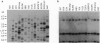Abstract
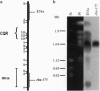
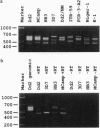
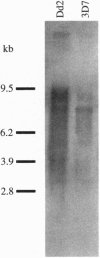
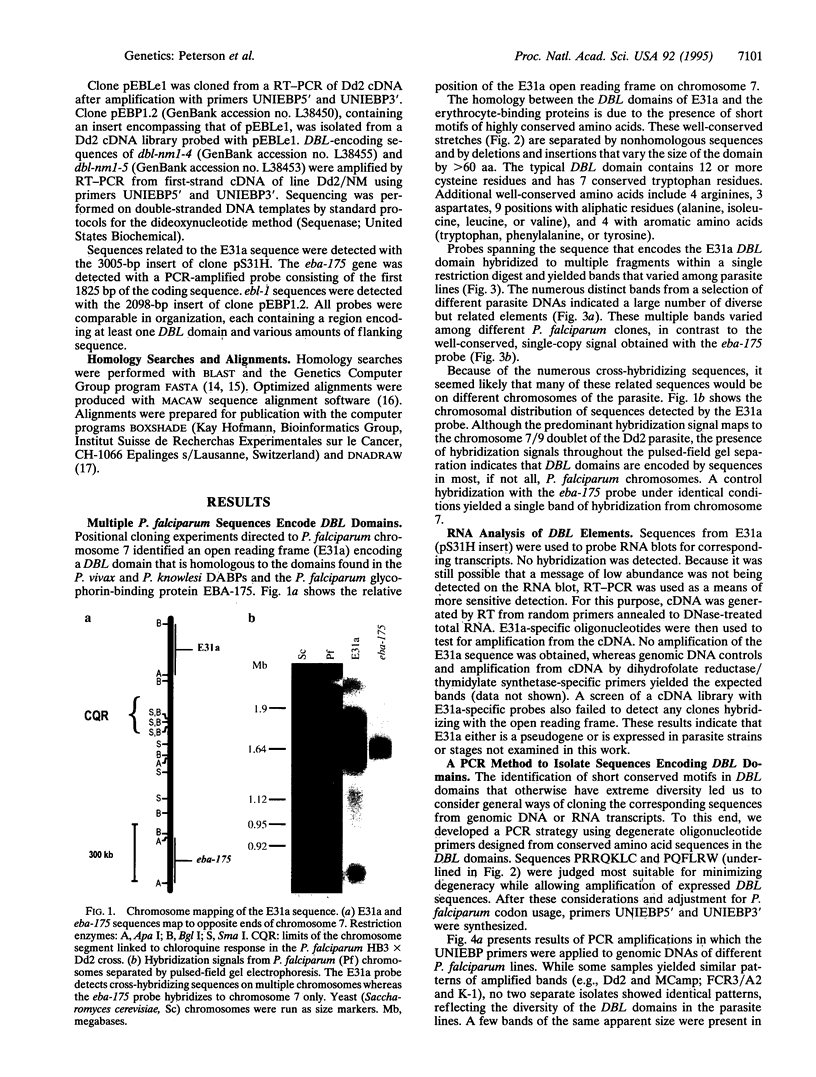
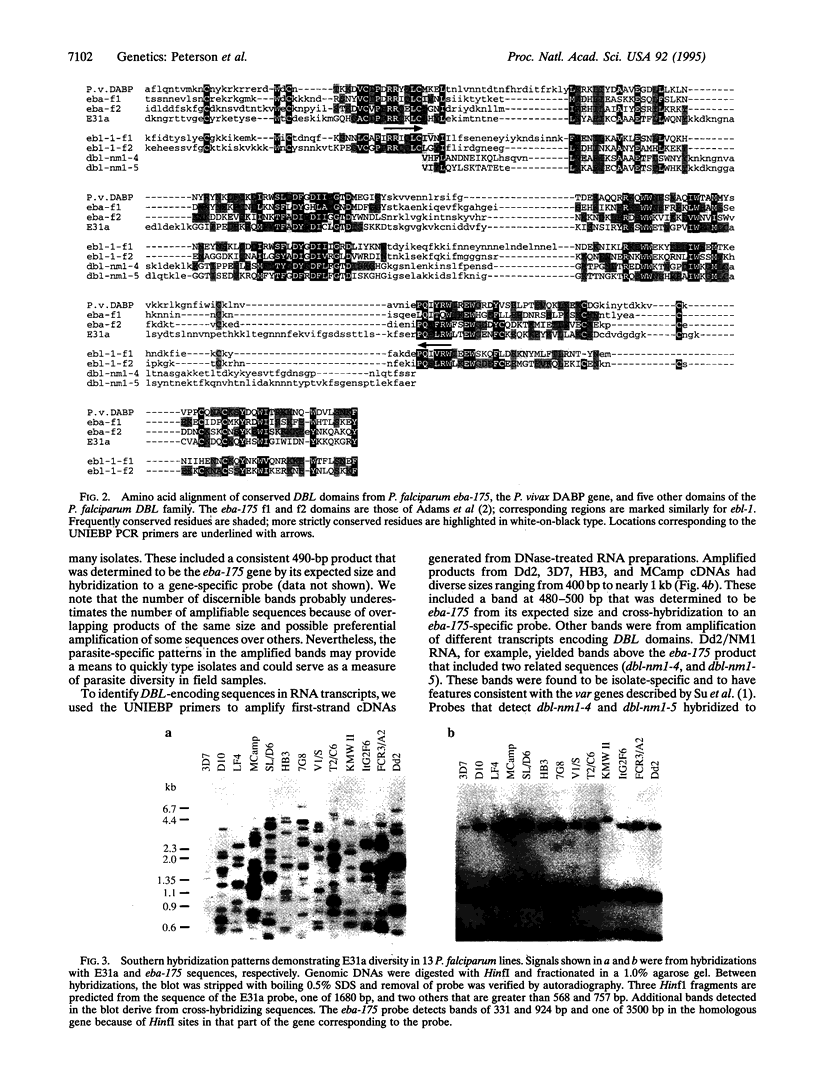
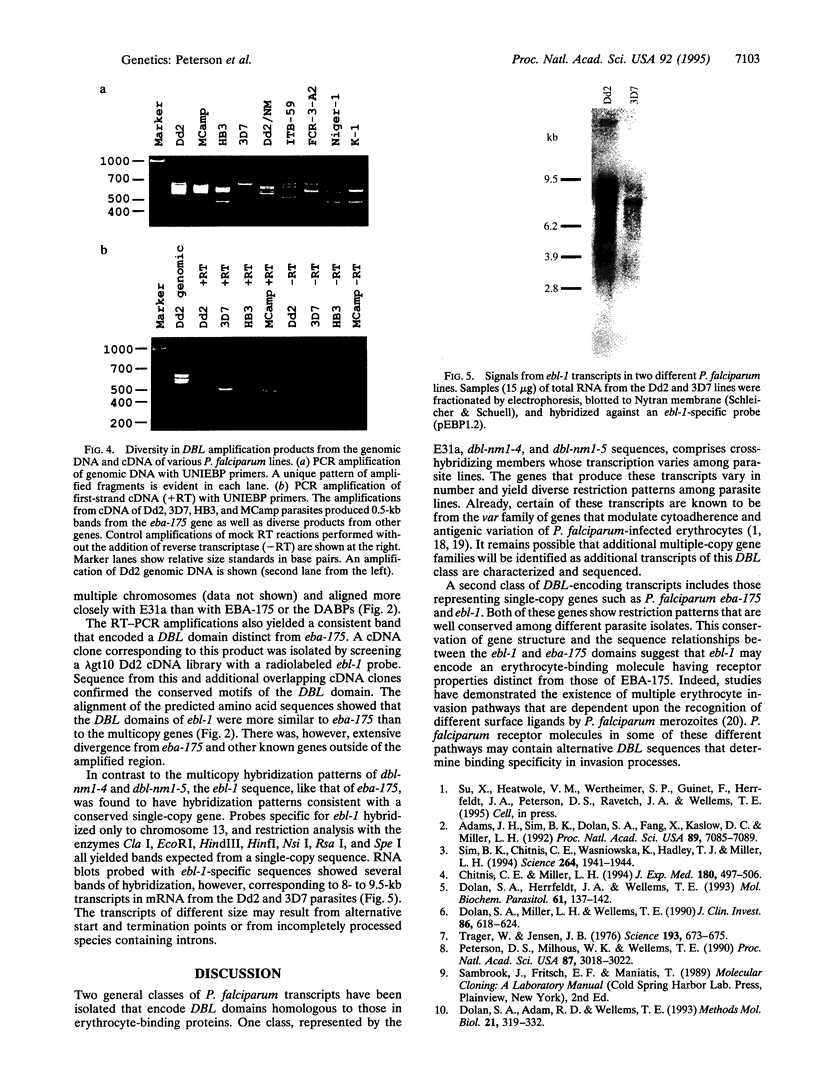
Open reading frames in the Plasmodium falciparum genome encode domains homologous to the adhesive domains of the P. falciparum EBA-175 erythrocyte-binding protein (eba-175 gene product) and those of the Plasmodium vivax and Plasmodium knowlesi Duffy antigen-binding proteins. These domains are referred to as Duffy binding-like (DBL), after the receptor that determines P. vivax invasion of Duffy blood group-positive human erythrocytes. Using oligonucleotide primers derived from short regions of conserved sequence, we have developed a reverse transcription-PCR method that amplifies sequences encoding the DBL domains of expressed genes. Products of these reverse transcription-PCR amplifications include sequences of single-copy genes (including eba-175) and variably transcribed genes that cross-hybridize to multiple regions of the genome. Restriction patterns of the multicopy genes show a high degree of polymorphism among different parasite lines, whereas single-copy genes are generally conserved. Characterization of the single-copy genes has identified a gene (ebl-1) that is related to eba-175 and is likely to be involved in erythrocyte invasion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Sim B. K., Dolan S. A., Fang X., Kaslow D. C., Miller L. H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Chitnis C. E., Miller L. H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994 Aug 1;180(2):497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle C. E., Macfarlane D. E. Isolation of RNA from cells in culture using Catrimox-14 cationic surfactant. Biotechniques. 1993 Dec;15(6):1102–1105. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S. A., Adam R. D., Wellems T. E. Chromosome mapping methods for parasitic protozoa. Methods Mol Biol. 1993;21:319–332. doi: 10.1385/0-89603-239-6:319. [DOI] [PubMed] [Google Scholar]
- Dolan S. A., Herrfeldt J. A., Wellems T. E. Restriction polymorphisms and fingerprint patterns from an interspersed repetitive element of Plasmodium falciparum DNA. Mol Biochem Parasitol. 1993 Sep;61(1):137–142. doi: 10.1016/0166-6851(93)90166-u. [DOI] [PubMed] [Google Scholar]
- Dolan S. A., Miller L. H., Wellems T. E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990 Aug;86(2):618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S. A., Proctor J. L., Alling D. W., Okubo Y., Wellems T. E., Miller L. H. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994 Mar;64(1):55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Milhous W. K., Wellems T. E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. Automated preparation of DNA sequences for publication. Nucleic Acids Res. 1986 Jan 10;14(1):65–73. doi: 10.1093/nar/14.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim B. K., Chitnis C. E., Wasniowska K., Hadley T. J., Miller L. H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994 Jun 24;264(5167):1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Walker-Jonah A., Dolan S. A., Gwadz R. W., Panton L. J., Wellems T. E. An RFLP map of the Plasmodium falciparum genome, recombination rates and favored linkage groups in a genetic cross. Mol Biochem Parasitol. 1992 Apr;51(2):313–320. doi: 10.1016/0166-6851(92)90081-t. [DOI] [PubMed] [Google Scholar]