell line NG108-15; Inhibits neurotransmission in cultured hippocampal neurons | 50,57,69,70 |
| JWH-250 | Potent selective CB1 agonist (also weaker CB2 agonist) | 11 | 4x | 7-9, 13-16 | ? | 62 |
| JWH-398 | Very potent non-selective CB1/CB2 agonist | 2.3 | 17x | ? | ? | 62 |
| Potency similar to that of Δ9-THC: | ||||||
| WIN 55,212-2 | Potent selective CB2 agonist (also potent CB1 agonist) | 62.3 | 0.63x | ? | Modulates NMDA receptor mediated release of Ca2+ in hippocampal slices | 76-78 |
| No data on potency: | ||||||
| RCS-4 | ? | ? | 3-6, 11, 12 | ? | 47,49 | |
| RCS-4-C4 homologc | ? | ? | 3-6 | ? | 47,49 | |
| RCS-08d | ? | ? | ? | ? | 47,49 | |
Other cannabimimetics found in Spice branded products have relatively high potency, with potency ranging from 4- to 20-fold higher than Δ9-THC, and include CP47,497,[52,54,55,57,63–65] JWH-018,[53,58,62,66–69] and JWH-073,[50,57,69,70] amongst others[62] (Table 2). Notably, JWH-018 is the cannabimimetic most commonly found in current Spice blends[47–50] JWH-018 is found in all blends analyzed in Table 2 (i.e. from blend 1, which corresponds to Tai High Afgan Kush, to blend 21, which corresponds to Magic Gold); By contrast, other extremely potent synthetic cannabinoids are only found in one of the Spice blends mentioned herein, as is the case for (±)-CP-47,497-C8 and HU-210, which are found only in blend 18 [Spice Gold] (Table 2). JWH-018 activates multiple CB1 receptor signalling pathways, including ERK1/2 in CB1 receptor expressing HEK and CHO cells.[58,67] JWH-018 usage in humans is currently not detectable with the immunoassay screening methods employed for detecting marijuana use from urine specimens; determination of the parent drug in serum, or its metabolites in urine, is performed by gas chromatography–mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS).[54,68,69,71–75] Peak concentrations of JWH-018 vary between 5 and 35 μg/L depending on the Spice blend used.[68] Some cannabimimetics found in Spice blends have potency similar to that of Δ9-THC, as is the case for WIN 55,212-2.[76–78] A note to mention that for some cannabimimetics commonly found in Spice brands, there is no current available data in terms of their potency; such is the case for the RCS class of cannabimimetics, including RCS-04, RCS-4-C4 homolog and RCS-8.[47,79]
Most of the synthetic cannabinoids are found in different concentrations within any given Spice branded product. Due to their considerable potency in comparison with Δ9-THC, typical doses of any synthetic cannabinoid may be less than a 1 mg/g of Spice blend: JWH-018 is found in all Spice branded products analyzed to date, with a concentration of 0.2% to 3% of the total content of Spice content analyzed.[47–50] (±)-CP-47,497-C8 is reported to range between 1–17 mg/g, JWH-018 ranges between 2 and 36 mg/g and JWH-073 ranges between 6 and 23 mg/g following analysis of up to 46 products of different brands, including Spice.[50,80] As far as the duration of effects of CP-47,497-C8 and JWH-018 following human consumption in comparison to Δ9-THC, the effects seems to be considerably longer for CP-47,497-C8 (5–6 h) and shorter for synthetic cannabinoid JWH-018 (1–2 h), as reported in a self-experiment;[81] this corroborates the data in terms of potency of these synthetic cannabinoids compared to Δ9-THC, which are 5x and 48x at CB1 receptor (Table 2).
Current status on the scheduling of cannabimimetics found in Spice branded products
As of 1 March 2011, and with the exception of HU-210, which already falls under Schedule I,[82] most of the cannabimimetics found in current K2 and Spice branded products, were placed in Schedule I on a temporary basis (one-year ban), under the US House of Representatives (HR) Bill 1254.[83] Exempt of this proposed Bill and for reasons unknown, were a CB1 agonists (−)-CP-55,940 (about 70 times more potent than Δ9-THC;[49]), and WIN 55,212-2, amongst others. There are ample debates as a result of passage of this Bill, including arguments that it would criminalize numerous substances without any scientific or medical evidence to support doing so, or without evidence that criminalization would actually reduce demand or prevent deaths.[84] Others argue that, ‘the Federal Government (then followed by the State Government) should ban this entire class of substances, including the derivatives, analogues and homologues. Otherwise we will continue to see years of this ‘dance’ where the Government bans a small subset of synthetic cannabinoids, followed by the makers of Spice/K2 coming out with a new version of their product containing cannabinoids not yet banned’.[85] As of March 2012, the HR 1254 Bill had received a six-month extension period; it is currently up to individual states to provide Legislature as regards banning of cannabimimetics found in Spice.[86] For instance, as of April 2012, in the JWH series of cannabimimetics, only JWH-018 and JWH-073 are under US Drug Enforcement Agency (DEA) ban (with DEA numbers 7118 and 7173, respectively) and the remaining JWH compounds found in Spice blends, remaining on temporary ban.[87] It is important to mention that the composition of Spice blends constantly changes and these chemicals could be found in higher abundance and/or include novel, more potent cannabimimetics in future blends, without adequate DEA regulation under current DEA standards.[87,88]
Potential novel markets with cannabinoid research chemicals
In addition to marijuana and Spice cannabimimetics, there is now potential for a novel market, of cannabinoid research chemicals. These include (1) full and potent CB1 receptor agonists, such as O-2545-HCl (Ki=1.5 nM) and others, currently available from various websites,[89–92] with potency 10- to 40-fold higher than Δ9-THC (Table 3); as well as (2) inhibitors of the enzymes responsible for 2-AG and AEA degradation, currently not marketed on non-research websites. By preventing the degradation of eCBs such as 2-AG and AEA, these inhibitors enhance the effects of these eCBs on CB1 receptors in the CNS[93–123] (Table 4). These cannabinoid research chemicals, currently mostly used in laboratory research, as is the case for FAAH inhibitor URB597, which has antidepressant and anxiolytic activity in animal models,[121–123] have the potential to be exploited as street drugs in future Spice blends or otherwise. None of these research chemicals is currently under DEA Schedule I, and therefore these chemicals are readily available to the general population on a legal basis; most importantly, their potentially detrimental effects on the CNS are currently unknown.
Table 3.
Potent cannabimimetic research chemicals: These are currently available to research laboratories, but also accessible to the general population through various websites, for which there are currently no regulations as far as legality, and no Schedule under DEA. The effects of these chemicals following potential human consumption are currently unknown
| Compound | Potency and selectivity | Ki at CB1 (nM) | Fold potency | Refs |
|---|---|---|---|---|
| O-2545-HCl | potent CB1/CB2 agonist | 1.5 | 26x | 90 |
| ACEA | potent and highly selective CB1 agonist | 1.4 | 29x | 91 |
| ACPA | potent and selective CB1 agonist | 2.2 | 18x | 91 |
| AM-1235 | potent and selective CB1 agonist | 1.5 | 26x | 66 |
| AM-2232 | potent CB1/CB2 agonist | 0.3 | 133x | 66 |
| AM-2233 | full agonist at CB1 | 2.8 | 14x | 80 |
Table 4.
Inhibitors of endogenous eCB degradation, including MAGL, FAAH, and ABHD6 inhibitors; these cannabinoid research chemicals could potentially be used as potent ‘street drugs’, since they are known to inhibit 2-AG and AEA degradation in animal models and pharmacological studies. As is the case for cannabimimetics listed in Table 3, there are no Schedule regulations on these compounds, or known effects in human
| Compound | Specificity | IC50 (μM) | Observations | Refs |
|---|---|---|---|---|
| URB602 | Weak reversible MAGL inhibitor | 223 | Reduces MAGL activity to 73% and elevates 2-AG levels in hippocampal slices at 100 μM | 93,94 |
| URB602 carbamate analogue 16 | MAGL inhibitor | ? | Reduces MAGL activity to 26% at 100 μM in hippocampal slices | 95 |
| NAM | Irreversible MAGL inhibitor | 0.14 | Electively prevents metabolic inactivation of 2-AG by ∼85% in mouse cerebellum in vitro | 96-98 |
| JZL 184 | Irreversible MAGL inhibitor | 0.008 | Blocks hydrolysis of 2-AG in vivo in the mouse brain | 99,100 |
| (±)-oxiran- hexanoate | Reversible dual MAGL/FAAH inhibitor | 4.1 MAGL 5.1 FAAH |
101 | |
| (2R)-(−)-oxiran acetate | Reversible dual MAGL/FAAH inhibitor | 2.4 MAGL 0.3 FAAH |
101 | |
| URB602 carbamate analogue 12 | Reversible dual MAGL/FAAH inhibitor | 0.006 MAGL 0.012 FAAH |
102 | |
| URB602 carbamate analogue 26 | Reversible dual MAGL/FAAH inhibitor | 2.1 MAGL 1.0 FAAH |
Also a highly potent blocker of AEA uptake (0.082 μM) by RBL2H3 cells; reduce 2-AG hydrolysis by these cells at ≥ [0.030 μM] | 102 |
| WWL 70 | Potent ABHD6 inhibitor | 0.07 | 103,104 | |
| UCM710 | Potent dual ABHD6 and FAAH inhibitor |
2.4 ABHD6 4.0 FAAH |
Augments levels of 2-AG and AEA in neurons; no effect on MAGL | 105 |
| PMSF | Non-specific irreversible amidase (incl. FAAH) inhibitor | 290 | 106 | |
| AACOCF3 | cPLA2 and FAAH inhibitor | 0.008 | 107 | |
| AA-5-HT | ? | 5.6 | 108 | |
| JNJ 1661010 | Reversible FAAH inhibitor | 0.012 | Brain penetrant and active in vivo | 109 |
| LY 2183240 | FAAH inhibitor | 0.013 | Also a highly potent blocker of AEA uptake (270 pM) | 110 |
| MAFP | Potent, irreversible FAAH inhibitor | 0.003 | 111,112 | |
| NADA | FAAH inhibitor | 0.4 | Also a potent CB1 agonist (Ki = 0.25 μM) | 113 |
| PIA | FAAH inhibitor | 4.89 | 114 | |
| PF 3845 | FAAH inhibitor | 0.23 | 115 | |
| PF 750 | Irreversible FAAH inhibitor | 0.016 | Orally active, acts within the CNS | 116,117 |
| ST4070 | Reversible FAAH inhibitor | 0.033 | 118,119 | |
| VER-156084 | FAAH inhibitor | 0.1-10 | 120 | |
| URB597 | FAAH inhibitor | 0.0046 | 121-123 |
Δ9-THC, cannabimimetics and adult CNS: beneficial effects in neuropsychiatric diseases
Marijuana, marijuana-derived Δ9-THC, and some of the cannabimimetics listed above, have been studied in animal models and clinical trials, (and in some cases, approved for patient use in some countries), for their potential therapeutic, neuroprotective and neuroregenerative properties in psychotic, mood and neurodegenerative disorders.[124] For instance, HU-210 treatment exerts anxiolytic- and antidepressant-like effects, possibly through its stimulating effect on hippocampal neurogenesis in the adult rat.[61] Inhibitors of FAAH (URB597) (Table 4) and AEA transporter (AM-404) have shown to exert antidepressant and anxiolytic effects in rodent models.[121,122,125] Importantly, only lower doses of CP-55,940 and WIN 55212–2 have been shown to exert anxiolytic effect while Δ9-THC (0.25–10 mg/kg) produced a dose-dependent anxiogenic effect.[122] Sativex® (a preparation derived from cannabis leaf), is approved for the treatment of specific symptoms such as, spasticity and pain of multiple sclerosis patients, in various countries (excluding the USA).[126] In pilot studies, Nabilone® (a Schedule II synthetic cannabinoid), is effective in alleviation of Huntington's Disease (HD), presumably through its effects on GABAergic, domapinergic and glutamatergic systems present in the brain areas known to degenerate in HD.[127]
Ontogeny of the eCB system: pre-neuronal phase of CNS development
In this section, we review findings that components of the eCB system are present during early (i.e. pre-neuronal) CNS development of the developing embryo, a phase of CNS development which occurs at days 16–22 gestation in human, and during which the basic scaffold for the CNS is established with the formation of forebrain, midbrain and hindbrain (Table 5).
Table 5.
Stage comparison between chick, mouse, and human at pre-neuronal stages of embryonic CNS development. Comparison between chick (HH), mouse, (E), and human (days, weeks) embryonic stages is shown for neural plate formation, neurulation (i.e. formation of the neural folds), and brain primordia formation (i.e. formation of the forebrain, midbrain and hindbrain vesicles). The data is compiled from Carnegie Stages Comparison[132] and Psychoyos et al.[133]
| Species | Neural plate | Neurulation | Brain primordia | ||||
|---|---|---|---|---|---|---|---|
| Chick (HH) | 4 | 5 | 8 | 10 | 11 | 12 | 13 |
| Mouse (E) | 7 | 7.25 | 7.5 | 7.75 | 8 | 8.25 | 9 |
| Human (days) | 16 | 18 | 20 | 21 | 22 | 24 | 28 |
| Human (wks) | 2 | 2.5 | 2.5 | 3 | 3 | 3.5 | 4 |
Overview of pre-neuronal phase of CNS development
It is during the pre-neuronal phase of CNS development that the basic scaffold for brain structures such as the cerebral cortex, amygdala, hippocampus, tectum, cerebellum and basal ganglia originate, from a simple neuroepithelium, the neural plate[128,129] (Figure 2A). This is the earliest recognizable form of the CNS and appears at mouse E7 (chick HH4, day 16 gestation in human) (Table 5). The neural plate is subdivided into presumptive territories for the different precursors of the forming CNS, the forebrain, the midbrain and the hindbrain[130] (Figure 2A). A crease progressively appears along the midline of the neural plate, and deepens until its sides arch over and fuse with each other to form the neural tube, the anterior segment of which will form the CNS. As the embryo develops, the anterior neural tube becomes divided into presumptive areas for the forebrain, midbrain and hindbrain (chick HH11, mouse E8.0, day 22 gestation in human, Figures 2B and 2C). These events together constitute the pre-neuronal phase of embryonic CNS development[129–131] (in subsequent development, i.e. during the neuronal phase, these areas will further differentiate to become the domains of the brain mentioned in Table 1; thus, the forebrain will form the amygdala, mediodorsal thalamus etc, the midbrain will form the reticular formation, tectum etc, and the hindbrain will give rise to the cerebellum, pons etc) (Table 1)). Interestingly, chick (Figures 2A–2D), mouse and human (Figures 2E–2H) embryos share the same developmental cascades, as well as similar basic morphology during early stages of CNS development.[132–136]
Figure 2.
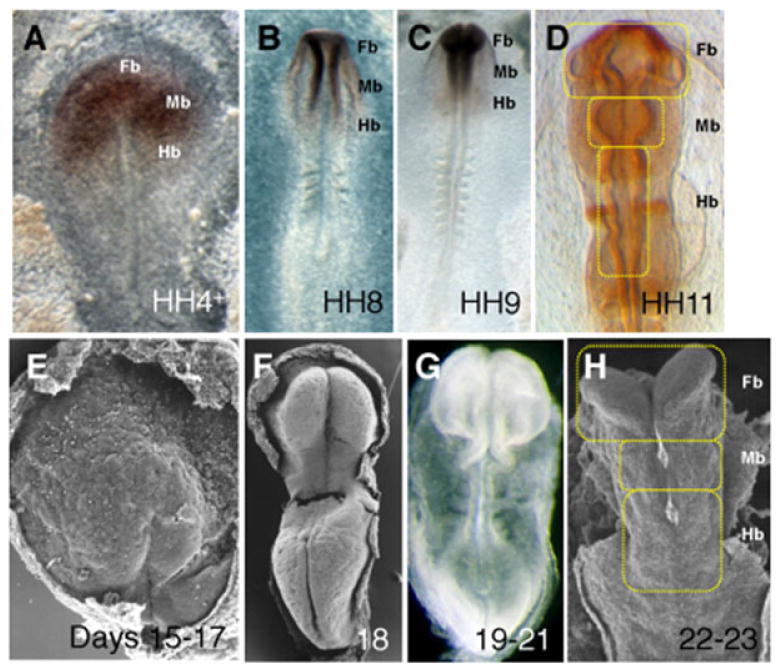
Pre-neuronal development of the CNS in chick and human embryos: A-D, chick embryos at pre-neuronal stages: A, Neural plate in chick at stage HH4+: presumptive territories for the forebrain (Fb), midbrain (Mb) and hindbrain (Hb) of the CNS; The forebrain and anterior midbrain territories express the neural marker Otx2; B, Neurulation, stage HH8 (B) and HH9 (C); D, Subdivisions of the developing CNS brain primordia, stage HH11; these 3 primitive vesicles will subsequently develop into the domains of the brain described in Table 1, during neuronal stages; In D, the embryo expresses neural marker En1. E-H, human embryos at pre-neuronal stages: E, 15–17 days; F, 18 days, G, 19–21 days, and H, 22–23 days (Table 5 indicates stage comparison). At pre-neuronal stages, chick (A-D), mouse (not shown) and human (E–H) embryos share the same basic morphology. (A-D) adapted from Psychoyos et al.[133] with permission from Bentham Open; (E-H) reproduced with permission from Prof. K. Sulik (University of Tennessee).
Ontogeny of the eCB system
Recent studies demonstrate that components of the eCB system are present in the developing CNS at its earliest stages of development, i.e. during the pre-neuronal phase. There are so far no available studies on the eCB system in human gestation weeks 2 to 4; Our current knowledge on this question comes from animal studies at equivalent developmental stages. Recently obtained data on eCB levels during the pre-neuronal phase of CNS development come from studies employing mass spectrometry and liquid chromatography: Both 2-AG and AEA are detectable in chick embryos at stages HH9-11, with levels of 2-AG of 1.1 nmol/g tissue and levels of AEA of 3.6 pmol/g tissue (earlier stages were not investigated in this study).[137] These stages are equivalent to stage E7.75-8 in mouse and the third week of gestation in human (Table 5). The qPCR, RT-PCR and immunohistological studies also demonstrate that some of the enzymes required for 2-AG and AEA metabolism in adult CNS, such as DAGLα/β, NAPE-PLD, MAGL and FAAH, are also found in embryonic CNS at pre-neuronal stages in chick HH3-11 and mouse E7.5-E9.5.[137] Although the enzymatic activities of these enzymes have not yet been examined, these preliminary results corroborate the findings that 2-AG and AEA are also detectable at pre-neuronal stages.
CB1 receptor mRNA is present in the chick embryo from the earliest stages of CNS development, i.e. from stage HH3- onwards (equivalent to the second week of gestation in human).[137] CB1 receptor mRNA and protein are subsequently expressed in the neural plate from HH4-5; during neurulation (stages HH8-10 in chick) and formation of the brain primordia (HH11-13 in chick), CB1 receptor is expressed in presumptive forebrain, midbrain and hindbrain (Figures 3A–3D) in addition to other tissues. A note to mention that these CB1 expressing regions are the very same areas that will give rise to the adult brain structures mentioned earlier in Table 1. Similarly, CB1 receptor is expressed in the neural plate and brain primordia precursors of mouse E7.5-9.5 embryos, in addition to other tissues.[137] In zebrafish, CB1 receptor mRNA is detected from the three-somite stage onwards (equivalent to mouse E7.5),[138] corroborating earlier studies in rat[139] and chick[140,141] (reviewed in Psychoyos et al.[133]). Pharmacological studies on the ability of the CB1 receptor agonist (−)-CP-55,940 to stimulate [[35]S]GTPγS binding in neural membranes derived from stage HH9-11 chick embryos, indicate that CB1 receptors are functionally active from the earliest stages of CNS development onwards, i.e. during the pre-neuronal phase[137] (Figure 3E). Most importantly, this last set of findings demonstrates that the developing brain of HH9-11 chick embryos (corresponding to the third week of gestation in human) is responsive to the effects of (-)-CP-55,940, a synthetic cannabinoid commonly found in Spice Gold.
Figure 3.
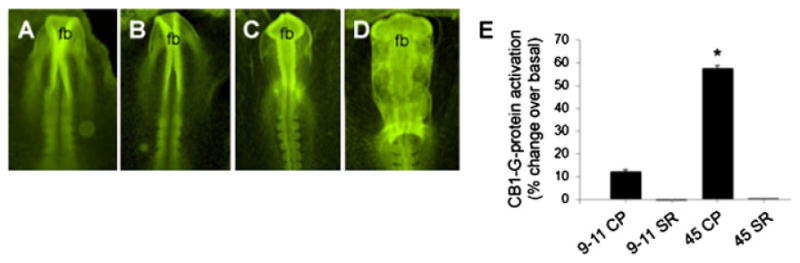
Functional studies on CB1 receptor in chick at pre-neuronal stages of CNS development, using CB1 agonist and potent Spice synthetic cannabinoid (-)-CP-55,940: A-D, whole mount immunohistochemistry with CB1 receptor antibody in chick. The CB1 receptor antigen is homogeneously distributed throughout the embryo, with intense localization in the anterior neural folds (A, B) and brain primordia, including the forebrain (fb) (C, D). E, Binding studies with CB1 receptor in chick. CP-55,940-stimulated [[35]S]GTPγS binding in membranes derived from stage HH9-11 and stage HH45 chick embryonic brain. A 12% increase in G-protein activation over basal levels is found in the brain primordia of stage HH9-HH11 embryos (9–11 CP). This effect is completely reversed following the addition of a CB1 antagonist SR141716A (9–11 SR). Similarly, in stage HH45 embryos, a 57% increase in G-protein activation (45 CP) is reversed following the addition of SR141716A (45 SR). Data presented as mean ± SEM. *Indicates a statistical difference (p < .05) from all other groups. CP; CP-55,940, SR; SR141716A. Reproduced with permission from from Bentham Open[133] and John Wiley & Sons, Inc.[137]
Potential role for the eCB system during pre-neuronal development of the CNS
Together, these data suggest a possible function for the eCB system at this earliest phase of in utero CNS development. This role is so far unknown, although it is clearly discernible from the role of the eCB system during neuronal development (reviewed below); such function(s) for the eCB system in early, pre-neuronal development of the future CNS, could potentially include, modulation of cell fate and gene transcription, resulting in the establishment of a neuronal scaffold within the neural tube, anteroposterior (AP), dorsoventral (DV) and mediolateral (ML) patterning of the forebrain, midbrain and hindbrain, and differentiation and/or apoptosis within the neuroepithelium of these three brain subdivisions, to generate the secondary vesicles which will subsequently form the brain. Clearly, this is currently an open question, and studies in this field of eCB research are warranted. An important point here is that an eCB system is indeed present in early neural development, and that this system is amenable to potential interference by exocannabinoids such as Δ9-THC, cannabimimetics found in Spice branded products, and other cannabinoid research chemicals (see below).
The eCB system during neuronal development
We have reviewed recent findings, which suggest that the eCB system is present during the initial, pre-neuronal phase of embryonic CNS development. So far, the function of the eCB system during the pre-neuronal phase of CNS development is not well characterized. On the other hand, the eCB system is well understood and extensively characterized in the later stages of CNS development, i.e. during the neuronal phase; we have therefore summarize its functions briefly herein, since most findings have been extensively reviewed elsewhere.[142–148]
Function of the eCB system during neuronal development of the CNS
During the neuronal phase of CNS development (from mouse E9.5 and week 4.5 human gestation), the forebrain differentiates into telencephalon and diencephalon; the telencephalon will then develop into amygdala, globus pallidus (part of basal ganglia), cerebral cortex and hippocampus; the diencephalon will become epithalamus, thalamus, hypothalamus, and pineal gland amongst others; the midbrain will differentiate into striatum (caudate and putamen), substantia nigra, tectum and VTA, and the hindbrain will give rise to cerebellum, medulla oblongata and pons[131] (Table 1). During neuronal development, the eCB system is required for the correct establishment of neuronal diversity and connectivity within the developing amygdala, cerebral cortex, caudate nucleus, hippocampus, nucleus accumbens, cerebellum and putamen; in theses areas, the eCB system is implicated in neurogenesis, neuronal migration, dendritogenesis, axon guidance, synaptogenesis, lineage specification and gliogenesis[145–148] (Table 6). As far as components of the eCB system. in mouse E10.5 and chick HH17, levels of 2-AG are 2.8 nmol/g and 2.1 nmol/g respectively; subsequently, levels of 2-AG reach 10 ng/g at mouse E14.5. At the end of gestation (mouse E18 and chick HH44), 2-AG levels are 17.6 nmol/g and 9.4 nmol/g respectively. Levels of AEA are also detectable in the embryonic CNS, albeit at much lower concentrations than 2-AG.[137,149,150]
Table 6. Summary of the functions of the eCB system is shown in animal models at various stages of neuronal development of the CNS.
| Species | Stage | Function of the eCB during neuronal development of the CNS | Refs |
|---|---|---|---|
| Developing cortex (forebrain derivative): | |||
| Rat | E5-20 | Maturation of glutamatergic system | 172 |
| Mouse | E12 | Pyramidal cell specification; radial migration of immature pyramidal cells in VZ/SVZ | 157 |
| Mouse | E14.5 | Establishment of long-range axonal connections from pyramidal cells in VZ/SVZ | 157 |
| Rat | E19 | Interneuron specification and migration via regulation of BDNF/TrkB and GABA receptor | 164,165 |
| Rat | E21 | Maturation of serotogenic system | 171 |
| Rat | P2 | Inhibits neurogenesis via attenuation of ERK and promotes astroglial differentiation | 163 |
| Developing hippocampus (forebrain derivative): | |||
| Rat | E17 | Promotes neurogenesis | 61 |
| Rat | E17-E18 | Inhibits synapse loss between hippocampal neurons in models for neurodegenerative diseases; protects hippocampal neurons from excitotoxicity; modulates synaptogenesis in intact E17 hippocampus | 165–168 |
| Developing telencephalon (forebrain derivative): | |||
| Rat | E14 | Modulation of TH activity (cathecolaminergic system) | 158 |
| Rat | E14 | Modulation of PENK mRNA (opioidergenic system) | 145 |
| Developing cerebellum (hindbrain derivative): | |||
| Zebrafish | P1 | Required for FGF-dependent axonal elongation and fasciculation of cerebellar neurons | 169,170 |
In humans, the earliest stage of investigation is 14 weeks gestation, where CB1 receptor is expressed with preferential expression in the cerebral cortex, hippocampus, caudate nucleus, and putamen.[151] By 19–20 weeks, intense expression is evident in CA2–CA3 of hippocampus and in the basal nuclear group of the amygdala.[152,153] In the developing mouse brain, CB1 receptor is expressed at E10.5.[137] During subsequent neuronal development, CB1 receptors are expressed in early neural progenitors in hippocampus and cortex,[154,155] with receptor levels increasing throughout neuronal specification and synaptogenesis, and CB1 being progressively localized to developing axonal projections.[152,153,156,157] In cultured mesencephalic neurons of E12 mouse embryos, CB1 receptors co-localize with tyrosine hydroxylase (TH) containing neurons.[158] At E12.5, CB1 receptor protein is localized in Cajal-Retzius cells and newly differentiated postmitotic glutamatergic neurons of the mouse telencephalon[156] and to the subpial area of the ganglionic eminence and marginal zone of the neocortex.[159] From E13.5-E21, abundant CB1 receptor protein is detected in several long-range axonal tracts including corticofugal tracts such as corticothalamic and corticospinal tracts.[156,157] From E16 onwards in rat, CB1 receptors are also highly expressed in hippocampus during the initiation of gliogenesis.[160] Using cultured chick cerebral cortex neurons derived from E3 embryos (HH18-20), Nilsson et al. demonstrated that CB1 receptor agonist and Spice constituent (−)-CP-55,940, was able to reduce cAMP activity, and that this reduction is blocked by CB1 receptor antagonist AM251, indicating the presence of functional CB1 receptors in the cultures.[161] Finally, pharmacological studies demonstrate the ability of CB1 receptor agonist and Spice constituent WIN 55212–2, to stimulate [[35]S]GTPγS binding in various areas of rat E16.5 (including cortex, midbrain and brainstem), rat E18 (same areas and also hippocampus) and in human 19 weeks gestation.[152] Together, the above studies indicate that CB1 receptors are functionally active during neuronal development[160] similar to the situation at pre-neuronal stages.
Enzymes required for 2-AG and AEA metabolism, including DAGLα/β, NAPE-PLD, MAGL and FAAH, are present in the developing CNS at the earliest stages of neuronal development (as early as E10.5 and HH17),[137] and in the presumptive cortical and hippocampal neurons of E14.5, E16.5 and E18.5 embryos,[149,162] suggesting that these enzymes play an essential role in determining the availability of 2-AG and AEA in the developing brain. In particular, DAGLα/β protein levels peak at E14.5 and E16.5, and then dramatically decrease at P1. In contrast, MAGL levels transiently decrease at E18.5.[149]
Functional studies: the eCB system and neuronal circuitry during cortex and hippocampus development
During corticogenesis (i.e. formation of the cortex), eCBs constitute a novel class of morphogens required for the genesis, proliferation, migration, and axonal guidance of neocortical pyramidal cells. In the telencephalon of mouse E12, the eCB system is functional in the ventricular zone (VZ) / subcortical proliferative ventricular zone (SVZ), to control the commitment and proliferation of pyramidal cell progenitors, and the radial migration of immature pyramidal cells[157] (Table 6). Later during gestational development, the eCB system is responsible for the pyramidal cells of the newly formed cortical plate to initiate the elongation and fasciculation of their long-range glutamatergic axons.[157] In the developing cortex of postnatal (P) 2 rat embryos, eCBs inhibit lineage commitment and differentiation program of neural progenitor cells into mature neurons, via attenuation of ERK pathway by CB1 receptor, and promote astroglial differentiation.[163] Furthermore, eCBs are responsible for controlling interneuron specification and migration in the developing cortex of rat E19 embryos, by regulating BDNF/TrkB and glutamatergic receptor signalling.[164] In the developing hippocampus of E17 rat embryos, eCBs promote neurogenesis via ERK1/2 MAPK.[61] The eCB system also inhibits network-driven synapse loss between hippocampal neurons in cultures of rat E18 hippocampal neurons.[165–168] Finally, CB1 signalling is required for FGF-dependent axonal growth and fasciculation of cerebellar in zebrafish[169,170] (Table 6).
The eCB system and the ontogeny of neurotransmitter systems
In addition to its role in shaping neuronal circuitry in the developing embryo, the eCB system is also responsible for modulating the ontogeny of various neurotransmitter systems during development, mostly the catecholaminergic (i.e. dopaminergic) and opioidergenic systems. The eCB system is responsible for regulating the expression of genes encoding for key components involved in the synthesis of these neurotransmitters, namely, tyrosine hydroxylase (responsible for dopamine synthesis) and pro-enkephalin (PENK) mRNA, a precursor of enkephalin A.[145,158] Additional studies demonstrate that the eCB system is also involved in the maturation of serotogenic,[171] GABAergic,[164,165] and glutamatergic (NMDA receptor,[172]) systems. These functions are summarized in Table 6.
Exposure TO Δ9-THC and other cannabimimetics during the pre-neuronal phase of CNS development
In this section we review current knowledge on the effects of Δ9-THC and other cannabimimetics, such as those found in Spice branded products and cannabinoid research chemicals, on gestational early CNS development.
Increased risk for anencephaly following peri-conceptional exposure to marijuana in humans
In utero exposure to marijuana during the peri-conceptional period (i.e. 1 to 4 weeks of gestation) is associated with an increased risk for anencephaly, a non life-sustaining condition in which the forebrain fails to form[7] (Figure 1A): A study performed by the US National Birth Defects Prevention Center, which included 10241 infants with major congenital malformations and 4967 infants without major congenital malformations born between 1997 and 2003, found a clear correlation between gestational marijuana exposure and the risk of anencephaly: periconceptional marijuana use (first trimester) was found to be associated with an increased risk of anencephaly. Restricting the analysis to marijuana use in the first month after conception, during which the neural tube closes, confirmed this finding. Marijuana use in the other months of the peri-conceptional period was not associated with an increased risk of anencephaly.[7] It is noteworthy that this study was carried out using data collected in the period 1997–2004. During this time, Δ9-THC content in marijuana averaged 5.2% (varying between 4.5% and 6.4%), compared to 3.1% in the period 1983–1994, and 5.8 to 9.3% in 2008.[13] From these results, we can predict that the risk of infants born with anencephaly will increase in the coming years, considering that not only the number of childbearing women potentially exposed to marijuana has increased, but also has the Δ9-THC content found in marijuana preparations.
In utero exposure to the synthetic cannabinoids found in Spice branded products and to other cannabimimetics in human and animal models
So far, nothing is known as whether cannabimimetics found in Spice branded products, such as JWH-018 and others, have the potential to cross the placental barrier following human consumption in pregnant users, and if it does, what the implications on CNS development might be. Our only knowledge is inferred from animal studies, which will be discussed herein. Classical studies on marijuana embryotoxicity describe cases of anencephaly and exencephaly (an early stage of anencephaly) in rodent models.[173–177] Furthermore, experiments with chick embryo report anencephaly and other CNS malformations following exposure to the potent cannabimimetic research chemical O-2545-HCl (Ki = 1.5 nM)[178] Figure 4A,B). In embryos treated with low dose of O-2545-HCl, the neural folds fail to elevate and to fuse, a phenotype comparable to exencephaly in rodent systems. In embryos treated with moderate dose of O-2545-HCl, the brain is poorly segmented into forebrain, midbrain and hindbrain primordia, in a phenotype comparable to anencephaly in rodents. These studies also reveal that O-2545-HCl also affects gene expression in the developing CNS. The spatial and temporal expression of genes required for correct establishment of brain primordia (Otx2, Krox20 and Pax6) is altered in embryo treated with this cannabimimetic Figure 4A,B). Finally, these studies also show that the time point most susceptible to the deleterious effects of O-2545-HCl coincides with the formation of the neural plate, equivalent to days 15–19 gestation in human.
Figure 4.
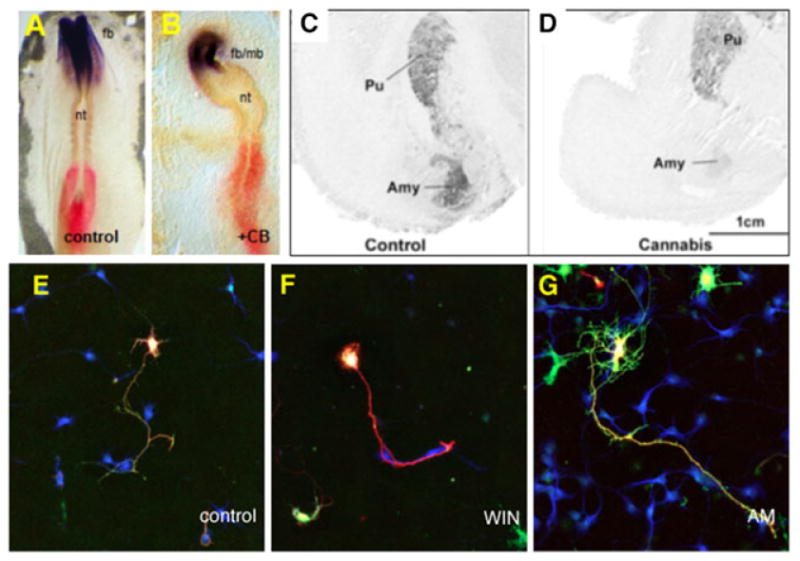
Effect of cannabimimetics at pre-neuronal and neuronal stages of CNS development: A-B, Effect of potent cannabimimetic O-2545-HCl (+CB; Table 4) on CNS development at pre-neuronal stages in chick, equivalent to days 17–19 in human. Exposure to O-26545-HCl at HH4 results in anencephaly observed at HH8, as evidenced by disruption in the expression pattern of Otx2, a marker for forebrain (fb) and midbrain (mb) at pre-neuronal stages;[133,178] Note that the neural tube (nt), i.e. the future spinal cord, is also disrupted. C-D, Human studies on the effect of gestational exposure to Δ9-THC on the mRNA expression of dopamine D2 in the amygdala (Amy) and Putamen (Pu) of 18–22 weeks gestation embryos. Δ9-THC is shown to down-regulate the expression of D2 mRNA;[193]E-G, Effect of WIN 55,212-2, a research chemical found in some Spice preparations, on cultured hippocampal neurons derived from E17 rat embryos: WIN 55,212-2 is shown to 2 inhibits dendritogenesis, via reduction of both length and number of primary dendrites (F), while CB1 antagonist AM-281 exerts opposite effects (G).[156] Reproduced with permission from John Wiley & Sons, Inc.,[178] Elsevier,[193] Wiley-Blackwell (E-G)[156] and Bentham Open.[135]
Thus, it is possible that severe marijuana consumption in users who have conceived and are at their 15th–19th day post-conception, results in aberrant embryonic CNS development (i.e. an embryo in which the neural plate fails to develop, in a phenotype similar to that observed in chick following cannabimimetic exposure). This aberrant phenotype could easily be misinterpreted as lack of implantation in human with the conclusion that, Δ9-THC is deemed devoid of any detrimental effects, except ‘lack of implantation’, a phenotype which has been described in mice.[179,180] It is also possible that less severe consumption of marijuana at 15–19 days after conception might result in implantation of embryos partly lacking anterior neural tissue and developing anencephalic phenotype; in principle, this hypothesis corroborates the findings concerning the association between an increased risk of anencephaly and marijuana consumption during the peri-conceptional period in human[7] described earlier. Another variable is that with some human studies, subjects might be selected after pregnancy is confirmed, and therefore it is not possible to investigate the possibility that exposure to marijuana early in gestation, is associated with lethality for severely malformed fetuses. What is certain is that, at early stages of pregnancy (15–19 days after conception), most women are unaware of their pregnancy and of the potential risks of concommitant use of marijuana and/ or potential cannabimimetics such as those found in Spice brands.
There is some evidence that CB1 and other components of the eCB system are present in chick embryos at pre-neuronal stages,[137] i.e. at stages during which, cannabimimetic O-2545-HCl is shown to exert its detrimental effects. At these pre-neuronal stages, the chick embryo expresses CB1 receptors which are responsive to the effects of CB1 agonist and Spice Gold constituent (−)-CP-55,940 (Figure 3E). It is therefore possible that cannabimimetics, such as O-2545-HCl or others, exert their deleterious effects on pre-neuronal development by interfering with an eCB system of hitherto unknown function, present in the embryo at the earliest stages of CNS ontogeny. Because the basic molecular mechanisms that control early CNS development are evolutionary conserved amongst species[133–136] the knowledge gained by analyzing the mechanism of cannabimimetic mediated embryotoxicity in animal models is applicable to our understanding of the effects of Δ9-THC, and potentially other cannabimimetics, in human.
Exposure to Δ9-THC and other cannabimimetics during the neuronal phase of CNS development
Neurobehavioural deficiencies following exposure to marijuana during human pregnancy
In humans, gestational marijuana exposure is associated with a plethora of neurobehavioural deficiencies including visual behavioural alterations in neonates;[181] mental, motor and neurobehavioral deficiencies,[182] as well as aggressive behavior and attention problems[183] in 18-month olds; lower scores in verbal and memory domains[184] in 3 year olds; lower performance at intelligence tests[185] and social behavioral disturbances[186] at age 6–7; decrease in learning abilities and in academic achievements,[187] neuropsychological problems,[188] ADHD (inattention, impulsivity)[189] and depressive symptoms[190] at age 10; as well as long-term abnormal cognitive and behavioral function in young adults.[191,192] It is not known whether the neurobehavioural deficiencies observed in the offspring following gestational Δ9-THC exposure, stem from developmental defects in the cognitive and emotional centres in which the eCB system is present during neuronal development, i.e. cortex, hippocampus, amygdala and nucleus accumbens. In addition, there is no data available at the moment on the possible outcome of pregnancy following exposure to cannabimimetics and other research chemicals during gestation, although we can predict symptoms similar to those observed following Δ9-THC exposure. Some human studies and several rodent studies have provided a partial answer to these questions and will be reviewed in the following section.
Human studies on the effect of Δ9-THC on neurotransmitter synthesis
Gestational exposure to Δ9-THC results in interference with the ontogeny of various neurotransmitter systems during development, mostly the catecholaminergic and opioidergenic systems, leading to abnormal neuronal circuitry during development in the centres in which these neurotransmitters are required, and consequent neurobehavioural abnormalities in the offspring. In humans, there is evidence that in utero marijuana exposure impairs dopamine D2 mRNA expression in the amygdala and in the nucleus accumbens of 18–22 weeks gestation (Figure 4C,D).[193] It is possible that defective dopamine D2 signalling in the amygdala and nucleus accumbens following Δ9-THC exposure, results in abnormal neuronal circuitry in those areas, which are cognitive and emotional centres. This in turn would lead to abnormal outcome in the offspring, such as the neurobehavioural deficiencies observed by Fried et al.,[181] Richardson et al.,[188] and Noland et al.,[189] and the neuropsychiatric disorders observed by Stein et al.,[188] and Gray et al.[189] Similarly, gestational Δ9-THC exposure increases μ opioid receptor mRNA in the amygdala, decreases κ opioid receptor mRNA in the mediodorsal thalamus and decreases PENK mRNA levels in the striatum of 18–22 weeks gestation.[194] As is the case for dopamine D2, it is possible that defective opioidergic signalling in centres required for the wiring of emotion, impulsivity and attention, result in defective neuronal circuitry in those centres, and subsequent neurobehavioural defects. Exposure to Δ9-THC could also affect other neurotransmitter systems during gestation, such as opioidergic, GABAergic, and glutamatergic (NMDA) systems as well as others, although this possibility has not been investigated so far. A note to mention that so far nothing is known on possible effects of gestational exposure to Spice branded products and to cannabinoid research chemicals in humans (all our current knowledge on this issue stems from animal studies, reviewed below).
Animal studies: Effect of Δ9-THC, Spice cannabimimetics and other research chemicals during neuronal development
Animal studies examining the effects of gestational exposure to Δ9-THC, cannabimimetics found in Spice branded products and to other research chemicals on gestational neurotransmitter signalling, gestational development of neurons, and subsequent neurobehavioural development in the offspring and/or adult animal will be summarized herein. Δ9-THC and Spice constituent WIN 55,212-2 both induce deficits in memory and learning, as well as alterations in motor activity, exploratory behaviour, emotional hyperactivity, social interaction, neuroendocrine control, stress responses, nociception, anxiogenic-like profile, and heroin seeking profiles in rat offspring and/or adult exposed during gestational E12.5-E20.[146,155,194–198]
Interference with neurotransmitter synthesis: At the molecular level, these neurobehavioural deficiencies are associated with impaired neurotransmitter synthesis and signalling in the areas of the developing CNS associated with cognitive circuitry, emotional behaviour, impulsivity, reward, addiction and movement and attention/multitasking (i.e. amygdala, cortex, dorsal striatum/caudate putamen, hippocampus, mediodorsal thalamus amongst others) (Table 7). Such examples include impaired dopamine function in the striatum, substantia nigra and VTA, via increase of tyrosine hydroxylase activity,[155,158,199–201] impaired glutamatergic receptor function and signalling in the cerebellum,[172] cortex,[195,197] and hippocampus,[196] and impaired noradrenergic signalling in the hippocampus[196] and the cortex,[197] all of which were accompanied by neurobehavioural deficiencies in the offspring. For instance, gestational exposure to WIN 55,212-2 in rat at E5-20 results in deficient glutamatergic neurotransmission in the offspring cortex and hippocampus, i.e. in the brain centres required for higher cognitive functions such as memory and learning; these biochemical deficiencies are associated with neurobehavioural impairment in the adult, including memory impairment, alterations in LTP, and emotional reactivity.[195,196] Gestational exposure to Δ9-THC and other cannabimimetics also results in perturbations in the GABAergic, opioidergic and serotonergic systems during neuronal development associated with neurobehavioural deficiencies in the off-spring.[171,193,194,202–206] For instance, Δ9-THC inhibits PENK mRNA expression in the nucleus accumbens, caudal putamen and rostral dorsal striatum during early neurodevelopment in rodent. This is associated with long-lasting neurobiological impairments in neuronal systems linked with opioid/reward/ stress limbic function in the offspring,[194,203,204] suggesting that impairment of PENK signalling during gestation via exposure to Δ9-THC. This might result in deficient circuitry in nucleus accumbens, and henceforth, aberrant limbic function in the offspring. Interestingly, PENK is highly expressed in proliferating neuronal and glial progenitors in rat at E14, its levels of expression later decrease sharply and are hardly detectable until E2. This suggests that this neurotransmitter might be responsible for proliferation and commitment of neuronal precursors within the developing cortex,[206] a function, which could also be potentially, impeded following gestational Δ9-THC exposure. As far as interference with serotogenic system in rats exposed gestationally to Δ9-THC, lower serotonin levels were noted in the diencephalon, hippocampus, midbrain raphe nuclei and septum in these offspring, and neurobehavioural deficiencies were noted in adult animals[171] (Table 7).
Interference with neuronal circuitry: In addition to their ability to interfere with neurotransmitter synthesis during neuronal development, Δ9-THC and cannabimimetics also impede with the formation of neuronal circuitry in the developing embryo, perhaps by modulating the expression of genes encoding for neuron-glia cell adhesion molecules, molecules which are required for cell proliferation, neuronal migration or axonal elongation. In cultured hippocampal neurons derived from E17-E18 embryos, the cannabimimetic and Spice constituent WIN 55,212-2 inhibits new synapse formation[168] and dendritogenesis,[156] the latter via reduction of both length and number of primary dendrites, while CB1 antagonist AM-281 exerted opposite effects (Figure 4E–G). CB1 receptor was found to translocate from the axonal termini to the somatic compartment of hippocampal neurons in E16.5 embryos following exposure to cannabimimetic (−)-CP-55,940,[156] which is also a compound commonly found in Spice blends.9 Similar results are observed in hippocampal interneurons derived from rat neonates exposed to Δ9-THC during gestation. Δ9-THC was found to interfere with the specification and migration of these interneurons, which failed to migrate within the hippocampus and remained within the strata radiatum, lacunosum-moleculare of the CA1-CA3 subfields.[164,165] At the molecular level, HU-210, a synthetic cannabinoid commonly found in Spice blends, was found to have (1) neuroregenerative effects (i.e. proliferative) in cultures of E17 hippocampal neurons, via CB1/ERK1/2 MAPK activation[61] and proliferative effects in granule cell precursors (GCPs) derived from the cerebellum of P4 pups, via CB1/AKT/glycogen synthase kinase-3β/β-catenin activation.[205] Together, the above studies suggest that disturbance of neuronal development in cortex, hippocampus and possibly amygdala and nucleus accumbens, following gestational cannabimimetic exposure, might in part result in disruptions in neurotransmitter signalling, as well as interference with neuronal morphogenesis and proper circuitry. These aberrations would in turn lead to subtle defects in cognitive, neurobehavioural and emotional processing in the offspring, which is the phenotype we observe in the offspring born to marijuana users. Recent studies in zebrafish are of particular interest in illustrating this point whereas previous studies focused on behaviour of neurons/axons at the earliest E14 in rat brain slices following treatment with cannabimimetics, this study used 1 to 4 cell stage embryos, in other words a period corresponding to peri-implantation in human (week 1 of gestation): In CB1-morpholino treated zebrafish embryos, the medial longitudinal fascicule, which corresponds to the anterior and posterior commissures of the forebrain in human, and which normally runs along the AP axis as segmented tight bundles of axons, appears clearly disorganized, spreading along the ML and DV axis of the CB1-morpholino treated embryo, suggesting that these embryos are receiving the wrong cues/or fail to receive cues upon CB1 receptor inactivation.[170] The anterior and posterior commissures of the forebrain are responsible for transferring information between the two cerebral hemispheres to coordinate localized functions in the adult, such as memory establishment[207] and visual discrimination,[208] both functions which are impaired in offspring following gestational exposure to marijuana.[7,156,182,184,209]
Table 7.
Summary of known effects of Δ9-THC and other cannabimimetics on neuronal development of the CNS, subdivided on effects in neuronal circuitry, and effects on neurotransmitter signalling. All experiments were performed in rat
| Compound | Stage | Area | Effect on neuronal development of developing CNS in rat | Refs |
|---|---|---|---|---|
| Δ9-THC, WIN-55,212-2 | E14 | Mesencephalic neuron cultures | Modulates the activity of TH | 155,158,199-201 |
| Δ9-THC | E5-P20 | Cerebellum | Modulates transcription of glutamate receptor subunits | 172 |
| Δ9-THC, WIN 55,212-2 | E15-P9 | Cortex | Reduces levels of glutamatergic neurotransmitters | 195,197 |
| WIN55,212-2 | E5-20 | Hippocampal neuron cultures | Reduction in glutamate outflow | 196 |
| Δ9-THC, WIN 55,212-2 | E15-P9 | Cortex, amygdala | Reduces levels of noradrenergic neurotransmitters | 193,197 |
| Δ9-THC | – | Nucleus accumbens, caudal putamen and rostral dorsal striatum | Inhibition of PENK mRNA expression | 194,202-204 |
| HU-210 | P4 | Cerebellar neurons | GABA mRNA expression | 205 |
| Δ9-THC | – | Diencephalon, hippocampus, midbrain raphe nuclei and septum | Reduces serotonin levels | 171 |
Future perspectives
To summarize, (1) the eCB system is present from conception onwards in the developing CNS; (2) Δ9-THC and other cannabimimetics interfere with the eCB system to cause anencephaly and neurobehavioural deficiencies in the offspring; and (3) pregnant users are now potentially exposed to high potency marijuana and to a new market for ‘synthetic marijuana’ i.e. Spice, a market to which drug enforcement regulations are struggling to adapt, and for which the risks on pregnancy and the developing fetus are not yet understood.
In view of these findings, awareness should be brought to the following: (1) the necessity to better inform potential pregnant users on the availability of high potency marijuana which significantly differs from the marijuana inhaled in the 70s' and 80s', and of synthetic marijuana such as that found in Spice branded products); (2) the necessity to inform users who are planning to conceive, since detrimental effects of marijuana (and potentially cannabimimetics found in Spice branded products), potentially occur already at peri-conceptional age (from conception to week 4 of pregnancy); and (3) the necessity to revise Bills and Legislations in terms of the specificity of banned research chemicals, and to broaden these legislations, as to include potential novel cannabimimetics and cannabinoid research chemicals.
Concluding remarks
The argument that marijuana is a “harmless” drug is no longer valid. Although some of the cannabimimetics and constituents of marijuana appear to be useful in some conditions, the recent advances in registry and statistical evaluation of effects, which now take into account confounding variables, has enabled us to clearly affirm that marijuana is detrimental to pregnancy. This is enhanced by the recent discovery of an eCB system in the developing embryo, a system of which the function is impeded following maternal exposure to marijuana. Most alarmingly, Δ9-THC content of marijuana has increased from 1.3% in the 1970s to an average content of 8.2% in modern preparations, with some preparations containing up to up to 37.2% Δ9-THC.[13] Marijuana has regained its popularity from the 1970s, especially amongst teens/young adults, where it has regained its social and cultural status as the most popular drug of abuse; As a result, this poses not only a potential risk for the fetus of pregnant teen/young adults, but also for teens in general.[210–212] Clearly, additional awareness should be provided to teens and young adults in particular, concerning the health deficits caused by marijuana, especially given the current debates on rescheduling, legalization and decriminalization of marijuana based on its medical applications.[213,214] Finally, very little is known on the potentially detrimental effects of gestational exposure to the psychoactive constituents of Spice blends and to cannabinoid research chemicals and further studies are warranted.
Acknowledgments
Grant sponsor: Our research was supported by Ruth L. Kirschstein, National Research Service Award in Neurosciences F32DA021977 from the National Institute on Drug Abuse (DP), and NIH grants DA020531, MH085079 and AA018709 (KYV).
Appendix
Abbreviations
- ABHD6/12
α/β-hydrolase domain-containing serine hydrolases 6 and 12
- AEA
N-arachydonylethanolamide
- 2-AG
2-arachidonoylglycerol
- AP
anteroposterior
- CNS
central nervous system
- DAGLα
sn-1 specific diacylglycerol lipase, alpha
- DV
dorsoventral
- eCB
endocannabinoid
- ERK1/2
extracellular-signal-regulated kinase 1/2
- FAAH
fatty acid amide hydrolase
- HD
Huntington's Disease
- HH
Hamburger and Hamilton
- Ki
dissociation constant
- LC-MS
liquid chromatography-mass spectrometry
- MAGL
monoacylglycerol lipase
- ML
mediolateral
- NAPE-PLD
N-acyl phosphatidyl ethanolamine phospholipase D
- NAT
Ca2+-dependent N-acyltransferase
- PKA
protein kinase A
- NBDP
National Birth Defects Prevention
- NS/PCs
neural stem/progenitor cells
- PENK
pro-enkephalin
- P
postnatal
- qPCR
real-time quantitative RT-PCR
- RT-PCR
reverse transcription polymerase chain reaction
- SVZ
subcortical proliferative ventricular zone
- Δ9-THC
Δ9-tetrahydrocannabinol
- Δ1-THC
Δ1-tetrahydrocannabinol
- THCAS
cDNA encoding tetrahydrocannabinol synthase
- VZ
ventricular zone
- VTA
ventral tegmentum area
Chemical names
- AACOCF3
1,1,1-Trifluoro-6Z,9Z,12Z,15Z-heneicosateraen-2-one
- AA-5HT
N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-5,8,11, 14-eicosatetraenamide
- ACEA
N-(2-chloroethyl)-5Z,8Z,11Z, 14Z-eicosatetraenamide
- ACPA
N-(cyclopropyl)-5Z,8Z,11Z, 14Z-eicosatetraenamide
- AM-251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1 -(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM-281
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- AM-404
4-hydroxyphenylarachidonylamide
- AM-694
1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole
- AM-1235
[1-(5-fluoropentyl)-6-nitro-1H-indol-3-yl]-1-naphthalenyl-methanone
- AM-2201
[1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone
- AM-2232
3-(1-naphthalenylcarbonyl)-1H-indole-1-pentanenitrile
- AM-2233
2-iodophenyl[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]-methanone
- (±)-CP-47497
rel-5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]phenol
- (−)-CP-55,940
2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl) phenol
- HU-210
(6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1 -ol
- JNJ 1661010
N-phenyl-4-(3-phenyl-1,2,4-thiadiazol-5-yl)-1-piperazinecarboxamide
- JWH-018
1-pentyl-3-(1-naphthoyl)indole
- JWH-073
naphthalen-1-yl-(1-butylindol-3-yl)methanone
- JWH-250
2- (2-Methoxyphenyl)-1-(1-pentylindol-3-yl)ethanone
- JWH-398
(4-chloronaphthalen-1-yl)(1-pentylindolin-3-yl)-methanone
- JZL 184
4-[bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester
- LY 2183240
5-[(1,1′-biphenyl]-4-yl) methyl]-N,N-dimethyl-1H-tetrazole-1-carboxamide
- MAFP
(5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraenyl-methyl ester phosphonofluoridic acid
- Nabilone
(6aR,10aR)-rel-1-hydroxy-6, 6-dimethyl-3-(2-methyloctan-2-yl)-(dibenzo[b,d]pyran-1-ol)
- NADA
N-[2-(3,4-dihydroxyphenyl)ethyl]-5Z,8Z,11Z,14Z-eicosatetraenamide
- NAM
1-(5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraen-1-yl-1H-pyrrole-2,5-dione
- O-2545-HCl
(6aR,10aR)-6a,7,10, 10a-tetrahydro-3-[5-(1H-imidazol-1-yl)-1,1-dimethylpentyl]-6, 6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol
- PF 750
N-phenyl-4-(3-quinolinylmethyl)-1-piperidinecarboxamide
- PF 3845
N-3-pyrdinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl] methyl]-1-piperidine-carboxamide
- PIA
N-(1-methylethyl)-hexadecanamide
- RCS-04
1-pentyl-3-[(4-methoxy)-benzoyl]indole
- RCS-4-C4 homolog
(4-methoxyphenyl)(1-butyl-1H-indol-3-yl)-methanone
- RCS-8
1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole
- SR141716
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- ST4070
1-biphenyl-4-ylethenyl piperidine-1-carboxylate
- Δ9-THC
(-)-(6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol
- URB597
[3-(3-carbamoylphenyl)-phenyl] N-cyclohexylcarbamate
- URB602
biphenyl-3-ylcarbamic acid cyclohexyl ester
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Mental Health Findings. Center for Behavioral Health Statistics and Quality, NSDUH, Series H-39, HHS Publication No. SMA10-4609. 2010 Available at: http://www.oas.samhsa.gov/NSDUH/2k9NSDUH/MH/2k9MHResults.htm [20 April 2012]
- 2.Gaoni Y, Mechoulam R. The isolation and structure of delta-1- tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 3.Harbison RD, Mantilla-Plata B. Prenatal toxicity, maternal distribution and placental transfer of tetrahydrocannabinol. J Pharmacol Exp Ther. 1972;180:1446. [PubMed] [Google Scholar]
- 4.Kennedy JS, Waddell WJ. Whole-body autoradiography of the pregnant mouse after administration of 14C-9-THC. Toxicol Appl Pharm. 1972;22:252. doi: 10.1016/0041-008x(72)90175-5. [DOI] [PubMed] [Google Scholar]
- 5.Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- 6.Anencephaly. Wikipedia. Almazi 2012 Jun 17; Available at: http://en.wikipedia.org/wiki/Anencephaly.
- 7.van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology. 2008;20:60. doi: 10.1097/EDE.0b013e31818e5930. [DOI] [PubMed] [Google Scholar]
- 8.Day NL, Richardson G. Prenatal marijuana use: Epidemiology, methodological issues and infant outcome. In: Chasnoff IJ, editor. Clinics in Perinatology. WB Saunders; Philadelphia: 1991. pp. 77–92. [PubMed] [Google Scholar]
- 9.United Nations Office on Drugs and Crime. Synthetic cannabinoids in herbal products, 2011-06-06. 2012 Apr 24; Available at: http://www.unodc.org/documents/scientific/Synthetic_Cannabinoids.pdf.
- 10.Fattore L, Fratta W. Beyond THC: The new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuro-Psychoph. 2012 Apr 26; doi: 10.1016/j.pnpbp.2012.04.017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seely KA, Prather PL, James LP, Moran JH. Marijuana-based drugs: Innovative therapeutics or designer drugs of abuse? Mol Interv. 2011;11:36. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ElSohly M. Quarterly Report, Potency Monitoring Project, Report 100, December 16, 2007 thru March 15, 2008. National Institute on Drug Abuse, Contract Number N01DA-5-7746. 2012 Apr 21; Available at: http://www.infocenters.co.il/ada/multimedia/full-pdf/marijuana_potency_usa.pdf.
- 14.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 15.Sirikantaramas S, Taura F, Morimoto S, Shoyama Y. Recent advances in Cannabis sativa research: biosynthetic studies and its potential in biotechnology. Curr Pharm Biotechnol. 2007;8:237. doi: 10.2174/138920107781387456. [DOI] [PubMed] [Google Scholar]
- 16.Taura F, Dono E, Sirikantaramas S, Yoshimura K, Shoyama Y, Morimoto S. Production of Δ1-tetrahydrocannabinolic acid by the biosynthetic enzyme secreted from transgenic. Pichia pastoris Biochem Bioph Res Co. 2007;361:675. doi: 10.1016/j.bbrc.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 17.Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Wada Y, Shoyama Y, et al. The gene controlling marijuana psychoactivity: Molecular cloning and heterologous expression of delta-1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J Biol Chem. 2004;279:39767. doi: 10.1074/jbc.M403693200. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Shoyama Y, Aramaki H, Azuma T, Nishioka I. Tetrahydrocannabinolic acid, a genuine substance of tetrahydrocannabinol. Chem Pharm Bull. 1967;15:1075. doi: 10.1248/cpb.15.1075. [DOI] [PubMed] [Google Scholar]
- 19.Pertwee RG. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addict Biol. 2008;13:147. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- 20.Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 22.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 24.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 25.Onaivi ES. Commentary: Functional neuronal CB2 cannabinoid receptors in the CNS. Curr Neuropharmacol. 2011;9:205. doi: 10.2174/157015911795017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert DM, Fowler CJ. The endocannabinoid system: Drug targets, lead compounds, and potential therapeutic applications. J Med Chem. 2005;48:5059. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 28.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 29.Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54:36. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, et al. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001;104:923. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 33.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 34.Alger BE. Endocannabinoids and their implications for epilepsy. Epilepsy Curr. 2004;4:169. doi: 10.1111/j.1535-7597.2004.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. P Natl Acad Sci USA. 2002;99:10819. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf) 2012;204:267. doi: 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121:135. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 41.Laaris N, Good CH, Lupica CR. Δ9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59:121. doi: 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katona I, Sperlágh B, Maglóczky Z, Sántha E, Köfalvi A, Czirják S, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 45.National Institute on Drug Abuse, NIH. Research Report Series: Marijuana Abuse. Publication Number 05-3859, October 2005. 2012 May 17; Available at: http://www.virginia.edu/case/ATOD/RRMarijuana.pdf.
- 46.U.S. Department of Justice Drug Enforcement Administration. Title 21 United States Code (USC) Controlled Substances Act. Pub. L. 91-513, 84 Stat. 1236, enacted October 27, 1970. 2012 May 15; Available at: http://www.deadiversion.usdoj.gov/21cfr/21usc/index.html.
- 47.Kikura-Hanajiri R, Uchiyama N, Goda Y. Survey of current trends in the abuse of psychotropic substances and plants in Japan. Leg Med (Tokyo) 2011;13:109. doi: 10.1016/j.legalmed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 48.European Monitoring Centre for Drugs and Drug Addiction. Understanding the Spice Phenomenon. 2012 Apr 24; Available at: http://www.emcdda.europa.eu/attachements.cfm/att_80086_EN_Spice%20Thematic%20paper%20—%20final%20version.pdf.
- 49.Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF. Hijacking of Basic Research: The Case of Synthetic Cannabinoids. Research Report. Research Triangle Institute Press publication OP-0007-1111. 2012 Apr 24; doi: 10.3768/rtipress.2011.op.0007.1111. Available at: http://www.rti.org/pubs/op-0007-1111-wiley.pdf. [DOI] [PMC free article] [PubMed]
- 50.Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198:31. doi: 10.1016/j.forsciint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Makriyannis A, Deng H. Cannabimimetic indole derivatives. WO Patent No. 01/28557 A1,1-25 PCT/US00/28832, 2001. 2012 Apr 26; Available at: http://worldwide.espacenet.com/publicationDetails/biblio?CC=WO&NR=0128557&KC=&FT=E&locale=en_EP.
- 52.Melvin LS, Milne GM, Johnson MR, Subramaniam B, Wilken GH, Howlett AC. Structure-activity relationships for cannabinoid receptor-binding and analgesic activity: Studies of bicyclic cannabinoid analogs. Mol Pharmacol. 1993;44:1008. [PubMed] [Google Scholar]
- 53.Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ammann J, McLaren JM, Gerostamopoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: part 1 -synthetic cannabinoids. J Anal Toxicol. 2012;36:372. doi: 10.1093/jat/bks048. [DOI] [PubMed] [Google Scholar]
- 55.Compton MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201. [PubMed] [Google Scholar]
- 56.Guhring H, Schuster J, Hamza M, Ates M, Kotalla CE, Brune K. HU-210 shows higher efficacy and potency than morphine after intrathecal administration in the mouse formalin test. Eur J Pharmacol. 2001;429:127. doi: 10.1016/s0014-2999(01)01313-9. [DOI] [PubMed] [Google Scholar]
- 57.Tomiyama K, Funada M. Cytotoxicity of synthetic cannabinoids found in ‘Spice’ products: The role of cannabinoid receptors and the caspase cascade in the NG 108-15 cell line. Toxicol Lett. 2011;207:12. doi: 10.1016/j.toxlet.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. Brit J Pharmacol. 2010;160:585. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molina-Holgado F, Pinteaux E, Heenan L, Moore JD, Rothwell NJ, Gibson RM. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Neurosci. 2005;28:189. doi: 10.1016/j.mcn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bal G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina-Holgado F, Rubio-Araiz A, García-Ovejero D, Williams RJ, Moore JD, Arévalo-Martín A, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- 62.Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H. 1 -Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Weissman A, Milne GM, Melvin LS., Jr Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol. J Pharmacol Exp Ther. 1982;223:516. [PubMed] [Google Scholar]
- 64.Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046. [PubMed] [Google Scholar]
- 65.Shim JY, Welsh WJ, Howlett AC. Homology model of the CB1 cannabinoid receptor: Sites critical for nonclassical cannabinoid agonist interaction. Biopolymers. 2003;71:169. doi: 10.1002/bip.10424. [DOI] [PubMed] [Google Scholar]
- 66.Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995. [PubMed] [Google Scholar]
- 67.Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291:837. [PubMed] [Google Scholar]
- 68.Coulter C, Garnier M, Moore C. Synthetic cannabinoids in oral fluid. J Anal Toxicol. 2011;35:424. doi: 10.1093/anatox/35.7.424. [DOI] [PubMed] [Google Scholar]
- 69.Poklis LJ, Amira D, Wise LE, Wiebelhaus JM, Haggerty BJ, Poklis A. Detection and disposition of JWH-018 and JWH-073 in mice after exposure to “Magic Gold” smoke. Forensic Sci Int. 2012;220:91. doi: 10.1016/j.forsciint.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 2000;60:133. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 71.Möller I, Wintermeyer A, Bender K, Jübner M, Thomas A, Krug O. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2011;3:609. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- 72.Teske J, Weller JP, Fieguth A, Rothämel T, Schulz Y, Tröger HD. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chrom. 2010;878:2659. doi: 10.1016/j.jchromb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Beuck S, Möller I, Thomas A, Klose A, Schlörer N, Schänzer W, et al. Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Anal Bioanal Chem. 2011;2:493. doi: 10.1007/s00216-011-4931-5. [DOI] [PubMed] [Google Scholar]
- 74.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kacinko SL, Xu A, Homan JW, McMullin MM, Warrington DM, Logan BK. Development and validation of a liquid chromatography-tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. J Anal Toxicol. 2011;35:386. doi: 10.1093/anatox/35.7.386. [DOI] [PubMed] [Google Scholar]
- 76.Kuster JE, Stevenson JI, Ward SJ, D'Ambra TE, Haycock DA. Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids. J Pharmacol Exp Ther. 1993;264:1352. [PubMed] [Google Scholar]
- 77.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443. [PubMed] [Google Scholar]
- 78.Hampson RE, Miller F, Palchik G, Deadwyler SA. Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: Implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology. 2011;60:944. doi: 10.1016/j.neuropharm.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uchiyama N, Kikura-Hanajiri R, Goda Y. Identification of a novel cannabimimetic phenylacetylindole, cannabipiperidiethanone, as a designer drug in a herbal product and its affinity for cannabinoid CB and CB receptors. Chem Pharm Bull (Tokyo) 2011;59:1203. doi: 10.1248/cpb.59.1203. [DOI] [PubMed] [Google Scholar]
- 80.Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, et al. Spice: A never ending story? Forensic Sci Int. 2009;191:58. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. Spice' and other herbal blends: Harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 82.Office of Diversion Control US Department of Justice Drug Enforcement Administration. Drugs and Chemicals of Concern. HU-210. 2012 Apr 30; Available at: http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_hu210.htm.
- 83.House of Representatives, 112th Congress. H.R.1254 [Report No. 112-295, Parts I and III] To Amend the Controlled Substances Act to Place Synthetic Drugs in Schedule I. 2012 Apr 26; Available from: http://www.gpo.gov/fdsys/pkg/BILLS-112hr1254ih/pdf/BILLS-112hr1254ih.pdf.
- 84.Committee Reports, 112th Congress. House Report 112-295 - Part 2: Synthetic Drug Control Act of 2011, The Library of Congress-Thomas. 2012 Apr 23; Available at: http://thomas.loc.gov/cgi-bin/cpquery/2?&sid=cp112KGP4l&refer=&r_n=hr295p2.112&db_id=112&item=2&&sid=cp112KGP4l&r_n=hr295p2.112&dbname=cp112&hd_count=2&item=2&&sel=TOC_63972&.
- 85.Duncan G. Emerging Substances of Abuse - K2/Spice (Synthetic Cannabinoids) 2012 Apr 23; Available at: http://www.slideshare.net/Guedde/spicek2-synthetic-cannabinoids-6988186.
- 86.National Conference of State Legislatures. Cannabinoids (a.ka. “K2” / “Spice”) Enactments. 2012 Apr 23; Available at: http://www.ncsl.org/issues-research/justice/synthetic-cannabinoids-enactments.aspx.
- 87.Office of Diversion Control US Department of Justice Drug Enforcement Administration. Controlled Substances - Alphabetical Order. 2012 Apr 29; Available at: http://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf.
- 88.United States Drug Enforcement Administration. Chemicals Used in “Spice” and “K2” Type Products Now Under Federal Control and Regulation. United States Drug Enforcement Administration, Publication Number: 202-307-79772011. 2012 Apr 25; Available at: http://www.justice.gov/dea/pubs/pressrel/pr030111.html.
- 89.Thrive Chemical Research. 2012 Apr 26; Available at: http://www.thrivechem.com/o-2545-hydrochloride-7509-p.asp.
- 90.Martin BR, Wiley JL, Beletskaya I, Sim-Selley LJ, Smith FL, Dewey WL, et al. Pharmacological characterization of novel water-soluble cannabinoids. J Pharmacol Exp Ther. 2006;318:1230. doi: 10.1124/jpet.106.104109. [DOI] [PubMed] [Google Scholar]
- 91.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427. [PubMed] [Google Scholar]
- 92.Deng H, Gifford AN, Zvonok AM, Cui G, Li X, Fan P, et al. Potent cannabinergic indole analogues as radioiodinatable brain imaging agents for the CB1 cannabinoid receptor. J Med Chem. 2005;48:6386. doi: 10.1021/jm050135l. [DOI] [PubMed] [Google Scholar]
- 93.King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachi-donoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szabo M, Agostino M, Malone DT, Yuriev E, Capuano B. The design, synthesis and biological evaluation of novel URB602 analogues as potential monoacylglycerol lipase inhibitors. Bioorg Med Chem Lett. 2011;21:6782. doi: 10.1016/j.bmcl.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 96.Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Järvinen T, et al. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 97.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, et al. N-arachidonyl maleimide potentiates the pharmalocogical and biochemical effects of the endocannabinoid 2-arachidonylyglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, et al. Blockade of 2-arachidonylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cisneros JA, Björklund E, González-Gil I, Hu Y, Canales A, Medrano FJ, et al. Structure–activity relationship of a new Series of reversible dual monoacylglycerol lipase/fatty acid amide hydrolase inhibitors. J Med Chem. 2012;55:824. doi: 10.1021/jm201327p. [DOI] [PubMed] [Google Scholar]
- 102.Minkkilä A, Myllymäki MJ, Saario SM, Castillo-Melendez JA, Koskinen AM, Fowler CJ, et al. The synthesis and biological evaluation of para-substituted phenolic N-alkyl carbamates as endocannabinoid hydrolyzing enzyme inhibitors. Eur J Med Chem. 2009;44:2994. doi: 10.1016/j.ejmech.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Blankman JL, Simon M, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonyl-glycerol. Chem Biol. 2007;14:1347. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li W, Blankman JL, Cravatt BF. A functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. J Am Chem Soc. 2007;129:9594. doi: 10.1021/ja073650c. [DOI] [PubMed] [Google Scholar]
- 105.Marrs WR, Horne EA, Ortega-Gutierrez S, Cisneros JA, Xu C, Lin YH, et al. Dual inhibition of alpha/beta-hydrolase domain 6 and fatty acid amide hydrolase increases endocannabinoid levels in neurons. J Biol Chem. 2011;286:28723. doi: 10.1074/jbc.M110.202853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vann RE, Walentiny DM, Burston JJ, Tobey KM, Gamage TF, Wiley JL. Enhancement of the behavioral effects of endogenous and exogenous cannabinoid agonists by phenylmethyl sulfonyl fluoride. Neuropharmacology. 2012;62:1019. doi: 10.1016/j.neuropharm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koutek B, Prestwich GD, Howlett AC, Chin SA, Salehani D, Akhavan N, et al. Inhibitors of arachidonoyl ethanolamide hydrolysis. J Biol Chem. 1994;269:22937. [PubMed] [Google Scholar]
- 108.Bisogno T, Melck D, De Petrocellis L, Bobrov MY, Gretskaya NM, Bezuglov VV, et al. Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Comm. 1998;248:515. doi: 10.1006/bbrc.1998.8874. [DOI] [PubMed] [Google Scholar]
- 109.Keith JM, Apodaca R, Xiao W, Seierstad M, Pattabiraman K, Wu J, et al. Thiadiazolopiperazinyl ureas as inhibitors of fatty acid amide hydrolase. Bioorg Med Chem Lett. 2008;18:4838. doi: 10.1016/j.bmcl.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 110.Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, et al. Identification of a high-affinity binding site involved in the transport of endocannabinoids. P Natl Acad Sci USA. 2005;102:17852. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Petrocellis L, Melck D, Ueda N, Maurelli S, Kurahashi Y, Yamamoto S, et al. Novel inhibitors of brain, neuronal, and basophilic anandamide amidohydrolase. Biochem Biophys Res Commun. 1997;231:82. doi: 10.1006/bbrc.1997.6000. [DOI] [PubMed] [Google Scholar]
- 112.Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, et al. Methyl arachidonyl fluorophosphate: A potent irreversible inhibitor of anandamide amidase. Biochem Pharmacol. 1997;53:255. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- 113.Bisogno T, Melck D, Bobrov MY, Gretskaya NM, Bezuglov VV, De Petrocellis L, et al. N-acyl-dopamines: Novel synthetic CB(1) can-nabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817. [PMC free article] [PubMed] [Google Scholar]
- 114.Jonsson KO, Vandevoorde S, Lambert DM, Tiger G, Fowler CJ. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Brit J Pharmacol. 2001;133:1263. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahn K, Johnson DS, Fitzgerald LR, Liimatta M, Arendse A, Stevenson T, et al. Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry. 2007;46:13019. doi: 10.1021/bi701378g. [DOI] [PubMed] [Google Scholar]
- 117.Mileni M, Johnson DS, Wang Z, Everdeen DS, Liimatta M, Pabst B, et al. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. P Natl Acad Sci USA. 2008;105:12820. doi: 10.1073/pnas.0806121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gattinoni S, De Simone C, Dallavalle S, Fezza F, Nannei R, Amadio D, et al. Enol carbamates as inhibitors of fatty acid amide hydrolase (FAAH) endowed with high selectivity for FAAH over the other targets of the endocannabinoid system. Chem Med Chem. 2010;5:357. doi: 10.1002/cmdc.200900472. [DOI] [PubMed] [Google Scholar]
- 119.Caprioli A, Coccurello R, Rapino C, Di Serio S, Tommaso M, Vertechy M, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. J Pharmacol Exp Ther. 2012;342:188. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- 120.Roughley SD, Browne H, Macias AT, Benwell K, Brooks T, D'Allessandro J, et al. Fatty acid amide hydrolase inhibitors. 3: Tetra-substituted azetidine ureas with in vivo activity. Bioorg Med Chem Lett. 2012;22:901. doi: 10.1016/j.bmcl.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 121.Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17:179. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 122.Tambaro S, Bortolato M. Cannabinoid-related agents in the treatment of anxiety disorders: current knowledge and future perspectives. Recent Pat. CNS Drug Discov. 2012;7:25. doi: 10.2174/157488912798842269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci. 2009;1:347. doi: 10.1007/978-3-540-88955-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vinod KY, Xie S, Psychoyos D, Hungund BL, Cooper TB, Tejani-Butt SM. Dysfunction in Fatty Acid amide hydrolase is associated with depressive-like behavior in wistar kyoto rats. PLoS One. 2012;7:e36743. doi: 10.1371/journal.pone.0036743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;5:607. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 127.Curtis A, Mitchell I, Pavel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington's disease. Mov Disord. 2009;24:2254. doi: 10.1002/mds.22809. [DOI] [PubMed] [Google Scholar]
- 128.Spratt NT. Localization of the prospective neural plate in the early chick blastoderm. J Exp Zool. 1952;120:109. [Google Scholar]
- 129.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 130.Wittler L, Kessel M. The acquisition of neural fate in the chick. Mech Dev. 2004;121:1031. doi: 10.1016/j.mod.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 131.Balinsky BL. An Introduction to Embryology. WB Saunders Co.; New York: 1975. [Google Scholar]
- 132.Carnegie Stages Comparison. University of South Wales. 2012 May 29; Available at: http://embryology.med.unsw.edu.au/OtherEmb/CStages.htm.
- 133.Psychoyos D, Hungund B, Finnell RH. Endocannabinoid signaling in early neurodevelopment: Effect of gestational Δ9-THC exposure. Open Neuropsychopharmacol J. 2009;2:64. [Google Scholar]
- 134.Corbin JG, Gaiano N, Juliano SL, Poluch S, Stancik E, Haydar TF. Regulation of neural progenitor cell development in the nervous system. J Neurochem. 2008;106:2272. doi: 10.1111/j.1471-4159.2008.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Acampora D, Barone P, Simeone A. Otx genes in corticogenesis and brain development. Cereb Cortex. 1999;9:533. doi: 10.1093/cercor/9.6.533. [DOI] [PubMed] [Google Scholar]
- 136.Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Psychoyos D, Vinod KY, Cao J, Xie S, Hyson RL, Wlodarczyk B, et al. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Res B Dev Reprod Toxicol. 2012;95:137. doi: 10.1002/bdrb.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Migliarini B, Carnevali O. A novel role for the endocannabinoid system during zebrafish development. Mol Cell Endocrinol. 2009;299:172. doi: 10.1016/j.mce.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 139.Buckley NE, Hansson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1998;82:1131. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- 140.Begbie J, Doherty P, Graham A. Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J Anat. 2004;205:213. doi: 10.1111/j.0021-8782.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Christiansen J, Coles E, Robinson V, Pasini A, Wilkinson DG. Screening from a subtracted embryonic chick hindbrain cDNA library: Identification of genes expressed during hindbrain, midbrain and cranial neural crest development. Mech Dev. 2001;102:119. doi: 10.1016/s0925-4773(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 142.Galve-Roperh I, Palazuelos J, Aguado T, Guzman M. The endocannabinoid system and the regulation of neural development: Potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2009;259:371. doi: 10.1007/s00406-009-0028-y. [DOI] [PubMed] [Google Scholar]
- 143.Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Ben-Shabat S. The endocannabinoid system during development: Emphasis on perinatal events and delayed effects. Vitam Horm. 2009;81:139. doi: 10.1016/S0083-6729(09)81006-6. [DOI] [PubMed] [Google Scholar]
- 144.Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology. 2011;214:5. doi: 10.1007/s00213-010-1892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox Res. 2004;6:389. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- 146.Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 147.Anavi-Goffer S, Mulder J. The polarised life of the endocannabinoid system in CNS development. Chembiochem. 2009;10:1591. doi: 10.1002/cbic.200800827. [DOI] [PubMed] [Google Scholar]
- 148.Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008;286:S84. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 149.Keimpema E, Barabas K, Morozov YM, Tortoriello G, Torii M, Cameron G, et al. Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J Neurosci. 2010;30:13992. doi: 10.1523/JNEUROSCI.2126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 151.Biegon A, Kerman IA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- 152.Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 153.Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 154.Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez-Ruiz JJ, Berrendero F, Hernandez ML, Romero J, Ramos JA. Role of endocannabinoids in brain development. Life Sci. 1999;65:725. doi: 10.1016/s0024-3205(99)00295-7. [DOI] [PubMed] [Google Scholar]
- 156.Vitalis T, Lainé J, Simon A, Roland A, Leterrier C, Lenkei Z. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurones and negatively regulates neurite growth in vitro. Eur J Neurosci. 2008;28:1705. doi: 10.1111/j.1460-9568.2008.06484.x. [DOI] [PubMed] [Google Scholar]
- 157.Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. P Natl Acad Sci USA. 2008;105:8760. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hernández M, Berrendero F, Suárez I, García-Gil L, Cebeira M, Mackie K, et al. Cannabinoid CB(1) receptors colocalize with tyrosine hydroxylase in cultured fetal mesencephalic neurons and their activation increases the levels of this enzyme. Brain Res. 2000;857:56. doi: 10.1016/s0006-8993(99)02322-7. [DOI] [PubMed] [Google Scholar]
- 159.Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;19:I78. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Berrendero F, García-Gil L, Hernández ML, Romero J, Cebeira M, de Miguel R, et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 161.Nilsson O, Jacobsson SO, Fowler CJ. Cannabinoid CB1 receptor activation does not prevent the toxicity of glutamate towards embryonic chick telencephalon primary cultures. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:245. doi: 10.1016/s1532-0456(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 162.Wu CS, Zhu J, Wager-Miller J, Wang S, O'Leary D, Monory K, et al. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32:693. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. P Natl Acad Sci USA. 2005;102:19115. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, et al. Hard- wiring the brain: Endocannabinoids shape neuronal connectivity. Science. 2007;316:1212. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 166.Kim HJ, Waataja JJ, Thayer SA. Cannabinoids inhibit network-driven synapse loss between hippocampal neurones in culture. J Pharmacol Exp Ther. 2008;325:850. doi: 10.1124/jpet.107.131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol. 1998;54:459. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- 168.Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Watson S, Chambers D, Hobbs C, Doherty P, Graham A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci. 2008;38:89. doi: 10.1016/j.mcn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 171.Molina-Holgado F, Amaro A, Gonzalez MI, Alvarez FJ, Leret ML. Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur J Pharmacol. 1996;316:39. doi: 10.1016/s0014-2999(96)00753-4. [DOI] [PubMed] [Google Scholar]
- 172.Suarez I, Bodega G, Fernandez-Ruiz J, Ramos JA, Rubio M, Fernandez B. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and perinatal delta9-tetrahydrocannabinol exposure. Cerebellum. 2004;3:66. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- 173.Persaud TV, Ellington AC. Cannabis in early pregnancy. Lancet. 1967;2:1306. doi: 10.1016/s0140-6736(67)90416-3. [DOI] [PubMed] [Google Scholar]
- 174.Jonega MG. Effects of delta9-tetrahydrocannabinol on hamster fetuses. J Toxicol Environ Health. 1977;2:1031. doi: 10.1080/15287397709529501. [DOI] [PubMed] [Google Scholar]
- 175.Geber WF, Schramm LC. Effect of marihuana extract on fetal hamsters and rabbits. Toxicol Appl Pharmacol. 1969;14:276. doi: 10.1016/0041-008x(69)90108-2. [DOI] [PubMed] [Google Scholar]
- 176.Mantilla-Plata B, Clewe CL, Harbison HD. 9-tetrahydrocannabinol-induced changes in prenatal growth and development of animals. Toxicol Appl Pharmacol. 1975;33:333. doi: 10.1016/0041-008x(75)90099-x. [DOI] [PubMed] [Google Scholar]
- 177.Rosenkrantz H. Effects of cannabis on fetal development of rodents. Adv Biosc. 1978;22:479. doi: 10.1016/b978-0-08-023759-6.50042-1. [DOI] [PubMed] [Google Scholar]
- 178.Psychoyos D, Hungund B, Cooper T, Finnell RH. A cannabinoid analogue of Delta9-tetrahydrocannabinol disrupts neural development in chick. Birth Defects Res B Dev Reprod Toxicol. 2008;83:477. doi: 10.1002/bdrb.20166. [DOI] [PubMed] [Google Scholar]
- 179.Wang H, Xie H, Dey SK. Endocannabinoid signaling directs periimplantation Events. AAPS J. 2006;8:E425. doi: 10.1007/BF02854916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park B, McPartland JM, Glass M. Cannabis, cannabinoids and reproduction. Prostaglandins Leukot Essent Fatty Acids. 2004;70:189. doi: 10.1016/j.plefa.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 181.Fried PA, Makin JE. Neonatal behavioral correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol. 1987;9:1. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- 182.Richardson GA, Day NL, Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: Infant mental and motor development. Neurotoxicol Teratol. 1995;17:479. doi: 10.1016/0892-0362(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 183.El Marroun H, Hudziak JJ, Tiemeier H, Creemers H, Steegers EA, Jaddoe VW. Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend. 1990;118:470. doi: 10.1016/j.drugalcdep.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Fried PA, Watkinson B. Cognitive development: memory and verbal outcome measures were negatively associated with prenatal marijuana use 36- and 48-month neurobehavioural follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. 1990;11:49. [PubMed] [Google Scholar]
- 185.Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254. doi: 10.1097/CHI.0b013e318160b3f0. [DOI] [PubMed] [Google Scholar]
- 186.Stein MT, Drahota A, Chavira DA. Ian: a 7-year old with prenatal drug exposure and early exposure to family violence. J Dev Behav Pediatr. 2008;29:512. doi: 10.1097/DBP.0b013e3181903153. [DOI] [PubMed] [Google Scholar]
- 187.Goldschmidt L, Richardson GA, Cornelius MD, Day NL. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 2004;26:521. doi: 10.1016/j.ntt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 188.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 189.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, et al. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 190.Gray KA, Day NL, Leech S, Richardson GA. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27:439. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 191.Smith AM, Fried PA, Hogan MJ, Cameron I. Effects of prenatal marijuana on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol. 2004;26:533. doi: 10.1016/j.ntt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 192.Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 193.Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 194.Spano MA, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 195.Antonelli T, Tomasini MC, Tattoli M, Ferraro L. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex. 2005;15:2013. doi: 10.1093/cercor/bhi076. [DOI] [PubMed] [Google Scholar]
- 196.Mereu G, Fà M, Ferraro L, Antonelli T, Tattoli M, Ghiglieri V. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. P Natl Acad Sci USA. 2003;100:4915. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, Ubaldi M, et al. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol. 2007;12:485. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 198.Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratù MR, et al. Effects of perinatal exposure to delta-9-tetrahydro-cannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl) 2008;198:529. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 199.Rodriguez de Fonseca F, Cebeira M, Fernandez-Ruiz JJ, Navarro M, Ramos JA. Effects of pre- and perinatal exposure to hashish extracts on the ontogeny of brain dopaminergic neurones. Neuroscience. 1991;43:713. doi: 10.1016/0306-4522(91)90329-m. [DOI] [PubMed] [Google Scholar]
- 200.Bonnin A, de Miguel R, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. The prenatal exposure to delta 9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci. 1995;56:2177. doi: 10.1016/0024-3205(95)00205-k. [DOI] [PubMed] [Google Scholar]
- 201.Bonnin A, de Miguel R, Castro JG, Ramos JA, Fernandez-Ruiz JJ. Effects of perinatal exposure to delta 9-tetrahydrocannabinol on the fetal and early postnatal development of tyrosine hydroxylase-containing neurones in rat brain. J Mol Neurosci. 1996;7:291. doi: 10.1007/BF02737066. [DOI] [PubMed] [Google Scholar]
- 202.Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Fernández-Ruiz JJ, et al. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavioural and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998;807:101. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- 203.Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 204.Perez-Rosado A, Manzanares J, Fernandez-Ruiz J, Ramos JA. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: Sex-dependent differences. Brain Res Dev Brain Res. 2000;120:77. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 205.Trazzi S, Steger M, Mitrugno V, Bartesaghi R, Ciani E. CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/Glycogen synthase kinase-3β/β-Catenin signaling. J Biol Chem. 2010;285:10098. doi: 10.1074/jbc.M109.043711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Olenik C, Meyer DK. Development of proenkephalin gene expression in rat neocortex: A non-radioactive in situ hybridization study. Brain Res Mol Brain Res. 1997;44:83. doi: 10.1016/s0169-328x(96)00190-8. [DOI] [PubMed] [Google Scholar]
- 207.Sullivan MV, Hamilton CR. Memory establishment via the anterior commissure of monkeys. Physiol Behav. 1973;11:873. doi: 10.1016/0031-9384(73)90283-7. [DOI] [PubMed] [Google Scholar]
- 208.Golub MS, Sassenrath EN, Chapman CF. Regulation of visual attention in offspring of female monkeys treated chronically with D-9-tetrahydrocannabinol. Dev Psychobiol. 1981;14:507. doi: 10.1002/dev.420140603. [DOI] [PubMed] [Google Scholar]
- 209.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioural and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 210.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43:189. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Br Med J. 2002;325:1212. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. Br Med J. 2002;325:1195. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.California Senate Bill 420 (HS 11362.7) on Medical Marijuana Implementation. 2012 May 16; Available at: http://info.sen.ca.gov/pub/03-04/bill/sen/sb_0401-0450/sb_420_bill_20031012_chaptered.html.
- 214.The National Organization for the Reform of Marijuana Laws (NORML) 2008 updated. Available at: http://norml.org/ [15 May 2012]


