Abstract
Background
Surgical resection is the cornerstone of therapy in patients with nonmetastatic breast cancer. Previous studies have reported underuse of adjuvant therapy among African Americans (AA). This study explores the independent effect of race on surgical resection in a recent, population-based sample of breast cancer patients.
Methods
All cases of nonmetastatic breast cancer reported to the state Cancer Registry between 1996 and 2002 were identified and linked to the state Inpatient/Outpatient Surgery Files and the 2000 Census. Characteristics between Caucasian and AA patients were compared using Student’s t and chi-square tests. Odds ratios (OR) of resection and 95% confidence intervals (CI) were calculated using logistic regression.
Results
We identified 12,404 Caucasian and 3,411 AA women. AA patients were more likely to be younger, non-married, have greater comorbidity, reside in rural communities, be less educated, live in poverty, and be uninsured or covered by Medicaid (all P < 0.0001). AA patients were slightly less likely to undergo resection compared to Caucasian patients (94.9% versus 96.4%, P < 0.0001). An interaction effect between race and urban/rural patient residence was observed (P = 0.003). After controlling for other factors, the adjusted OR for resection for urban AA patients was 0.58 (95% CI 0.41–0.82). In contrast, race had no effect on resection among rural patients (OR = 1.02; 95% CI 0.70–1.47).
Conclusions
AA race is an independent predictor of underuse of surgery among urban patients with breast cancer, while rural residence is associated with underuse of surgery, irrespective of race. Interventions designed to optimize surgical cancer care should target these vulnerable populations.
Keywords: Breast cancer, Race, Residence, Socioeconomic status, Resection
It is estimated that breast cancer will account for 178,480 new cancer cases and 40,460 cancer deaths among women in the United States of America in 2007.1 Recent statistics indicate that age-adjusted breast cancer mortality rates are higher among African American than Caucasian women.2 Population-based studies suggest that while survival rates for women with breast cancer have improved over the past two decades, survival rates in African American women have lagged behind, and the observed disparity is increasing.2–4
Several factors may account for the widening racial disparity in breast cancer mortality over time, including changes in risk factors, changes in access or quality of treatment, and differences in response to new treatments. Jatoi and colleagues recently combined breast cancer incidence data from the Connecticut and National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registries and mortality data from the National Center for Health Statistics to determine whether birth cohort mortality trends (reflecting differences in risk factors) or calendar period mortality trends (reflecting differences in access or effectiveness of new treatments) could explain the observed racial dis-parity in breast cancer mortality over the last several decades.5 The authors found that breast cancer mortality rates were similar for blacks and whites until the late 1970s, after which time the mortality rates among black women increased. The fact that this increase in mortality was associated with a synchronous increase in the calendar period mortality curves for blacks and non-whites, but not the birth cohort curves, suggested that the increasing racial disparity in mortality was likely attributable to differences in access or response to new treatments during this period.
Numerous studies suggest that there are no apparent racial/ethnic differences in efficacy or effectiveness of specific breast cancer treatments, including surgery and systemic therapy.6–9 African American and Caucasian women with early-stage breast cancer treated with breast-conservation therapy (BCT) appear to have similar rates of local control.10 An analysis of outcomes for African American and Caucasian women who participated in several National Surgical Breast and Bowel Project (NSABP) trials between 1982 and 1994 revealed no differences in disease-free survival between racial groups.11 However, a small difference in overall survival was observed, which was felt to be secondary to greater mortality from noncancer causes among African Americans. The author concluded that African American and Caucasian women with breast cancer who are matched for disease stage and receive equal treatment experience the same prognosis.
There is a growing body of literature suggesting that racial and ethnic disparities in cancer treatment may explain much of the observed variation in cancer outcomes.12 Underuse of surgical, radiation, and systemic therapy among African American patients with breast cancer could thus explain the observed lag in cancer survival. A recent study of 677 white, black, or Hispanic women with early-stage breast cancer analyzed the association between race and underuse of necessary adjuvant treatments, defined as radiation after BCT or systemic chemotherapy or hormonal therapy after surgery.13 Despite similar rates of oncological consultation, women from minority groups were half as likely to receive adjuvant therapy as Caucasian women, even when controlling for comorbidity and insurance status.
Surgical resection is the cornerstone of therapy in patients with localized or locoregional breast cancer. As such, failure to perform resection in these patients represents a serious breach in the standard of care and poses a serious threat to patients’ quality of life and long-term survival. The majority of studies to date have focused on disparities in the use of BCT versus mastectomy among African American and Caucasian women with breast cancer, with mixed results.9,14–18 Some of these studies have been criticized because they were hospital-rather than population-based, limited to Medicare patients, or failed to control for comorbidity or socioeconomic status. In this study, we explored the independent effect of African American race on receipt of surgical resection in a large, racially diverse, population-based sample of women with nonmetastatic breast cancer, while controlling for other important demographic, clinical, socioeconomic, and tumor variables.
METHODS
A dataset was created by identifying all cases of nonmetastatic, invasive breast cancer reported to the South Carolina Central Cancer Registry (SCCCR) from 1996 to 2002. Cases diagnosed at the time of autopsy were excluded. This dataset was then linked to the South Carolina Inpatient Files and Outpatient Surgery Files and the 2000 Census by the South Carolina Office of Research and Statistics (SCORS).
Patient demographics, tumor factors, year of diagnosis, and type of surgery performed (if any) were obtained from the Cancer Registry file. Age, gender, race (i.e., white, black, other, unknown), marital status (i.e., single, married, separated, divorced, widowed, unknown) were self-reported and abstracted from the medical record for submission to the SCCCR. Tumor location and stage, and type of surgery performed to the primary tumor site were submitted to the SCCCR using nationally standardized data items, coding definitions, and transmission format specifications as defined by the North American Association of Central Cancer Registries. Anatomic location was reported following the definitions provided by the World Health Organization’s International Classification of Disease for Oncology, Third Edition.19 SEER summary stage at the time of initial diagnosis was assigned in each case according to the 2000 SEER Summary Staging Manual.20 Type of surgery performed to the primary tumor site as part of the first course of treatment was coded according to the Commission of Cancer’s 1996 Registry Operations and Data Standards manual and its 1998 supplement.21,22
Comorbidity, patient residence (i.e., urban/rural status), and insurance status were obtained from the linked South Carolina Inpatient Files and Outpatient Surgery Files. Romano-Charlson comorbidity index was calculated using ICD-9-CM diagnosis and procedure codes from all inpatient admissions and out-patient surgeries during the 12 months prior to and including the date of breast cancer diagnosis.23
Patient residence and insurance status were obtained from files corresponding to the index breast cancer surgery, or from the last inpatient/outpatient surgery files prior to the date of diagnosis in patients who were not resected. Patient residence was defined as urban or rural based on the Metropolitan Statistical Area (MSA) of the county in which patients resided, as defined by the US Department of Health and Human Services Office of Rural Health Policy. Insurance status was based on payor and categorized as self-pay, commercial insurance, health maintenance organization, Medicare, Medicaid, worker’s compensation, indigent/charitable organization, other government (e.g., CHAMPUS, state, county), or unknown.
Educational level and income were estimated at the census tract and zip code level using 2000 Census data linked to the relevant Inpatient or Outpatient Surgery files. Patients who resided in census tracts or zip codes where more than 20% of individuals age 25 years or older had not completed high-school were classified as having a low level of education, which corresponded to the lowest quartile. Patients who resided in census tracts or zip codes where the median income, adjusted for household size, was less than 200% of the federal poverty guideline (as defined by the Department of Health and Human Services and issued each year in the Federal Register) were defined as living in poverty.24 This poverty threshold was chosen because it is the current basis for determining financial eligibility for a number of means-tested federal programs.
The study was approved by the Institutional Review Boards of the Medical University of South Carolina and the South Carolina Department of Health and Environmental Control (DHEC).
Statistical Analysis
The analysis was limited to female and Caucasian or African American patients for the purposes of this study. Because we were interested in receipt of surgery among patients with potentially resectable cancers at the time of diagnosis, we excluded patients with tumors that did not clearly constitute primary or solid breast cancers (morphology codes 8000, 8001, 8004, 8041, 8401, 8540, 8541, 8543, 8800, 8810, 8830, 8850, 8890, 8930, 8940, 8980, 8982, 9020, 9260) and patients with inflammatory breast cancer (morphology code 8530). In order to minimize confounding by previous or synchronous cancers, the analysis was limited to patients with a single occurrence of primary breast cancer during the study period. Due to sample size limitations, patients who were separated or divorced and patients with a Romano-Charlson comorbidity index of 2 or greater were collapsed into single groups, respectively. Due to the large amount of missing data for educational level and income at the census tract level (missing for 5,557 [35.1%] of patients), zip-code-level information was used for the analyses. Patients with payor status reported as self-pay or indigent/charitable organization and patients with payor status reported as worker’s compensation or other government were combined into two groups: “self-pay” or “other”, respectively. Patients with tumors involving the nipple or central portion of the breast and patients with tumors involving the upper outer quadrant or axillary tail were classified as having “central” or “upper outer quadrant” tumors, respectively. Stage at presentation was collapsed into two groups based on SEER summary stage at the time of initial diagnosis: localized/regional (due to direct extension only), or regional (due to lymph node involvement or not otherwise specified). Patients who underwent no cancer-directed surgery of their primary site or underwent local tumor destruction alone were classified as “not resected”. All other patients were classified as having been resected.
Differences in patient demographics, comorbidity, socioeconomic status, and tumor factors by race were compared using Student’s t (for mean age) or overall chi-square tests (for all other ordinal and categorical variables). Unadjusted odds ratios (OR) and 95% confidence intervals (95% CI) of resection for the various patient and tumor factors were estimated using the maximum-likelihood method and logistic regression. Adjusted odds ratios of resection for race were generated using a series of logistic regression models sequentially controlling for demographics, comorbidity, socioeconomic status, and tumor factors (including year of diagnosis). At each step, ordinal and categorical variables were checked for the linearity assumption with the log odds of resection. Variables were retained in the models based upon whether or not they were a confounder (i.e., changed the crude OR by 10% or more, either by themselves or collectively with the other covariates). Variables were removed from the models if they were not a confounder, did not improve the model fit, or increased the standard error of the odds ratio of resection for race. Potential interactions of interest that were tested once the final models were developed included race by age, gender, comorbidity, patient residence, income, education, and insurance status. The SAS System version 9.1 (Cary, NC) was used to conduct all analyses. A P-value < 0.05 (two-sided) was used as the cut-off point for statistical significance for individual variables, and interactions were considered statistically significant for P-value < 0.10.
This study was approved by the Institutional Re-view Boards of the Medical University of South Carolina and the South Carolina Department of Health and Environmental Control (DHEC).
RESULTS
A total of 16,296 patients with a single occurrence of nonmetastatic, invasive breast cancer diagnosed between 1996 and 2002 were identified from the Cancer Registry files. Patients with race reported as “other” or “unknown”, and patients with inappropriate morphology codes were excluded, leaving 15,982 patients. Because we were primarily interested in analyzing the effect of race on receipt of surgery in women with breast cancer, 165 male patients (1.03% of patients) and two patients with missing information regarding gender were excluded, leaving 15,815 patients in our final sample.
There were a total of 12,404 Caucasian and 3,411 African American patients in our study. The distribution of patient demographics, comorbidity scores, socioeconomic status, year of diagnosis, and tumor factors according to race are shown in Table 1. African American patients were slightly younger, more likely to be single, separated, or divorced, and had higher comorbidity scores compared to Caucasian patients. African Americans patients were also more likely than Caucasian patients to reside in rural communities, be less educated, live in poverty, and be uninsured or covered by Medicaid. There was no association between year of diagnosis or tumor location and race.
TABLE 1.
Characteristics of study population by patient race (%)
| Characteristic (percentage of Caucasian [C] and African American [AA] patients with missing data) |
Caucasian (N = 12,404) |
African American (N = 3,411) |
P |
|---|---|---|---|
| Age, years (mean ± SD) | 62.0 ± 13.7 | 57.5 ± 14.7 | <0.0001 |
| Age, years | <0.0001 | ||
| <50 | 20.1 | 33.3 | |
| 50–59 | 23.2 | 24.0 | |
| 60–69 | 24.4 | 18.9 | |
| 70–79 | 21.7 | 16.0 | |
| ≥80 | 10.6 | 7.8 | |
| Marital status (C 5.3%, AA 6.7%) | <0.0001 | ||
| Married | 63.1 | 42.7 | |
| Single | 6.2 | 18.1 | |
| Separated or divorced | 8.5 | 15.6 | |
| Widowed | 22.2 | 23.6 | |
| Romano-Charlson comorbidity index (C 8.2%, AA 6.4%) | <0.0001 | ||
| 0 | 85.3 | 78.0 | |
| 1 | 11.0 | 16.0 | |
| 2+ | 3.7 | 5.0 | |
| Residency (C 5.2%, AA 3.4%) | <0.0001 | ||
| Urban | 72.7 | 60.6 | |
| Rural | 27.3 | 39.4 | |
| Education, zip code level (C 7.2%, AA 5.8%) | <0.0001 | ||
| High education | 75.5 | 59.1 | |
| Low education | 24.5 | 40.9 | |
| Income, zip code level (C 7.2%, AA 5.7%) | <0.0001 | ||
| Not living in poverty | 91.8 | 70.2 | |
| Living in poverty | 8.2 | 29.8 | |
| Insurance status (C 5.8%, AA 3.8%) | <0.0001 | ||
| Commercial | 42.1 | 35.9 | |
| HMO | 6.1 | 7.6 | |
| Medicare | 44.7 | 36.7 | |
| Medicaid | 1.6 | 8.6 | |
| Other | 2.1 | 2.1 | |
| Self-pay | 3.4 | 9.1 | |
| SEER summary stage | <0.0001 | ||
| Localized or regional (direct extension only) | 71.7 | 60.5 | |
| Regional (lymph node involvement or NOS) | 28.3 | 39.5 | |
| Tumor location | 0.202 | ||
| Central | 5.9 | 5.6 | |
| Upper inner quadrant | 8.5 | 9.0 | |
| Lower inner quadrant | 4.7 | 5.0 | |
| Upper outer quadrant | 35.7 | 34.7 | |
| Lower outer quadrant | 5.8 | 5.9 | |
| Overlapping lesion | 21.3 | 20.1 | |
| NOS | 18.1 | 19.7 |
SEER, Surveillance, Epidemiology, and End Results; HMO, health maintenance organization; NOS, not otherwise specified.
Receipt of surgery (percentage of patients resected and odds ratios of resection) according to patient and tumor characteristics is shown in Table 2. Age had no apparent effect on receipt of surgery except in patients age 80 years or older. African American race had a small but statistically significant adverse effect of resection (unadjusted OR 0.69 [95% CI 0.58–0.83]). Patients who were single or widowed were also less likely to undergo resection (compared to married patients), as well as patients with greater comorbidity. Patients who resided in rural communities were less likely to undergo surgery compared to patients who resided in urban communities (94.5% versus 97.2%, P < 0.0001). Poverty status had no apparent effect on receipt of surgery, while low education and lack of medical insurance or coverage by Medicare, Medicaid, or other insurance had a negative effect.
TABLE 2.
Receipt of surgery by patient and tumor characteristics
| Characteristic (percentage of resected [R] and nonresected [NR] patients with missing data) |
Percentage resected | Unadjusted OR (95% Cl) |
P |
|---|---|---|---|
| Age, years | |||
| <50 | 96.5 | 1.0 | |
| 50–59 | 97.3 | 1.29 (0.99–1.68) | 0.060 |
| 60–69 | 96.4 | 0.97 (0.76–1.25) | 0.819 |
| 70–79 | 95.7 | 0.81 (0.64–1.04) | 0.100 |
| ≥80 | 92.6 | 0.45 (0.35–0.59) | <0.0001 |
| Race | |||
| Caucasian | 96.4 | 1.0 | |
| African American | 94.9 | 0.69 (0.58–0.83) | <0.0001 |
| Marital status (R 5.1%, NR 17.6%) | |||
| Married | 97.3 | 1.0 | |
| Single | 95.9 | 0.66 (0.48–0.89) | 0.006 |
| Separated or divorced | 96.3 | 0.73 (0.54–0.98) | 0.037 |
| Widowed | 95.2 | 0.55 (0.45–0.67) | <0.0001 |
| Romano-Charlson comorbidity index (R 7.2%, NR 22.0%) | |||
| 0 | 96.9 | 1.0 | |
| 1 | 96.2 | 0.80 (0.62–1.05) | 0.105 |
| 2+ | 94.4 | 0.55 (0.38–0.79) | 0.001 |
| Residency (R 4.6%, NR 11.5%) | |||
| Urban | 97.2 | 1.0 | |
| Rural | 94.5 | 0.49 (0.41–0.58) | <0.0001 |
| Education, zip code level (R 6.6%, NR 14.6%) | |||
| High education | 96.7 | 1.0 | |
| Low education | 95.9 | 0.80 (0.67–0.97) | 0.020 |
| Income, zip code level (R 6.5%, NR 14.6%) | |||
| Not living in poverty | 96.5 | 1.0 | |
| Living in poverty | 96.1 | 0.89 (0.69–1.14) | 0.354 |
| Insurance status (R 5.1%, NR 12.7%) | |||
| Commercial | 97.8 | 1.0 | |
| HMO | 97.7 | 0.95 (0.60–1.49) | 0.809 |
| Medicare | 95.4 | 0.46 (0.38–0.57) | <0.0001 |
| Medicaid | 93.2 | 0.30 (0.20–0.45) | <0.0001 |
| Other | 94.8 | 0.41 (0.24–0.70) | 0.001 |
| Self-pay | 94.5 | 0.39 (0.27–0.56) | <0.0001 |
| SEER summary stage | |||
| Localized or regional (direct extension only) | 95.8 | 1.0 | |
| Regional (lymph node involvement or NOS) | 96.7 | 1.28 (1.07–1.54) | 0.008 |
| Tumor location | |||
| Central | 96.6 | 1.0 | |
| Upper inner quadrant | 96.9 | 1.10 (0.68–1.76) | 0.700 |
| Lower inner quadrant | 96.8 | 1.06 (0.62–1.82) | 0.837 |
| Upper outer quadrant | 96.9 | 1.09 (0.74–1.61) | 0.668 |
| Lower outer quadrant | 97.4 | 1.31 (0.76–2.25) | 0.327 |
| Overlapping lesion | 97.4 | 1.29 (0.85–1.96) | 0.229 |
| NOS | 92.1 | 0.41 (0.28–0.60) | <0.0001 |
SEER, Surveillance, Epidemiology, and End Results; HMO, health maintenance organization; NOS, not otherwise specified; OR, odds ratio; CI, confidence interval.
A series of logistic regression models were used to assess the independent effect of race on resection by sequentially controlling for the other patient and tumor factors. None of the variables confounded the association between race and resection when adjusted for individually. Due to an interaction effect between patient residence and race (P = 0.003, Fig. 1), separate multivariate models were constructed for urban and rural patients (Table 3). African American race was independently associated with a lower probability of resection among urban patients (adjusted OR = 0.58; 95% CI 0.41–0.82) even before controlling for other patient demographics, socioeconomic status, and tumor factors. In contrast, race had no effect on receipt of surgery among rural patients in either the crude or adjusted models (adjusted OR = 1.02; 95% CI 0.70–1.47).
FIG. 1.
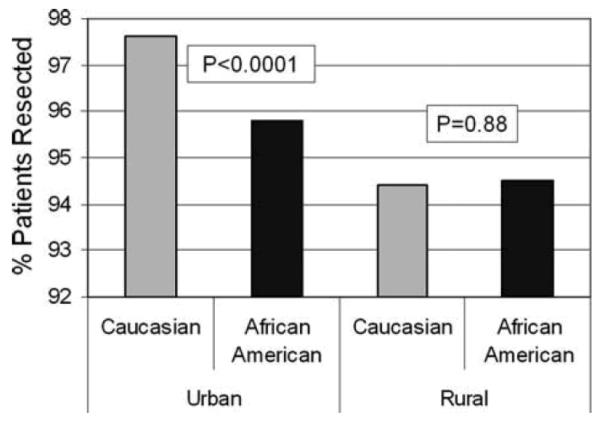
Percentage of patients resected according to patient race and urban/rural residence.
TABLE 3.
Crude and adjusted odds ratios of resection for African American race among urban and rural patients
| Models | OR (95% CI) for urban patients | OR (95% CI) for rural patients |
|---|---|---|
| 1. Race | 0.56 (0.44–0.73) | 1.02 (0.77–1.36) |
| 2. Model 1 + demographicsa | 0.58 (0.43–0.78) | 1.03 (0.75–1.43) |
| 3. Model 2 + comorbidityb | 0.56 (0.41–0.77) | 1.10 (0.79–1.55) |
| 4. Model 3 + SESc | 0.59 (0.42–0.82) | 1.08 (0.76–1.55) |
| 5. Model 4 + tumor factorsd | 0.58 (0.41–0.82) | 1.02 (0.70–1.47) |
Age, marital status.
Romano-Charlson comorbidity index.
Socioeconomic status: income, education, insurance status.
Tumor location, SEER summary stage, year of diagnosis.
SEER, Surveillance, Epidemiology, and End Results; OR, odds ratio; CI, confidence interval.
DISCUSSION
Using a large, racially diverse, population-based sample of women with nonmetastatic breast cancer diagnosed between 1996 and 2002, we found that African American race was associated with a slight underuse of curative resection, and that urban/rural residence moderated the effect of race on receipt of surgery. A significant proportion of the patients in our study (21.6%) were African American, and a higher proportion of these patients lived in rural counties compared to Caucasian patients (39.4% versus 27.3%, P < 0.001). Among urban patients, African Americans women were slightly less likely to undergo resection, even when controlling for other important patient factors, including socioeconomic status and insurance status. Among rural patients, however, the proportion of Caucasian and African American patients resected was similar, and race had no apparent effect on receipt of surgery.
Previous studies have reported underuse of surgical resection in patients with early-stage breast cancer. A 1988 study of 41,680 women with breast cancer from the National Cancer Data Base (NCDB) reported that 6.2%, 5.4%, and 10.4% women with American Joint Committee on Cancer (AJCC) stage I, II, and III breast cancer failed to undergo surgery.25 In a more recent study from the NCDB of 191,714 non-Hispanic white patients treated between 1995–1996, as many as 1.7%, 2.2%, and 2.4% of patients with AJCC stage I, II, and IIIA breast cancer, respectively, were not resected.26 Of note, although the authors noted underuse of BCT among non-Hispanic whites from lower-income zip codes, there was no appreciable difference in the overall use of surgical resection between income groups, as in our study.
A previous report from 2002 also reported under-use of surgical therapy among African American patients with breast cancer. Bradley and colleagues analyzed the association between race, socioeconomic status, and surgical treatment by linking data from the Metropolitan Detroit SEER registry to Michigan Medicaid enrollment files to identify 5,719 women with breast cancer diagnosed between 1996 and 1997.18 Ninety-three percent of the patients had nonmetastatic disease, 19% of patients were African American, and 11% were enrolled in Medicaid. Similar to our study, poverty status was defined as residence within a census area with median income below the federal poverty line. In a multivariate analysis controlling for age, marital status, Medicaid enrollment, poverty status, and stage, African American race was associated with an adjusted OR of resection of 0.62 (95% CI 0.42–0.90). The fact that this figure is almost identical to the one in our study suggests that underuse of resection among urban African American women with breast cancer appears to extend across geographically diverse communities, independent of comorbidity or socioeconomic status. Although the racial disparity in surgery for localized breast cancer in this and other studies may appear small, it is important to remember that long-term survival is impossible without attempted curative resection. Therefore, in cancers where surgical resection is clearly indicated, even minor differences in surgical treatment could be considered clinically significant, particularly if surgery carries minimal risks to the patient.
The observed underuse of surgery for localized breast cancer in this and other studies could be attributed to several patient-, physician-, and health-system-related factors. Patients’ misconceptions about cancer and its treatment may contribute to their willingness to undergo surgery. In a recent study by Margolis et al., African American patients were more likely to believe that lung cancer surgery causes tumor spread compared to Caucasian patients. Furthermore, 14% of these patients stated that they would not trust their physicians’ reassurance that this belief was false, and 19% of them stated that they would avoid surgery based on this belief. Patients’ risk perception of surgery may also be central to their willingness to undergo surgery. If so, higher risk aversion to surgery among African American could account for apparent underuse of surgical treatment among certain patients. Whether African American patients with breast cancer hold similar misconceptions about cancer surgery and its risks remains to be determined by future studies.
Healthcare-system-related factors, such as access to care or differences in referrals for surgical consultation, could also explain our findings. It is possible that some of the African American women in our study were either not referred for surgical consultation, or were referred to less experienced surgeons who may have been more likely to recommend more radical (and perhaps less “palatable”) surgery. Unfortunately, we did not have access to patients’ outpatient files, and therefore, could not determine whether differences in surgical treatment in our cohort were due to differences in surgical referral. Finally we cannot rule out the possibility that racial bias on the part of physicians, or perceived racism on the part of patients (as described in a previous study of Medicare recipients with breast cancer), were involved.27
To our knowledge, this is the first study to report the interaction between urban/rural residence, race, and underuse of surgical resection in patients with nonmetastatic breast cancer. Several studies have reported an association between rural residence and underuse of BCT among women with invasive breast cancer.16,28,29 In the Carolina Breast Cancer Study, a population-based case-control study of 788 women in North Carolina, African American patients were less likely to undergo BCT compared to white patients.29 When the authors controlled for area of residence, however, the association with race was no longer statistically significant. The failure of these authors to detect an interaction between area of residence, race, and surgical treatment in that study may have been due to the smaller size and greater homogeneity of their patient sample (i.e., all the women in the study were enrolled in the same managed care program).
The observed underuse of surgical resection in rural residents in this study may be explained by several factors. A previous study reported that rural women tend to have more negative attitudes about breast cancer, despite having a similar knowledge base about the disease.30 In addition, rural women were less likely to have had a recent mammogram or breast examination compared to urban women, even when they reported similar access to patient care. A similar fatalistic attitude about the efficacy of breast cancer treatment might partly explain why rural women in our study, who were diagnosed with non-metastatic, potentially curable breast cancer, were less likely to undergo resection. Finally, rural residents experience limited access to health care due to longer travel distances, fewer benefits (such as paid sick leave), fewer support services (such as childcare), and scant access to specialty physicians, including surgeons.31–34
Our observation that rural residence was associated with a lower probability of resection has significant public health implications. The 1990 Census reported that 61 million people lived in rural America. Furthermore, data collected by the US Department of Agriculture reported that, between 1990 and 1999, 2.2 million more people moved from urban to rural communities than from rural to urban communities.35 The results of our study are particularly sobering when one considers that rural communities have a higher proportion of elderly residents, and that the risk of breast cancer increases with age.
In response to the National Breast and Cervical Cancer Early Detection Program of 1991, the state of South Carolina’s Best Chance Network (BCN) contracted with radiology facilities, outpatient care centers and hospitals, and health care practitioners to provide funding for screening and diagnostic follow-up services to low-income women with abnormal breast screening results. In 2001, the state also began providing treatment coverage under Medicaid to women with breast cancer identified through the BCN. Approximately 60% of women enrolled in the BCN have been African American, and a similar percentage resided in rural communities. The persistent disparity in resection rates within these groups (including Medicaid patients) observed in our study suggests that, although such programs may be helpful, they are probably not sufficient. Further research is needed to identify and address persistent barriers to surgical evaluation and resection faced by rural women with breast cancer.
Our study has several potential limitations. Our analysis was limited to women diagnosed in South Carolina, which may limit its generalizability to other parts of the USA. The distribution of the demographic and tumor characteristics in our study, however, was not dissimilar to that recently reported in a large, nationwide sample of breast cancer patients from the NCDB (except for the higher proportion of African American patients in our study).36 The fact that the adjusted OR for resection for African American race in our study was almost identical to the one published in a previous study from the Metropolitan Detroit SEER registry, however, further supports our findings and speaks to their generalizability.18 Due to the relatively paucity of Hispanics in South Carolina (2.4% of the state population compared to 12.5% of the US population in 2000), we did not have a sufficient sample size to analyze the effect of Hispanic ethnicity on resection. Stage at presentation was recorded in our state cancer registry using SEER summary stage, rather than AJCC stage. Although this may have somewhat limited our ability to control for tumor stage at presentation, all the patients in our study had nonmetastatic disease and therefore should eventually have undergone resection. Due to the large percentage of rural patients in our study, we were unable to obtain exact addresses for many of the women and relied on zip code, rather than census-tract-level data, to estimate educational level and poverty status. Although use of census-tract data would have been optimal,37 the direction and magnitude of the univariate associations between education and poverty status and resection at the two levels were similar (data not shown). Therefore, we do not feel that the use of census-tract-level data would have changed our conclusions significantly. Although the percentage of Caucasian and African Americans with missing data (i.e., marital status, comorbidity, residence, education, income, and insurance status) was small and roughly similar (Table 1), patients who did not undergo resection were more likely to have missing data (Table 2). Although this could have deflated the regression coefficients for these variables in our multivariate models, it should not have significantly affected the adjusted regression coefficients for race listed in Table 3. Finally, we did not have access to patients’ outpatient files (other than the Outpatient Surgery Files). As a result, we could not determine whether underuse of resection among urban African Americans and rural patients were related to lower rates of surgical consultation.
Our study suggests that African American race is an independent predictor of underuse of surgical resection among urban women with nonmetastatic breast cancer. Therefore, interventions designed to decrease racial disparities in breast cancer care and mortality should specifically target urban populations. Our study also shows that rural residence is associated with underuse of surgery, irrespective of race. Future studies are needed to identify the patient-, physician-, and healthcare-system-related factors underlying these observations and optimize surgical cancer care in these vulnerable populations.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471–3. doi: 10.1093/jnci/94.7.471. [DOI] [PubMed] [Google Scholar]
- 3.Chu KC, Tarone RE, Brawley OW. Breast cancer trends of black women compared with white women. Arch Fam Med. 1999;8:521–8. doi: 10.1001/archfami.8.6.521. [DOI] [PubMed] [Google Scholar]
- 4.Chevarley F, White E. Recent trends in breast cancer mortality among white and black US women. Am J Public Health. 1997;87:775–81. doi: 10.2105/ajph.87.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jatoi I, Anderson WF, Rao SR, et al. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–41. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 6.Kimmick G, Muss HB, Case LD, et al. A comparison of treatment outcomes for black patients and white patients with metastatic breast cancer. The Piedmont Oncology Association experience. Cancer. 1991;67:2850–4. doi: 10.1002/1097-0142(19910601)67:11<2850::aid-cncr2820671124>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Roach M, 3rd, Cirrincione C, Budman D, et al. Race and survival from breast cancer: based on Cancer and Leukemia Group B trial 8541. Cancer J Sci Am. 1997;3:107–12. [PubMed] [Google Scholar]
- 8.Dignam JJ, Redmond CK, Fisher B, et al. Prognosis among African-American women and white women with lymph node negative breast carcinoma: findings from two randomized clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP) Cancer. 1997;80:80–90. doi: 10.1002/(sici)1097-0142(19970701)80:1<80::aid-cncr11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Du WSM. Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243–8. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 10.Connor CS, Touijer AK, Krishnan L, et al. Local recurrence following breast conservation therapy in African-American women with invasive breast cancer. Am J Surg. 2000;179:22–6. doi: 10.1016/s0002-9610(99)00258-5. [DOI] [PubMed] [Google Scholar]
- 11.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. (1):36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. 200. [DOI] [PubMed] [Google Scholar]
- 12.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 13.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–62. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 14.Muss HB, Hunter CP, Wesley M, et al. Treatment plans for black and white women with stage II node-positive breast cancer. The National Cancer Institute Black/White Cancer Survival Study experience. Cancer. 1992;70:2460–7. doi: 10.1002/1097-0142(19921115)70:10<2460::aid-cncr2820701012>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Heimann R, Ferguson D, Powers C, et al. Race and clinical outcome in breast cancer in a series with long-term follow-up evaluation. J Clin Oncol. 1997;15:2329–37. doi: 10.1200/JCO.1997.15.6.2329. [DOI] [PubMed] [Google Scholar]
- 16.Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79:314–9. [PubMed] [Google Scholar]
- 17.Velanovich V, Yood MU, Bawle U, et al. Racial differences in the presentation and surgical management of breast cancer. Surgery. 1999;125:375–9. [PubMed] [Google Scholar]
- 18.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 19.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. World Health Organization; Geneva: 2000. [Google Scholar]
- 20.SEER Summary Staging Manual. 2000 Available: http://www.seer.cancer.gov/tools/ssm/
- 21.Johnson C. Registry operations and data standards. American College of Surgeons; Chicago: 1996. [Google Scholar]
- 22.Johnson C. Registry operations and data standards, Revised (Appendix D, Surgery Codes) Vol. 2. American College of Surgeons; Chicago: 1998. [Google Scholar]
- 23.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–90. [DOI] [PubMed] [Google Scholar]
- 24.United States Department of Health and Human Services [accessed 6/24/2006];Poverty Guidelines, Research, and Measurement. Available: http://www.aspe.hhs.gov/poverty/index.shtml.
- 25.Osteen RT, Steele GD, Jr, Menck HR, et al. Regional differences in surgical management of breast cancer. CA Cancer J Clin. 1992;42:39–43. doi: 10.3322/canjclin.42.1.39. [DOI] [PubMed] [Google Scholar]
- 26.McGinnis LS, Menck HR, Eyre HJ, et al. National Cancer Data Base survey of breast cancer management for patients from low income zip codes. Cancer. 2000;88:933–45. [PubMed] [Google Scholar]
- 27.Mandelblatt JS, Kerner JF, Hadley J, et al. Variations in breast carcinoma treatment in older Medicare beneficiaries: is it black or white. Cancer. 2002;95:1401–14. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 28.Nattinger AB, Gottlieb MS, Veum J, et al. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–7. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 29.Dunmore C, Plummer P, Regan G, et al. Re: race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 2000;92:1690–1. doi: 10.1093/jnci/92.20.1690. [DOI] [PubMed] [Google Scholar]
- 30.Bryant H, Mah Z. Breast cancer screening attitudes and behaviors of rural and urban women. Prev Med. 1992;21:405–18. doi: 10.1016/0091-7435(92)90050-r. [DOI] [PubMed] [Google Scholar]
- 31.Rural Policy Research Institute Welfare Reform in Rural America: A Review of Current Research. 2001.
- 32.Fletcher CFJ, Gaddis B, Winter M, et al. Rural dimensions of welfare reform: A research conference on poverty, welfare, and food assistance. Georgetown University; Washington, DC: 2000. Small towns and welfare reform: Iowa case studies of families and communities. [Google Scholar]
- 33.Schur CFS. Access to health care. Rural health in the United States. Oxford University Press; New York: 1999. [Google Scholar]
- 34.National Advisory Committee on Rural Health Medicare Reform: A Rural Perspective. 2001.
- 35.HHS Rural Task Force Report to the Secretary One Department Serving Rural America. 2002.
- 36.Bland KI, Menck HR, Scott-Conner CE, et al. The National Cancer Data Base 10-year survey of breast carcinoma treatment at hospitals in the United States. Cancer. 1998;83:1262–73. doi: 10.1002/(sici)1097-0142(19980915)83:6<1262::aid-cncr28>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–82. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]


