Abstract
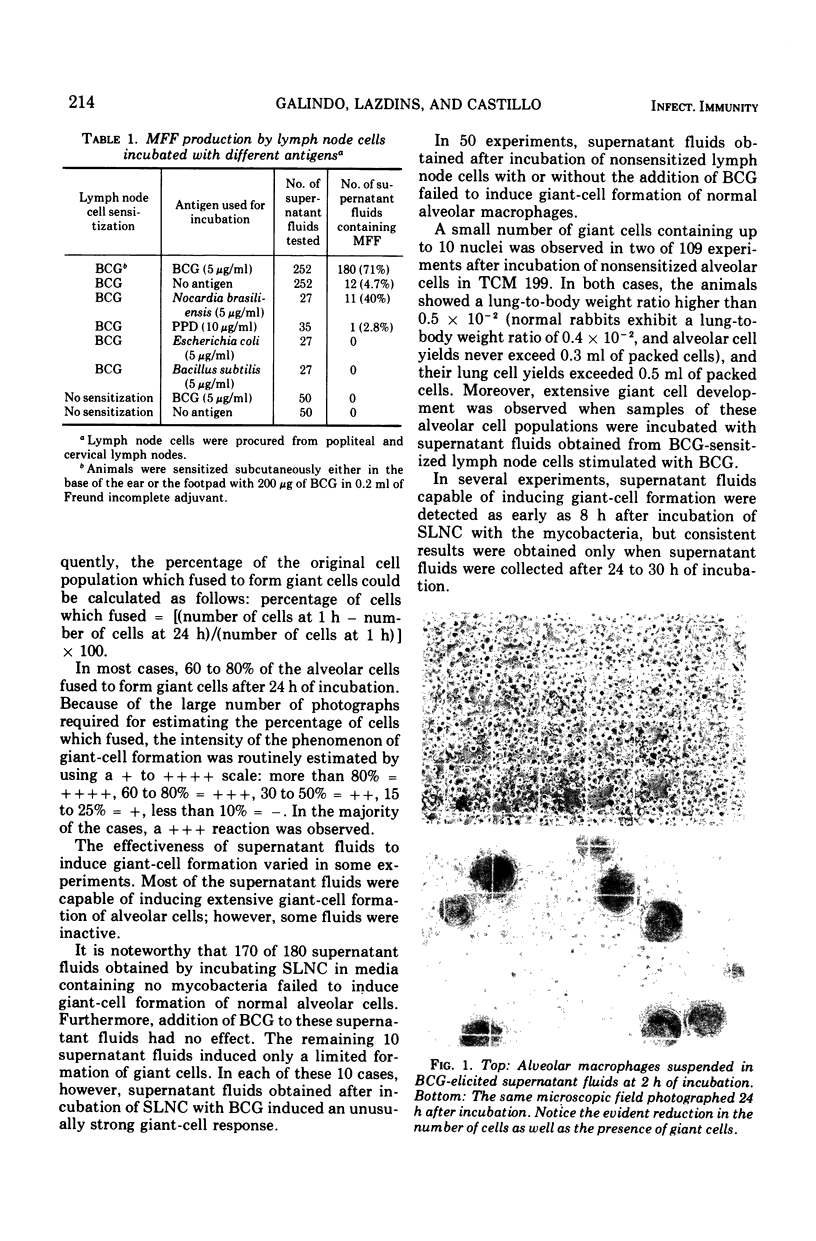
Cell-free supernatant fluids obtained from BCG-sensitized lymph node cells (6 × 106 cell/ml) incubated in tissue culture flasks containing heat-killed BCG (5 μg/ml) induced extensive development of multinucleated giant cells when incubated with normal alveolar macrophages. In contrast, supernatant fluids obtained after incubation of similar samples of the same lymph node cell population in flasks with (i) no mycobacteria, (ii) heat-killed Escherichia coli, or (iii) heat-killed Bacillus subtilis failed to produce giant cells when added to normal alveolar macrophages. Giant-cell formation was observed in experiments using Nocardia brasiliensis as the eliciting antigen, indicating cross-reactivity between the antigens of this organism and BCG. These experiments indicate that BCG-sensitized lymphoid cells produce a soluble macrophage fusion factor after specific antigen stimulation. The fusion factor is a nondialyzable substance which is resistant to heating at 80 C for 30 min.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANOCK R. M. Association of a new type of cytopathogenic myxovirus with infantile croup. J Exp Med. 1956 Oct 1;104(4):555–576. doi: 10.1084/jem.104.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- Galindo B. Antigen-mediated fusion of specifically sensitized rabbit alveolar macrophages. Infect Immun. 1972 Apr;5(4):583–594. doi: 10.1128/iai.5.4.583-594.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Harris H., Watkins J. F., Ford C. E., Schoefl G. I. Artificial heterokaryons of animal cells from different species. J Cell Sci. 1966 Mar;1(1):1–30. doi: 10.1242/jcs.1.1.1. [DOI] [PubMed] [Google Scholar]
- Lolekha S., Dray S., Gotoff S. P. Macrophage aggregation in vitro: a correlate of delayed hypersensitivity. J Immunol. 1970 Feb;104(2):296–304. [PubMed] [Google Scholar]
- MARSTON R. Q. Cytopathogenic effects of hemadsorption virus type I. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):853–856. doi: 10.3181/00379727-98-24207. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]