Abstract
Background
Alcohol use disorders (AUD) have long been considered to be some of the most disabling mental disorders; however, empirical data on the burden of disease associated with AUD have been sparse. The objective of this article is to quantify the burden of disease (in disability-adjusted life years [DALYs] lost), deaths, years of life lost due to premature mortality (YLL), and years of life lost due to disability (YLD) associated with AUD for the United States in 2005.
Methods
Statistical modeling was based on epidemiological indicators derived from the National Epidemiologic Survey on Alcohol and Related Conditions. Formal consistency analyses were applied. Risk relations were taken from recent meta-analyses and the disability weights from the burden of disease study of the National Institutes of Health. Monte Carlo simulations were used to derive confidence intervals. All analyses were performed by sex and age. Sensitivity analyses were undertaken on key indicators.
Results
In the United States in 2005, 65,000 deaths, 1,152,000 YLL, 2,443,000 YLD, and 3,595,000 DALYs were associated with AUD. For individuals 18 years of age and older, AUD were associated with 3% of all deaths (5% for men and 1 % for women), and 5% of all YLL (7% for men and 2% for women). The majority of the burden of disease associated with AUD stemmed from YLD, which accounted for 68% of DALYs associated with AUD (66% for men and 74% for women). The youngest age group had the largest proportion of DALYs associated with AUD stemming from YLD.
Conclusions
Using data from a large representative survey (checked for consistency) and by combining these data with the best available evidence, we found that AUD were associated with a larger burden of disease than previously estimated. To reduce this disease burden, implementation of prevention interventions and expansion of treatment are necessary.
Keywords: Alcohol Use Disorders, Mortality, Burden of Disease, Disability-Adjusted Life Years, United States
Alcohol consumption is one of the most important global risk factors for the burden of disease (Lim et al., 2012; Rehm et al., 2009; World Health Organization, 2009). Alcohol use disorders (AUD), which include alcohol dependence (AD) and alcohol abuse (or in the International Classification of Diseases [ICD] the harmful use of alcohol), were identified as the largest disease category contributing to the alcohol-attributable global burden of disease for the year 2004, making up approximately one-third of this burden (Rehm et al., 2009). These are the latest data available on the global burden of AUD, as the new Global Burden of Disease Study 2010 (GBD 2010) did not estimate the burden of alcohol abuse (Vos et al., 2012). For the United States, in addition to an older burden of disease study (Michaud et al., 2006), there have been several efforts to estimate alcohol-attributable deaths and years of life lost due to premature mortality (YLL; Danaei et al., 2009; McGinnis and Foege, 1993; Mokdad et al., 2000; Shield et al., 2013), but no study has tried to comprehensively examine the burden of disease associated with AUD. The current study tries to fill this gap.
In estimating the burden of disease and injury associated with AUD, we used (i) the last available data sources and (ii) a bottom-up approach focusing on U.S. data. Most of the estimates of the burden of AUD to date (Rehm et al., 2004; World Health Organization, 2009), including the new estimate for AD from the GBD 2010 (Vos et al., 2012), have been based on the 1998 meta-analyses of mortality associated with AD (Harris and Barraclough, 1998). However, more recent research has shown much higher standardized mortality rates (Roerecke and Rehm, 2013).
In addition, we used U.S.-specific estimates for disability weights (DWs) rather than the current global burden estimates from the GBD 2010 (Salomon et al., 2012). As DWs measure decrements in health associated with certain health states and as health states are impacted by social determinants, it is doubtful that the level of disability of the same health states can be considered the same in different regions of the world. Thus, global weights may not apply to the United States (Rehm and Frick, 2010; Üstün et al., 1999a,b).
To summarize, the aim of this study was to measure the burden of disease and injury associated with AUD based on the most up-to-date and accurate data for the United States. We used the age- and sex-specific AUD-associated mortality estimates from the most recent set of meta-analyses (Roerecke and Rehm, 2013) and the DWs derived from the U.S. National Institutes of Health (NIH) burden of disease study as the basis for our analysis (Rehm and Frick, 2013).
Materials and Methods
All results were based on population and mortality data for the United States for 2005. Population data were obtained from the United Nations (http://esa.un.org/wpp/unpp/panel_population. htm), and numbers of deaths were obtained from the National Center for Health Statistics (http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_10.pdi). Data from Waves 1 and 2 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) were used to estimate incidence, prevalence, duration, and the absolute number of people with AUD in the U.S. population (Hasin et al., 2007). U.S.-specific DWs for AUD were taken from Rehm and Frick (2013) and were based on the health state description from the review of Samokhvalov and colleagues (2010).
U.S.-specific life expectancies were taken from Arias and colleagues (2010). We used age- and sex-specific estimates when they were available. Using U.S.-specific data, we estimated the burden of disease for AUD in the United States for the year 2005. The exact procedures are detailed below.
Prevalence, Treatment Rate, Incidence, and Duration of AUD in the United States
We used data from Wave 1 of the NESARC, a large U.S. general population survey, to estimate the 12-month prevalence of AUD (Hasin et al., 2007). The Wave 1 NESARC, conducted in 2001 to 2002, surveyed a representative sample (n = 43,093, response rate = 81.0%) of the adult (18 years of age and older) population of the United States, oversampling Blacks, Hispanics, and young adults 18 to 24 years of age. General population surveys underestimate prevalence of AUD and especially of severe AUD, as the sampling frame does not include some of the special or marginalized populations (Shield and Rehm, 2012) where there is a multifold prevalence of AUD, such as people in hospitals (De Wit et al., 2010; Roche et al., 2006) including psychiatric hospitals, the homeless (Fazel et al., 2008), and people in prisons (Fazel et al., 2006).
The NESARC had a longitudinal component with n = 34,653 of the original respondents being re-interviewed in 2004 to 2005 (86.7% of those eligible for re-interviewing were re-interviewed, for a cumulative response rate of 70.2%). From these longitudinal data, we could empirically estimate the 12-month incidence of AUD (Grant et al., 2009).
To estimate the proportion of people with AUD in treatment (treatment rate), we estimated the prevalence of past-year treatment from the NESARC sample (for further discussion, see Cohen et al., 2007; Hasin et al., 2007). Using the same conservative definition as Hasin and colleagues (2007), 5.47% of people with AUD were estimated to be in treatment for AUD (excluding Alcoholics Anonymous, employee assistance programs, assistance from clergy or other religious figures, and “other” nonspecified sources of care). While the treatment rates by sex were similar, there were considerable differences in treatment rates by age, with people 35 to 44 years of age having the highest treatment rate (7.53%). This treatment rate may be an underestimate, as it is survey based and, as described above, general population surveys do not capture some of the populations with a high prevalence of AUD, particularly severe AUD.
Estimation of the duration of AUD is challenging. First, for any episode of AUD, where both AD and alcohol abuse were included, the relative weight of AD and alcohol abuse could not be determined from the NESARC data. Second, given the DSM-IV criteria for alcohol abuse, which can yield a diagnosis based on a small number of discrete, widely spaced events (e.g., drunk driving or legal problems due to alcohol), the duration may not be as meaningful for alcohol abuse as for AD.
As a result, and to be conservative, we decided to base all estimates for years of life lost due to disability (YLD) solely on individuals with AD; YLD for people with alcohol abuse were not included. The NESARC data offered 2 possible estimates of duration of AD. The first was based on the duration of the only or the longest episode as reported by the respondent; the second was based on the length of the interval from the onset of dependence to full remission (cessation of all symptoms as specified in the DSM-IV criteria) or the date of interview, whichever came first. However, when duration estimates are reconciled with incidence and prevalence rates using the DISMOD program inconsistencies are yielded (slightly excessive durations) as duration estimates included time that did not correspond to a dependence diagnosis, that is, when the individual was in partial remission (Barendregt et al., 2003). DISMOD is based on a set of differential equations that describe age-specific epidemiological parameters such as prevalence, incidence, remission, case fatality, and “all other causes” mortality. Based on the conceptual model shown in Fig. S1, there are 4 transition hazards between the age-specific disease states of being healthy (i.e., without the disease under consideration), diseased, dead from the disease, or dead from all other causes: incidence, remission, case fatality, and all other mortality. The parameters of the model depend on each other, and using these relationships, 1 parameter such as incidence can be modeled based on other parameters such as prevalence, case fatality, and duration (for formulas and details, see Barendregt et al., 2003). We used the derived estimates of duration that were consistent with prevalence, incidence and mortality, rather than the empirical estimates, as the derived estimates were not only consistent with other epidemiological indicators, but were also conservative (i.e., shorter duration). Dependent on age, the so derived durations varied between 2.6 and 3.7 years for men and between 1.8 and 2.5 years for women.
Risk Relations Associated with AUD
Mortality risks of AUD were derived from the meta-analyses of prospective and historical cohort studies (Roerecke and Rehm, 2013). In these meta-analyses, people with AUD were identified as either seeking AUD treatment or in general population surveys with validated instruments. Relative risks (RRs) were pooled for 2 mutually exclusive groups. For the first group, which comprised individuals with treated AUD, that is, AUD identified by a physician's diagnosis in treatment samples, mortality risks were separately stratified by age and by sex.
For the second group, people with AUD identified in population surveys (for a listing of all studies see Table S4), age stratification was not possible due to a lack of data. While there were several stratified studies for men to pool, no completely stratified estimates were available for women from population studies. We therefore used the pooled estimate for population studies that did not stratify by sex as the estimate for women. Because women with AUD showed markedly higher risk of mortality than men with AUD in all age categories in the treatment samples, the risk for women based on the joint samples is most likely a conservative estimate.
Overall, the mortality risk for individuals in the treatment sample was markedly higher than the risk for those with AUD in the general population (which we consider outside of treatment for this analysis; see also Table S5): For men, pooled RR = 3.38 (95% confidence interval [CI]: 2.98 to 3.84) in treatment samples compared with 1.91 (95% CI: 1.51 to 2.42) for AUD in the general population; for women, RR = 4.57 (95% CI: 3.86 to 5.42) compared with 1.95 (95% CI: 1.49 to 2.55). Thus, 94.5% of people with AUD were assigned an RR of 1.91 (men) or 1.95 (women), and 5.5% of people with AUD were assigned an age-specific RR with an average of 3.38 (men) or 4.57 (women). We conducted sensitivity analyses where all of the sample would receive the lower risk.
Calculations of YLL and YLD
The YLL component was estimated based on deaths associated with AUD and thus required prevalence of AUD and relative mortality risks. The YLD component was based on incidence, average duration, and the associated DW. As explained above, YLD was only based on AD.
Years of Life Lost Due to Premature Mortality
To estimate the number of deaths associated with AUD in the United States, AUD prevalence estimates obtained from NESARC (divided into those in treatment and those outside of treatment) were combined with the respective RR to derive age- and sex-specific AUD-associated fractions (Hanley, 2001; Walter, 1976) using the following formula:
where AAF = the age- and sex-specific AUD-attributable fraction or the proportion of deaths for a given sex and age group associated with AUD; i = the different subpopulations, that is, people with AUD in treatment for AUD, people with AUD outside of treatment, and people with no AUD, all separated by sex and age; pi = the proportion of the total age- and sex-specific population in the various subpopulations i; = the proportion of the total age-and sex-specific population in the various subpopulations i under the counterfactual exposure level, that is, no AD in either group and all RRs = 1, so that the term is actually 1; RRi = the relative risk of death for the subpopulation group i.
The age- and sex-specific AAFs were then multiplied by the respective number of deaths. Sex- and age-specific life expectancies for the age categories used in this analysis were obtained from the U.S. Department of Health and Human Services for 2005 (Arias et al., 2010). YLL are defined as the product of the number of deaths multiplied by the standard life expectancy at the age of death (in years). YLL were calculated using a time-discounting (3%) methodology; that is, each successive year of life expectancy was reduced by 3 % to account for peoples' preference for a healthy year now rather than a healthy year in the future (Mathers et al., 2006).
Years of Life Lost Due to Disability
As described above, we based the disability component (YLD) of the burden of disease solely on AD, using the following standard formula:
where I = the number of incident cases of AD; DW = disability weight; L = the average duration of AD until remission or death (years).
DWs were estimated based on the NIH burden of disease study for the United States (Rehm and Frick, 2010, 2013). As this study did not estimate age- and sex-specific DWs, we used the score of the Mental Component Summary (MCS) of the Short-Form 12-Item Health Survey, version 2 (SF-12; Kosinski et al., 2007; Ware et al., 1996), which was part of the NESARC survey, to obtain adjustments by sex and age. Age- and sex-specific DWs were calculated by adjusting the overall DW by the age- and sex-specific ratio for people with AD derived from the MCS. As a result, the average DW for all people with AD was the same as the DW of the NIH study.
We separated AD into 2 degrees of severity (Samokhvalov et al., 2010) and based the more severe cases on the incident cases of AD entering treatment (Hasin et al., 2007), that is, 5.5% of the people with AD. For a description of the health states underlying the severe and less severe cases, we used the review of Samokhvalov and colleagues (2010). This resulted in a DW of 0.147 for less severe cases, and of 0.656 for the severe cases (see also Table S6). As with YLL calculations, a time-discounting rate of 3% was used (Mathers et al., 2006).
Disability-Adjusted Life Years
Disability-adjusted life years (DALYs) were then calculated by adding YLL and YLD.
Estimation of Uncertainty
Monte Carlo simulations were used to determine the 95% CIs for the age- and sex-specific AAFs and YLD (Gmel et al., 2011b). Of 100,000 randomly generated sets of all underlying parameters were used to compute CIs for AAFs and YLD. The 95% CIs were defined as the range between the 2.5th percentile and 97.5th percentile of the 100,000 randomly generated AAFs and YLD.
Results
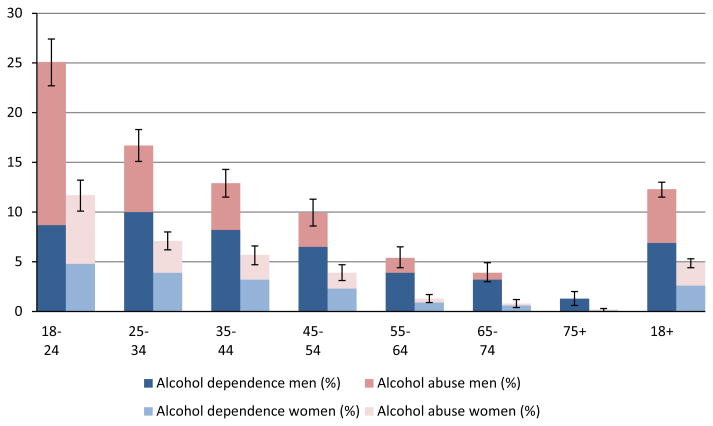
Prevalence of AUD in the U.S. population by sex and age, divided into the categories of AD and alcohol abuse based on NESARC data, showed consistent and expected patterns (Fig. 1). Men in all age categories had a higher prevalence than women, prevalence in both sexes decreased with age, and AD was more prevalent than alcohol abuse, except in the youngest age category (Fig. 1, Table S1). The numbers of deaths associated with AUD were considerable: We estimated that approximately 53,000 male deaths and 12,000 female deaths in the United States in 2005 were associated with AUD (Table 1).
Fig. 1.
Prevalence of alcohol use disorders by category, sex, and age in 2005.
Sources: NESARC data (Wave 1, 2001 to 2002, own calculations), and United Nations population estimates for 2005. For exact numbers and confidence intervals, see Table S1. NESARC, National Epidemiologic Survey on Alcohol and Related Conditions.
Table 1. Number of Deaths Associated with Alcohol Use Disorders in 2005, by Sex and Age.
| Deaths | 18 to 24 | 25 to 34 | 35 to 44 | 45 to 54 | 55 to 64 | 65+ | 18+ |
|---|---|---|---|---|---|---|---|
| Men | 4,785 | 5,135 | 8,102 | 11,941 | 8,361 | 15,117 | 53,442 |
| Lower CI | 3,259 | 3,373 | 5,493 | 7,448 | 4,342 | 6,558 | 30,473 |
| Upper CI | 6,366 | 7,036 | 10,970 | 17,085 | 13,304 | 26,588 | 81,350 |
| Women | 968 | 1,383 | 2,243 | 3,049 | 1,410 | 2,858 | 11,912 |
| Lower CI | 566 | 826 | 1,321 | 1,698 | 688 | 698 | 5,796 |
| Upper CI | 1,499 | 2,110 | 3,309 | 4,689 | 2,380 | 5,822 | 19,808 |
CI, 95% confidence interval.
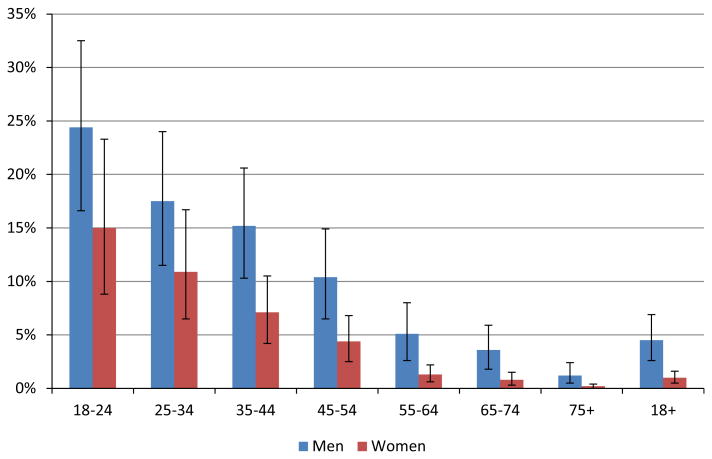
Estimates of the proportion of all deaths associated with AUD in the United States, that is, AUD-associated mortality by sex and age, are provided in Fig. 2 (see also Table S2). Given the sex- and age-specific prevalence of AUD indicated in Fig. 1, the proportion of AUD-associated deaths was higher for men than for women. From a maximum of 24% among men 18 to 24 years of age, the proportion declined steadily with increasing age, amounting to <1% for the age group 75 years and older (both sexes combined). However, the overall toll of AUD-associated mortality was quite high, comprising 4.5% of all-cause mortality for men and 1.0% of all-cause mortality for women (total: 2.7%; details in Table S2). The absolute number of AUD-associated deaths within any 10-year age group was highest among those 45 to 54 years of age for both sexes, where 10.4% of all deaths were associated with AUD in men and 4.4% in women (Tables 1 and S2). The reason for the apparent inconsistency between this observation and the result outlined in Fig. 2 (in which the proportion of AUD-associated deaths is highest in the youngest age group) is that mortality rates increase exponentially over a lifespan, so that at older ages a low AAF can translate into a large number of AUD-associated deaths.
Fig. 2.
Proportion of all deaths associated with alcohol use disorders in 2005, by sex and age. For exact numbers and confidence intervals, see Table S2.
In the sensitivity analysis, based on the general population risk parameters only, 15% less overall mortality was estimated, 47,000 men and 10,000 women.
For the United States in 2005, we estimated that 923,000 male YLL and 229,000 female YLL were associated with AUD (see Table 2). People 45 to 54 years of age also dis played the highest number of YLL. Given the nature of YLL, the younger age groups become proportionally more important (when compared to death as the outcome), and for both sexes, people 35 to 44 years of age were the second largest contributor to YLL. Because AUD often impact people early in their lives, the proportion of AUD-associated YLL of all YLL (total: 4.7%; men: 7.2%; women: 2.0%) is higher than the respective portion for deaths; as well, the ratio of YLL to deaths for AUD is much higher compared with other disease categories, such as most chronic diseases or tobacco use disorders (World Health Organization, 2008).
Table 2. Number of Potential Years of Life Lost to Premature Mortality (YLL) Associated with Alcohol Use Disorders in 2005, by Sex and Age.
| YLL | 18 to 24 | 25 to 34 | 35 to 44 | 45 to 54 | 55 to 64 | 65+ | 18+ |
|---|---|---|---|---|---|---|---|
| Men | 128,692 | 129,397 | 181,555 | 228,572 | 127,664 | 127,267 | 923,147 |
| Lower CI | 87,635 | 85,005 | 123,093 | 142,562 | 66,298 | 57,990 | 562,583 |
| Upper CI | 171,203 | 177,295 | 245,815 | 327,041 | 203,127 | 218,718 | 1,343,199 |
| Women | 26,884 | 36,116 | 53,060 | 62,922 | 23,707 | 25,914 | 228,603 |
| Lower CI | 15,703 | 21,573 | 31,235 | 35,040 | 11,572 | 7,524 | 122,647 |
| Upper CI | 41,622 | 55,117 | 78,258 | 96,756 | 40,007 | 51,265 | 363,026 |
CI, 95% confidence interval.
In terms of YLD (Table 3), in the United States in 2005, men experienced a total of 1,785,000 YLD associated with AUD (as estimated via AD only), while women experienced 658,000 YLD. This difference by sex is not surprising given the higher incidence and longer duration of AD (data not shown) in men. For both sexes, the burden of disability is highest in the younger age groups where the incidence of AUD is highest. This pattern exists even though the DWs for AD in the younger age groups are lower. For both sexes, a considerably larger burden of AD stems from YLD than from YLL.
Table 3. Number of Years of Life Lost Due to Disability (YLD) Associated with Alcohol Dependence in 2005, by Sex and Age.
| YLD | 18 to 24 | 25 to 34 | 35 to 44 | 45 to 54 | 55 to 64 | 65+ | 18+ |
|---|---|---|---|---|---|---|---|
| Men | 549,529 | 414,803 | 410,789 | 251,119 | 119,903 | 39,054 | 1,785,197 |
| Lower CI | 259,897 | 241,429 | 250,176 | 128,234 | 50,927 | 468 | 931,131 |
| Upper CI | 839,162 | 588,175 | 571,402 | 374,005 | 188,881 | 77,640 | 2,639,264 |
| Women | 204,617 | 156,649 | 158,482 | 86,832 | 47,583 | 3,686 | 657,849 |
| Lower CI | 43,665 | 88,596 | 86,438 | 38,295 | 6,476 | 0 | 263,471 |
| Upper CI | 365,568 | 224,703 | 230,527 | 135,368 | 88,690 | 10,085 | 1,054,940 |
CI, 95% confidence interval.
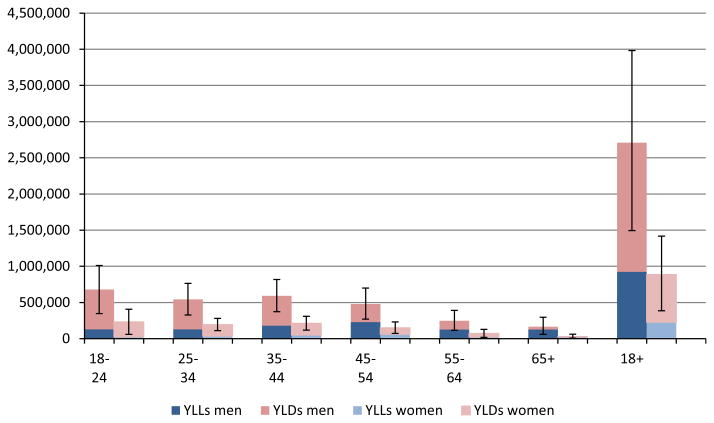
Figure 3 (see also Table S3) provides DALYs by sex and age, as well as the proportion of DALYs made up by YLD (the proportion made up by YLL by definition is 100% minus the proportion made up by YLD). Of the 3,595,000 DALYs associated with AUD in the United States in 2005, a greater burden was caused by disability from AD (YLD accounted for 68% [men 66%; women 74%] of the DALYs associated with AUD) as compared to premature mortality from AD. There is a steep age gradient in the composition of DALYs: The older the person, the lower the proportion of DALYs stemming from YLD. Thus, in young and middle adulthood, up to age 45 in men (but up to age 65 in women), the burden of disease associated with AUD is mainly constituted by its disabling effects, whereas in older age, the main effect is mortality.
Fig. 3.
Number of disability-adjusted life years lost associated with alcohol use disorders in 2005, by sex and age. For exact numbers and confidence intervals, see Table S3. YLL, years of life lost to premature mortality; YLD, years of life lost due to disability.
Discussion
AUD were found to be associated with a substantial burden of disease in the United States. Most of the burden stems from the disability associated with AUD, but AUD were linked also to 65,000 deaths per year. For many of the AUD-associated diseases, there is evidence that the impact of alcohol is causal (Rehm, 2011; Rehm et al., 2010). Heavy drinking, both continuous and episodic, has been causally linked to more than 200 diseases and injuries as measured in the 3-digit ICD, revision 10 codes (Gmel et al., 2011a; Rehm et al., 2010). More than 30 disease categories alone have alcohol or alcoholic in their name, meaning that without any alcohol consumption there would be no deaths or disability from these diseases. The only notable exception from clear causality seems to be mental disorders as mortality outcomes. While there is good evidence that alcohol consumption and AUD can cause mental disorders such as depression (Rehm et al., 2004), reverse causality occurs as well (Bolton et al., 2009). Additionally, underlying factors such as genetic vulnerability may be responsible for both AUD and co-occurring mental disorders (Kendler et al., 2003; Rehm et al., 2004). In addition, as there is considerable co-morbidity with AUD, both physical and mental co-morbid disorders (e.g., tobacco use disorders) may have been responsible for part of the mortality in people with AUD. There is no good methodology to correct for the impact of these co-morbidities. Thus, we cannot claim that all of the mortality associated with AUD was causally attributable to AUD.
The sensitivity analyses applying the lower RR estimates to all the population resulted in 57,000 deaths (15% less mortality). While this is still a large number, we believe it is an underestimate, as our prevalence estimates did not include special and vulnerable populations with multifold prevalence of AUD, especially more severe AUD, compared with the survey population (hospital and prison populations, homeless).
Notwithstanding the impact of AUD on mortality, the main component of the disease burden of AUD seems to be disability. AUD globally have been ranked among the most disabling disease conditions (World Health Organization, 2008), and current analyses corroborate this finding for the United States: AUD were estimated to be associated with almost twice as much disability as premature mortality in the United States in 2005 (overall YLD: 2.13 million, YLL: 1.15 million).
Before drawing conclusions from the results of this study, further potential limitations of this contribution must be considered. First, the underlying numbers for prevalence, incidence, and duration are based on the NESARC, and thus on subjective reporting. While this reliance may introduce some biases, the methodology of the NESARC has been subjected to various validity and reliability studies showing high quality (for details and references see Data S1). Estimating duration was the most challenging problem, as it could only be estimated from censored data and based on a very strict definition of remission, leading to potential bias in estimation. The resulting estimates for duration were higher than expected from prevalence data and other epidemiological indicators, but we were able to correct this potential overestimation by means of DISMOD internal consistency analysis (see methodology section above).
Another problem with prevalence estimates from surveys has been mentioned before. A sizable number of people with more severe AUD are not in the sampling frame, most notably homeless and imprisoned people, and some people cannot be interviewed as they are institutionalized at the time of the survey (Shield and Rehm, 2012). Recent reviews have shown that these groups have multifold AUD prevalence, for example for homeless above an average prevalence of AD of 38% was found (Fazel et al., 2008). Further studies may be able to better estimate the prevalence of AUD by combining studies from various populations (general household population, prison inmates, homeless) and may also have better means to enumerate severe dependence.
The second underlying element of the analysis is the metaanalysis on AUD and mortality (Roerecke and Rehm, 2013). Based on the comment of a reviewer, we performed a country-specific meta-analysis for studies from the United States only (Table S5), and while the CIs overlap for all 4 RRs, the U.S. studies show RRs, which are about 15 to 25% lower (except for men in the general population, where the RR is slightly higher). One reason for this is that U.S. studies are relatively old, and the mortality associated with AUD has increased over time (Roerecke and Rehm, 2013). However, it cannot be excluded that the estimates for YLL may be slightly overestimated.
Finally, we used the DWs of the NIH DALYs study to calculate AUD-associated YLD, as we believe these country-specific data are the best choice. Comparing our DWs with the GBD 2010 Study, the values of the NIH DALYs study were actually lower (see Table S6 for a comparison; for the DWs used in the GBD 2010 Study, see Salomon et al., 2012), that is, more conservative. Additionally, we also corroborated our results with a large Canadian general population study (Mcintosh et al., 2007), where town house meetings were held to derive DWs via standard gamble methodology. This sensitivity study yielded comparable, but somewhat lower, results (about 23% lower YLD). In sum, while we cannot exclude that even NIH experts may be subject to bias, the so derived DWs were comparable with other estimates derived via completely different methodology.
The numbers of deaths, YLL, YLD, and DALYs estimated in this article to be associated with AUD in 2005 in the United States are considerably larger than those numbers estimated in the GBD Study for the United States in 2004 (World Health Organization, 2008). The 2004 GBD Study estimated that 5,800 deaths, 101,500 YLL, 1,332,100 YLD, and 1,433,600 DALYs were attributable to AUD for people 18 to 64 years of age. In comparison, our estimates for the same age group for deaths and YLL were several times higher, and for YLD and DALYs were around twice as high.
The differences in numbers of deaths and YLL are simple to explain: The 2004 GBD Study was restricted to deaths where AUD were named as the cause of death (i.e., on the death certificate), while we defined AUD as a risk factor for mortality by applying AAFs based on current prevalence in the United States and RR relationships for mortality among AUD from a large meta-analysis (see Discussion above). Thus, our estimates of deaths should be more comparable with estimates of deaths caused by alcohol use or heavy alcohol use as a risk factor (for such comparisons in a different jurisdiction, see Rehm et al., 2013), and not just as a cause of death from a death certificate.
Estimates of YLD, however, should be comparable with the 2004 GBD Study estimates. The estimated number of YLD in this study was 1.8 times higher than the 2004 GBD Study estimates (1.8 times higher for men and 1.9 times higher for women). Both estimates are based on internally consistent data. Our study has the advantage of being based on empirical data for prevalence and incidence from the best U.S. survey data available as well as on DWs specifically for the United States, combined with empirical data on the prevalence of severity of treatment and other epidemiological indicators by sex and age, while the 2004 GBD Study data were derived top-down from global/regional data. Thus, we consider our estimates for the burden of disease as more valid and empirically evidenced than the 2004 GBD Study data. The 2 estimates are for different years (2004 and 2005), but as alcohol consumption in the United States was relatively stable in 2004 and 2005, the differences between our estimates and those in the 2004 GBD Study are not likely due to an increase in AUD.
AUD were shown to be responsible for a sizable portion of mortality and the burden of disease in the United States, a finding similar to recent European studies with slightly different methodology (Rehm et al., 2013; Wittchen et al., 2011). As all alcohol-attributable burden is in principle avoidable (Rehm et al., 2006), the question of how it can be reduced should be investigated. The usual measures aimed at preventing alcohol-attributable problems, such as taxation and restrictions on availability and marketing, have been shown to be effective in numerous studies (Anderson et al., 2009; Babor et al., 2010) and have been shown also to have an impact on AUD (Rehm and Greenfield, 2008). However, these measures may need to be supplemented by specific measures aimed at AUD to reduce heavy drinking and associated mortality and disability risks, such as brief interventions and treatment (Babor et al., 2010; Heather, 2012; Kaner et al., 2007; McQueen et al., 2011; Rehm and Roerecke, 2013). Currently, the treatment rate in the United States is quite low, with about 5% of all people with AUD in treatment in the same year (see above), and as treatments for AUD in general have been shown to be effective (as overviews, Berglund et al., 2003; Hester and Miller, 2003), an increase in the treatment rate for AUD can be expected to have positive public health consequences (Rehm et al., 2013).
In conclusion, as there are effective ways in which to reduce the alcohol-attributable burden, including the burden associated with AUD, we see no reason why the United States should accept the high level of the burden of disease currently associated with AUD.
Supplementary Material
Acknowledgments
This work was financially supported by Contract No. HHSN267200700041C of the National Institute of Alcohol Abuse and Alcoholism (NIAAA), Bethesda, MD. The first author also acknowledges salary support from the Ontario Ministry of Health and Long-Term Care.
The study on which this paper is based, the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), is sponsored by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, U.S. Department of Health and Human Services, with supplemental support from the National Institute on Drug Abuse. This research was supported in part by the Intramural Program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism.
The views and opinions expressed in this paper are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the U.S. government.
References
- Anderson P, Chisholm D, Fuhr D. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet. 2009;373:2234–2246. doi: 10.1016/S0140-6736(09)60744-3. [DOI] [PubMed] [Google Scholar]
- Arias E, Rostron BL, Tejada-Vera B. National vital statistics reports. 10. Vol. 58. National Center for Health Statistics; Hyattsville, MD: 2010. United States life tables, 2005. [PubMed] [Google Scholar]
- Babor T, Caetano R, Casswell S, Edwards G, Giesbrecht N, Graham K, Grube J, Gruenewald P, Hill L, Holder H, Homel R, Livingston M, Österberg E, Rehm J, Room R, Rossow I. Alcohol: No Ordinary Commodity. Research and Public Policy. 2nd. Oxford University Press; Oxford and London: 2010. [Google Scholar]
- Barendregt J, Van Oortmarssen G, Vos T, Murray CJL. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003;1:4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund M, Thelander S, Jonsson E. An Evidenced Based Review. Wiley-VCH Verlag; Weinheim, Germany: 2003. Treating Alcohol and Drug Abuse. [Google Scholar]
- Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord. 2009;115:367–375. doi: 10.1016/j.jad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding E, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of lifestyle, dietary, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101:181–191. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in Western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5:e225. doi: 10.1371/journal.pmed.0050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmel G, Kuntsche E, Rehm J. Risky single occasion drinking: bingeingis not bingeing. Addiction. 2011a;106:1037–1045. doi: 10.1111/j.1360-0443.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- Gmel G, Jr, Shield K, Frick H, Kehoe T, Gmel G, Sr, Rehm J. Estimating uncertainty for alcohol-attributable fractions for infectious and chronic disease. BMC Med Res Methodol. 2011b;11:48. doi: 10.1186/1471-2288-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, Saha TD, Smith SM, Pulay AJ, Pickering RP, Ruan WJ, Compton WM. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry. 2009;14:1051–1066. doi: 10.1038/mp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55:508–514. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Grant BF. Prevalence, correlates, disability and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heather N. Can screening and brief intervention lead to population-level reductions in alcohol-related harm? Addict Sci Clin Pract. 2012;7:15. doi: 10.1186/1940-0640-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RK, Miller WR. Handbook of Alcoholism Treatment Approaches. Effective Alternatives. 3rd. Allyn & Bacon; Boston, MA: 2003. [Google Scholar]
- Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, Heather N, Saunders J, Burnand B. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007:CD004148. doi: 10.1002/14651858.CD004148.pub3. [DOI] [PubMed] [Google Scholar]
- Kendler K, Prescott C, Myers J, Neale M. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kosinski M, Turner-Bowker DM, Gandek B. User's Manual for the SF-12v2 Health Survey. Quality Metric Inc.; Lincoln, RI: 2007. [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng TA, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassenbaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks RE, Martin RE, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Noua JM, Norman R, Olives C, Omer SB, Orchard J, Osborne Rh, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizarri PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield KD, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner P, Straif K, Straney L, Thurston GD, Tran JH, Dingenen RV, Van Donkelaar A, Lennert Veerman J, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CL, Ezzati M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Salomon JA, Ezzati M, Begg S, Lopez AD. Sensitivity and uncertainty analyses for burden of disease and risk factor estimates. In: Lopez AD, Mathers CD, Ezzati M, Murray CJL, Jamison DT, editors. Global Burden of Disease and Risk Factors Global Burden of Disease and Risk Factors. Oxford University Press; New York, NY: 2006. pp. 399–426. [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- McIntosh CN, Connor Gorber S, Bernier J, Berthelot JM. Eliciting Canadian population preferences for health states using the Classification and Measurement System of Functional Health (CLAMES) Chronic Dis Can. 2007;28:29–41. [PubMed] [Google Scholar]
- McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;(8):CD005191. doi: 10.1002/14651858.CD005191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, Ebrahim S, Ezzati M, Salomon JA, Kreiser JG, Hogan M, Murray CJ. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;18:11. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2000;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135–143. [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GLG, Graham K, Irving HM, Kehoe T, Parry CD, Patra J, Popova L, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B. The relation between different dimensions of alcohol consumption and burden of disease — an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Frick U. Valuation of health states in the U.S. study to establish disability weights: lessons from the literature. Int J Methods Psychiatr Res. 2010;19:18–33. doi: 10.1002/mpr.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Frick U. Establishing disability weights from pairwise comparisons for a US burden of disease study. Int J Method Psych. 2013;22:144–154. doi: 10.1002/mpr.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Greenfield TK. Public alcohol policy: current directions and new opportunities. Clin Pharmacol Ther. 2008;83:640–643. doi: 10.1038/sj.clpt.6100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Roerecke M. Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcohol. 2013;48:509–513. doi: 10.1093/alcalc/agt021. [DOI] [PubMed] [Google Scholar]
- Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, Sempos CT, Frick U, Jernigan D. In: Alcohol use, in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Vol. 1. World Health Organization; Geneva, Switzerland: 2004. pp. 959–1109. [Google Scholar]
- Rehm J, Shield KD, Rehm MX, Gmel G, Frick U. Modelling the impact of alcohol dependence on mortality burden and the effect of available treatment interventions in the European Union. Eur Neuropsychopharmacol. 2013;23:89–97. doi: 10.1016/j.euroneuro.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Patra J, Gmel G. Avoidable burden of disease: conceptual and methodological issues in substance abuse epidemiology. Int J Method Psych. 2006;15:181–191. doi: 10.1002/mpr.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche AM, Freeman T, Skinner N. From data to evidence, to action: findings from a systematic review of hospital screening studies for high risk alcohol consumption. Drug Alcohol Depend. 2006;83:1–14. doi: 10.1016/j.drugalcdep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. Alcohol use disorders and mortality — a systematic review and meta-analysis. Addiction. 2013;108:1562–1578. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Abdulhak AB, Blyth F, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hoegland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipshultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DJ, McGill N, McGrath J, Mensah GA, Meyer AC, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Prince M, Rehm JT, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soerjomataram I, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, Van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng ZJ, Murray CJL. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov AV, Popova S, Room R, Ramonas M, Rehm J. Disability associated with alcohol abuse and dependence. Alcohol Clin Exp Res. 2010;34:1871–1878. doi: 10.1111/j.1530-0277.2010.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield K, Gmel G, Kehoe T, Dawson DA, Grant BF, Rehm J. Mortality and potential years of life lost attributable to alcohol consumption by race and sex in the United States in 2005. PLoS ONE. 2013;8:e51923. doi: 10.1371/journal.pone.0051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield K, Rehm J. Difficulties with telephone-based surveys on alcohol in high-income countries: the Canadian example. Int J Method Psych. 2012;21:17–28. doi: 10.1002/mpr.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün TB, Rehm J, Chatterji S, Saxena S, Trotter R, Room R, Bickenbach J. Multiple-informant ranking of the disabling effects of different health conditions in 14countries. Lancet. 1999a;354:111–115. doi: 10.1016/s0140-6736(98)07507-2. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Saxena S, Rehm J, Bickenbach J. Are disability weights universal? WHO/NIH Joint Project CAR Study Group. Lancet. 1999b;354:1306. [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Abdulhak AB, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, Macintyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KV, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De León FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990– 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics. 1976;32:829–849. [PubMed] [Google Scholar]
- Ware JJ, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- World Health Organization. Global Health Risks. Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.