Abstract
Background
Emerging data have revealed a negative association between adiposity and muscle quality (MQ). There is a lack of research to examine this interaction among young, healthy individuals, and to evaluate the contribution of adiposity to adaptation after resistance exercise (RE).
Objective
The purpose of this investigation was to examine the influence of subcutaneous adipose tissue (SAT) on muscle function among non-obese individuals before and after RE.
Design
Analyses included 634 non-obese (body mass index < 30 kg m−2) subjects (253 males, 381 females; age = 23.3±5.2 years). SAT and muscle mass (magnetic resonance imaging-derived SAT and biceps muscle volume), isometric and dynamic biceps strength, and MQ (strength/muscle volume), were analyzed at baseline and after 12 weeks of unilateral RE.
Results
At baseline, SAT was independently associated with lower MQ for males (β = −0.55; P < 0.01) and females (β = −0.45; P < 0.01), controlling for body mass and age. Adaptation to RE revealed a significant negative association between SAT and changes for strength capacity (β = −0.13; p − 0.03) and MQ (β = −0.14; P < 0.01) among males. No attenuation was identified among females. Post-intervention SAT remained a negative predictor of MQ for males and females (β = − 0.47; P < 0.01).
Conclusions
The findings reveal that SAT is a negative predictor of MQ among non-obese, healthy adults, and that after 12 weeks of progressive RE this association was not ameliorated. Data suggest that SAT exerts a weak, negative influence on the adaptive response to strength and MQ among males.
Keywords: FAMuSS, adiposity, muscle quality, resistance exercise, strength
Introduction
Emerging data suggest that localized adiposity is related to acute muscular weakness and reduced muscle quality (MQ; that is, strength per unit of muscle mass),1–5 as well as incident mobility disability.6 Specifically, evidence now reveals ‘crosstalk’ between muscle and fat tissue may lead to perturbations in muscle regeneration and decreased functional capacity.7 Most research pertaining to the association between muscle mass and strength has demonstrated that physiological cross-sectional area is a robust predictor of force production.8–10 Certainly in the context of a heterogeneous population ample evidence exists to support this association, as well as the general belief that gains in strength are a direct result of gains in muscle mass.9 Conversely from a clinical perspective there is an increasing interest in defining disparate declines in strength and muscle mass, as a way to explicate the health risks of lifestyle and/or aging. At present, variability in the association between muscle mass and strength capacity is commonly attributed to sex differences,11,12 training status13 and/or the aging progression.2,14,15 These factors are also typically thought to influence the adaptive response to resistance exercise (RE).11,16–18
However, in view of the recent data to suggest an attenuating affect of adiposity on muscular function,1,4 further examination to delineate this interaction is certainly merited. Particularly relevant to clinical and public health outcomes, the causal mechanisms through which adiposity contributes to disability have not been fully elucidated, though evidence have confirmed a definitive link between obesity and functional deficit.6,19 In conjunction with general increased skeletal stress,20 the discrete impact of adiposity on muscular strength or MQ may contribute to gradual declines in the functional status and overall increased disability risk throughout adulthood.21 However, previous investigations attempting to distinguish this association, and/or the adaptive response to exercise between overweight/obese and normal-weight individuals, have yielded conflicting results. Cross-sectional studies have suggested that obesity may impair muscle function,22–24 whereas other investigations have demonstrated no differences between normal weight and obese subjects.25,26 Nevertheless, various studies have used body mass index (BMI) as a surrogate of fat mass,26 which is potentially problematic, as BMI cannot discriminate adipose tissue and muscle, and lacks sensitivity to identify non-obese individuals with excess body fat.27 Thus, the use of predetermined BMI cut-points may not sufficiently delineate the association between fat and muscle or between muscle mass and function, and is not an adequate predictor of respective cardiometabolic health risk among ‘normal weight obese’28 cohorts.
Certainly, understanding the independent contribution of depot-specific adiposity on muscle function and metabolic perturbation may better define risk for latent complications. In light of the well-documented interaction of fat and lean tissue, further examining the association between adiposity and muscular function before obesity and disease presentation will elucidate possible etiology for comorbidity and disability. Therefore, the purpose of this investigation was to examine the independent association between subcutaneous adipose tissue (SAT) and muscle strength, and quality among a large cohort of non-obese individuals. A secondary aim of this study was to evaluate adaptive responses to RE between individuals of varying degrees of SAT. On the basis of previous findings, we hypothesized that baseline MQ would be impaired but that muscular adaptation would be similar between individuals with elevated baseline adiposity as compared to lower adiposity.
Methods
This study was a subset of the Functional Polymorphisms Associated with Human Muscle Size and Strength (FAMuSS) investigation.29 Briefly the FAMuSS study was a large, National Institute of health funded multi-center effort to examine the genetic factors associated with baseline muscle, bone and fat tissue, as well as the subsequent adaptive response potential to 12 weeks of unilateral RE of the elbow flexors. The experimental design of FAMuSS has been previously described.11,29 Briefly, a 12-week periodized RE model was used to examine differential training adaptations for muscular hypertrophy of the upper arm among young, healthy, untrained males and females. Each subject was tested before and after the training intervention on precise measures of muscular mass and strength, as well as various anthropometric characteristics and subcutaneous fat mass. All subjects were asked to maintain normal dietary practices and refrain from additional exercise or weight loss practices. In all, 634 (n = 253 males, n = 381 females; age = 23.3 ± 5.2 years) non-obese (that is, BMI < 30 kg m−2) subjects were included in these analyses. Each subject signed an informed consent document, and all procedures were approved by the institutional review boards from the 10 sites involved with FAMuSS.
Materials and procedures
Baseline and follow-up anthropometric measures included body mass (kg), height (cm) and BMI (kg m−2). The upper arm volumetric measurements were assessed through standard magnetic resonance imaging (MRI) technique to specifically evaluate whole muscle volume and subcutaneous fat mass.
Volumetric measurements
MRI was performed before and after 12-week training intervention in order to assess the baseline of the subjects, and post-intervention whole muscle volume and subcutaneous fat volume as previously described.11 Subjects were scanned in the supine position with arms at their sides and palms facing up, on the scanner bed surface. Baseline MRI was performed 24–48 h before the first strength measurement. The hand was supinated and taped in place on the scanner bed surface, and the point of measurement centered to the alignment light of the MRI. To avoid any latent affects that strength testing/training might have on the outcomes (for example, cellular fluid retention), MRI scans for post assessment took place 48-96 h after the last session. MRI was performed at the maximum circumference of the upper arm (that is, belly of the muscle). The maximum circumference of the upper arm was identified with the biceps maximally contracted, the shoulder abducted to 90 F, and elbow flexed at 90°. After determining the circumference using an elastic measuring tape, the corresponding location was marked on the skin of the subject (point of measurement) using a radiographic bead (Beekley Spots, Beekley Corp., Bristol, CT, USA). This point of measurement was subsequently used for the alignment light of the MRI. Using an MRI scout image, six to nine slices were obtained to locate the long axis of the humerus. Subsequently, using the point of measurement as the central point, 15 serial fast spoiled gradient images of each arm were obtained (TE = 1.9 s, TR = 200 ms, flow artifact suppression, 30° flip angle). These image slices began at the top of the upper-arm and proceeded distal toward the elbow. This arrangement provided an image of the muscle belly that corresponded to slices 8 and 9. Each slice was 16mm thick, with a 0-mm interslice gap, 256 × 192 matrix resolution, 22 × 22 cm field of view and number of acquisitions is 6. This method allowed for 24-cm length images to be collected of each upper arm, which were subsequently analyzed volumetrically using a computer-based, three-dimensional interactive system called Rapidia (3D Med Co. Ltd., Seoul, Republic of Korea), and a custom-designed interactive processing and visualization program using Matlab (The Math Works Inc., Natick, MA, USA). To ensure accurate and reliable measurements, six slices from each image were analyzed using the metaphyseal–diaphyseal junction landmark, making sure the same regions were measured from pre- and post-images. Muscle and fat were isolated using image signal intensity differences between tissues, and once the region of interest was segmented, total volume was taken from the six evaluated slices. Repeatability and reliability of Rapidia volume measurements were verified using a phantom of known volume.
Serum cardiometabolic markers
Subjects completed one morning of testing (at baseline) after an overnight fast of at least 12 h. A venous blood sample was drawn into tubes (Vacutainer; BD Biosciences, Franklin Lakes, NJ, USA) for the determination of plasma glucose, serum insulin, C-reactive protein and lipid profile (triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein cholesterol and very-low-density lipoprotein concentrations), as previously described.30 The homeostasis model assessment on insulin resistance values were obtained from the following calculation: homeostasis model assessment on insulin resistance = (fasting glucose (mmoll−1) × fasting insulin (μUml−1))/22.5.31
Strength assessment
Baseline and post-intervention strength was evaluated through isometric and dynamic tests. Isometric maximal voluntary contraction (MVC) of the elbow flexors was tested using a strain gauge attached to a strength evaluation system (Model 32628CTL; Lafayette Instrument Company, Lafayette, IN, USA). Pre-intervention MVC was determined after three separate trials, and results were recorded as the average of the second and third trials. Three MVC tests lasting 3 s were performed on each arm, and were separated by 1-min rest periods. Peak force values were averaged for each testing day.
For dynamic strength, a modified one repetition maximum (1RM) protocol for dumbbell curl (Powerblocks; Intellbell Inc., Owatonna, MN, USA) was completed on a standard preacher curl bench. Before testing, an incremental warm-up took place and subjects were instructed to perform a full range of motion repetition (that is, from 180° to full elbow flexion) with a load that was estimated to be 100% of maximal ability. If this initial attempt was successful, it was followed by a small load increase (∼0.563–1.125 kg) and a 3-min rest period. Each failed attempt was succeeded by a small load decrease and also a 3 min rest. This process was repeated until a true 1RM was determined, which was identified if a subject failed to complete an additional incremental load increase after a given successful attempt. All 1RMs were determined within 3–5 attempts, and the maximal load was recorded in kilograms.
Muscle quality
Baseline and post-intervention 1RM strength scores were normalized to adjust for muscle size differences. Normalized force is a good indirect measure of MQ incorporating the dynamic strength capacity of muscle (1RM strength) and muscle size (whole muscle volume), and was determined using the following calculation: (strength/muscle volume). This method has been previously used30,32–35 as an index of relative strength per muscle mass, and may be considered a superior marker of upper body muscular function over absolute or body mass adjusted strength. MVC MQ was also calculated (data not shown). On comparing data from normalized 1RM (1RM-MQ) and MVC (MVC-MQ) strength, the results of the regression were unchanged, and thus we chose to use only 1RM-MQ in our final analysis. Moreover, given that 1RM strength is a dynamic assessment of maximal muscular function, it may be considered a more generalizable test to accompany a dynamic RE protocol.
Exercise training program
All RE took place with the non-dominant arm. Throughout the 12 week intervention, subjects met twice per week, for ∼45–60 min per session. Compliance to training was monitored by the research group and fitness staff responsible for training implementation. Exclusion criterion for analyses was set at anything in excess of two missed workouts during the entire training intervention. The specific details of the resistance training program have been documented previously29 Briefly, each session of RE was preceded by a specific warm-up of two sets of 12 repetitions with moderate resistance, for an arm flexion and extension exercise. Subsequent training incorporated dumbbell exercises (Power Blocks; Intellbell Inc.) for biceps preacher curls, standing biceps curls and seated incline biceps curls, as well as overhead triceps extension and triceps kickbacks. The tempo was controlled for every contraction, and included a 2 s concentric and 2 s eccentric repetition cadence. The specific progression of weekly training included: weeks 1–4: 3 sets of 12 repetitions, with a 12-repetition maximum weight (3 × 12RMs); weeks 5–9: 3 sets of 8 repetitions, with an 8-repetition maximum weight (3 × 8RMs); weeks 10–12: 3 sets of 6 repetitions, with a 6-repetition maximum weight (3 × 6RMs). In accordance with the principle of progressive overload, individual variations in load and repetitions occurred as subjects progressed in strength capacity. Specific load increases occurred on an incremental basis (that is, 0.5–2 kg), if an individual was able to complete two or more repetitions over his/her assigned repetition goal for any exercise, in the last set, for two consecutive workouts (the ‘2-for-2 rule’36). Complete repetitions were counted only if a participant was able to achieve full range of motion. Incomplete repetitions were recorded as one half of an attempt.
Dietary control
All participants were instructed to maintain habitual dietary practices during the course of the intervention. Individuals who were currently taking supplemental dietary protein and/or other supplements reported to build muscle or to cause weight gain (that is, dietary supplements containing protein, creatine or androgenic precursors) were not eligible for recruitment in the study. Moreover, data from individuals who had lost significant body mass during the course of the 12 weeks were not analyzed.
Statistical analyses
Pearson's product-moment correlations were used to examine the selected bivariate correlations. A minimum criterion α level of P ⩽ 0.05 was used to determine statistical significance. Data are reported as means and standard deviations (s.d.). Multiple linear regression was initially used to evaluate the association between adiposity and MQ (baseline and post-intervention MQ), after controlling for multiple confounding factors. The following standard covariates were included in the model: age and body mass. Previous data have demonstrated a sex-specific adaptation profile to RE,11 which we confirmed through an initial model testing the association of sex on MQ (β = 0.24; P < 0.01). Further, because sex was identified to be a significant predictor of baseline characteristics, all analyses were conducted separately for males and females. Specifically, multiple regression analyses were conducted to examine the sex-specific influence of adiposity on MQ, as well as the relation between muscle mass and strength, while controlling for fat mass.
Analysis of covariance was also conducted to assess the adaptive responses for outcomes following RE. Specifically, linear regression took place for each of the following dependent variables:(1) muscular hypertrophy, and (2) 1RM strength capacity. In each of these models, post-intervention means were entered as the dependent variable (for example, post-intervention mean whole muscle volume), and baseline muscle mass and strength characteristics were entered as covariates, along with the pre-specified significant correlates (age and body mass) as independent moderators. This method was completed to reduce the risk of regression to the mean, which may lead to an over- or underestimation of the intervention effect, and is a potential issue when assessing pre-to-post intervention change scores (that is, absolute mean differences).37 Collinearity was examined using the variance inflation factor, and tests revealed no issues of collinearity for any model. For each model standard regression coefficients (β) were determined, and paired t-tests were used to evaluate the respective zero difference. Further, percent variance attributable to the main outcome within each model was tested using an analysis of variance to determine the significance of each model.
Results
Sex-specific baseline comparisons
With the exception of subcutaneous fat mass, which was greater in females (P < 0.01), males had higher values for all baseline measures (P < 0.01) (Table 1). Further, among both males and females age and body mass were correlated with baseline muscle volume, strength and MQ (r = 0.15–0.25; P < 0.05).
Table 1. Baseline, post-intervention and pre-to-post intervention absolute changes for demographic data, whole muscle volume, SAT volume and muscle functional parameters.
| Group | Body mass (kg) | BMI (kg m−2) | Whole muscle volume (ml) | SAT volume (ml) | Isometric MVC (kg) | 1RM strength (kg) | Muscle quality (kg m−1) |
|---|---|---|---|---|---|---|---|
| All (N = 634) | |||||||
| Baseline | 66.1 ± 11.9 | 22.9 ± 2.8 | 469.1 ± 126.8 | 197.3 ± 85.5 | 39.7 ± 21.6 | 8.3 ± 0.1 | 1.8E-2 ± 0.7E-2 |
| Post training | 66.5 ± 12.3 | 23.1 ± 2.8 | 522.7 ± 137.8 | 201.3 ± 87.3 | 47.2 ± 24.7 | 12.1 ± 4.4 | 2.4E-2 ± 0.7E-2 |
| Pre/post change | 0.4 ± 5.2 | 0.2 ± 0.8 | 54.1 ± 38.6a | 3.9 ± 24.7b | 7.5 ± 7.5 | 4.0 ± 2.2b | 0.6E-2 ± 0.5E-2b |
| Males (N = 253) | |||||||
| Baseline | 73.5 ± 0.8a | 23.5± 0.2a | 522.2 ± 112.9a | 148.7 ± 69.5 | 57.9±21.2a | 11.7 ± 2.9a | 2.3E-2 ± 0.6E-2a |
| Post training | 74.3 ± 11.2 | 23.7 ± 2.9 | 594.5 ± 121.1 | 152.2 ± 73.0 | 67.8 ±24.6 | 16.0 ± 3.7 | 2.7E-2 ± 0.6E-2 |
| Pre/post change | 0.8 ± 5.5 | 0.2 ± 0.8 | 72.3 ± 40.4b,c | 3.5 ± 22.2b | 9.9 ± 9.1b,c | 4.5 ± 2.6b,c | 0.5E-2 ± 0.5E-2b |
| Females (N = 381) | |||||||
| Baseline | 61.2 ± 0.5 | 22.6 ± 0.1 | 433.6 ± 123.3 | 229.6 ± 79.7a | 27.8 ±10.9 | 5.9 ± 1.5 | 1.5E-2 ± 0.5E-2 |
| Post training | 61.3 ± 10.0 | 22.7 ± 2.7 | 475.2 ± 127.4 | 233.8 ± 80.5 | 33.6 ±12.4 | 9.5 ± 2.4 | 2.1E-2 ± 0.8E-2 |
| Pre/post change | 0.1 ± 4.9 | 0.2 ± 0.8 | 42.0 ± 32.2b | 4.2 ± 26.4b,c | 5.9±5.7b | 3.6 ± 1.9b | 0.7E-2 ± 0.4E-2b,c |
Abbreviations: 1RM, one repetition maximum; BMI, body mass index; MVC, maximal voluntary contraction; SAT, subcutaneous adipose tissue.
A significant gender difference at baseline (P < 0.05).
A significant effect by time (P < 0.05).
A significantly greater gender × time interaction (P < 0.05).
Baseline MQ
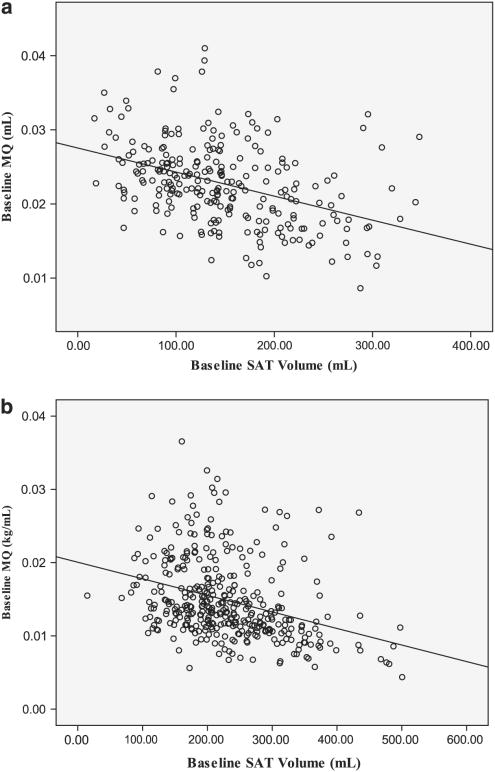
Initial multiple regression revealed that adiposity was an independent negative predictor of baseline MQ for males (β = −0.55; P < 0.01) and females (β = −0.45; P < 0.01), controlling for age and body mass (Figures 1a and b).
Figure 1.
The relationship between subcutaneous adipose tissue and muscle quality at baseline, for males (a) and females (b).
Data also revealed variability in the relationship between baseline muscle volume and strength capacity contingent on fat mass being entered as a moderating variable. Specifically through initial univariate regression, muscle mass predicted baseline strength capacity for males (r2 = 0.18; β = 0.44; P < 0.01) and females (r2 = 0.05; β = 0.22; P < 0.01). However, after entering fat mass into the model, a greater amount of additional variance in strength capacity was explained. For both males and females, SAT was negatively associated with baseline strength capacity (Table 2), when controlling for muscle volume.
Table 2. Sub-group linear regression for whole muscle volume (independent predictor) and strength capacity (dependent variable), without (Model 1) and with (Model 2) subcutaneous adipose tissue (SAT) volume entered into the model.
| Model | Predictor(s) | β | t | p | F | Adjusted R2 | VIF | |
|---|---|---|---|---|---|---|---|---|
| Muscular strength capacity (1RM) | ||||||||
| Males | 1 | Whole muscle volume | 0.44 | 7.70 | < 0.01 | 58.50 | 0.18 | 1.0 |
| 2 | Whole muscle volume | 0.61 | 9.90 | < 0.01 | 49.40 | 0.28 | 1.3 | |
| SAT volume | −0.35 | −5.70 | < 0.01 | — | — | 1.3 | ||
| Females | 1 | Whole muscle volume | 0.22 | 5.00 | < 0.01 | 19.40 | 0.05 | 1.0 |
| 2 | Whole muscle volume | 0.31 | 9.90 | < 0.01 | 12.70 | 0.07 | 1.6 | |
| SAT volume | −0.15 | −2.40 | 0.02 | — | — | 1.6 | ||
Abbreviations: 1RM, one repetition maximum; VIF, variance inflation factor.
Adaptive response to RE
Post-intervention descriptive data, as well as respective pre-to-post intervention changes in whole muscle volume, strength and MQ are presented in Table 1. Males experienced greater improvements than females in hypertrophy (that is, absolute pre-to-post change in whole muscle volume) and strength (P < 0.01), however less improvement in MQ (P < 0.01). With regard to the influence of adiposity on adaptive response to RE, regression did not identify a significant relationship between SAT and pre-to-post change for whole muscle volume for males or females. However, among males a significant association was determined between baseline SAT and pre-to-post intervention change for strength capacity (β = −0.13; P = 0.03; Table 3), and thus also for MQ (β = −0.14; P < 0.01). Accordingly, baseline SAT exerts a weak, yet significant reciprocal association with strength adaptation among males. Conversely, for females no specific relationships were identified between SAT and pre-to-post intervention change for strength or MQ (P > 0.05).
Table 3. Multiple regression models for hypertrophic (whole muscle volume) and strength (1RM) adaptive responses to resistance exercise, for both males and females.
| Model: predictor(s) | β | t | P | F | Adjusted R2 | VIF | |
|---|---|---|---|---|---|---|---|
| Post-intervention muscle volume | |||||||
| Males | Baseline muscle volume | 0.91 | 32.30 | < 0.01 | 507.04 | 0.89 | 1.8 |
| Baseline SAT | −0.02 | −0.80 | 0.42 | — | — | 1.5 | |
| Age | 0.03 | 1.40 | 0.18 | — | — | 1.0 | |
| Baseline body mass | 0.07 | 2.50 | 0.02 | — | — | 1.9 | |
| Females | Baseline muscle volume | 0.97 | 58.40 | < 0.01 | 1370.40 | 0.94 | 1.6 |
| Baseline SAT | 0.01 | 0.33 | 0.75 | — | — | 2.3 | |
| Age | −0.01 | −0.92 | 0.36 | — | — | 1.0 | |
| Baseline body mass | −0.02 | −0.92 | 0.36 | — | — | 2.0 | |
| Post-intervention muscular strength (1RM) | |||||||
| Males | Baseline strength (1RM) | 0.64 | 12.50 | < 0.01 | 71.50 | 0.59 | 1.6 |
| Baseline muscle volume | 0.18 | 3.14 | < 0.01 | — | — | 2.0 | |
| Baseline SAT | −0.13 | −2.30 | 0.03 | — | — | 1.9 | |
| Age | −0.14 | −3.30 | < 0.01 | — | — | 1.0 | |
| Baseline body mass | 0.09 | 1.50 | 0.14 | — | — | 2.1 | |
| Females | Baseline strength (1RM) | 0.61 | 13.90 | < 0.01 | 61.50 | 0.46 | 1.3 |
| Baseline muscle volume | 0.01 | 0.26 | 0.79 | — | — | 1.7 | |
| Baseline SAT | −0.04 | −0.56 | 0.57 | — | — | 2.6 | |
| Age | −0.14 | −3.70 | < 0.01 | — | — | 1.0 | |
| Baseline body mass | 0.13 | 2.10 | 0.04 | — | — | 2.4 | |
Abbreviations: 1RM, one repetition maximum; SAT, subcutaneous adipose tissue; VIF, variance inflation factor.
However, for post-intervention data, regression revealed that SAT (post-intervention subcutaneous fat volume) remained as an independent negative predictor of post-intervention MQ for both males (β = −0.47; P < 0.01) and females (β = −0.47; P < 0.01).
Discussion
To our knowledge, this is the first investigation to isolate and examine the discrete associations between adiposity, MQ and the morphological and functional adaptive responses to RE. Further noteworthy, this investigation incorporated precise imaging techniques to evaluate localized muscle and SAT volumetric data before and after a unilateral RE protocol within a large cohort of healthy, untrained, non-obese (BMI < 30 kg m−2) males and females. The principal findings of this study suggest that greater subcutaneous adiposity is negatively associated with MQ among young, non-obese individuals, and that after 12-weeks of progressive RE this influence was not ameliorated at post intervention. Specifically, our findings revealed a robust negative association between SAT and MQ, such that when controlling for age and body mass (two independent, significant correlates), SAT made a strong and unique contribution to explaining the variance in MQ for both males (r2 = 0.20; β = −0.53) and females (r2 = 0.12; β = −0.45). According to these data, a one standard deviation change in subcutaneous fat volume (69.5 ml-80.0 for males and females, respectively) was predictive of an ∼0.5 s.d. decrease in MQ.
Current finding are supportive of our hypothesis that subcutaneous adiposity is reciprocally associated with MQ among young, healthy, non-obese subjects. Previous reports pertaining to the impact of adipose tissue on MQ have been limited to assessment and implication of myosteatosis (that is, muscle ‘fat infiltration’).3 Most often characterized with cross-sectional data from aging adults (muscle attenuation on computed tomography3 or localized intermuscular adipose tissue (IMAT) with MRI),38 this fat infiltration also appears in conjunction with certain disease processes (for example, Duchenne muscular dystrophy, type 2 diabetes),39,40 and obesity,41–43 as well as during periods of reduced physical activity.44 Indeed, recent findings reveal a ‘crosstalk’ between myogenic and adipocyte cell progenitors, which seem to trigger simultaneous muscle degeneration and formation of adipocytes, scar tissue and/or collagen within the muscle.7,45 Although the MRI data analysis in this study did not allow us to distinguish IMAT from lean muscle tissue, it is plausible that IMAT depot may have been pronounced among individuals with greater SAT. Recent evidence confirm a robust association between total adipose tissue and ectopic fat (that is, adipose tissue accumulated within anatomic regions outside the subcutaneous depot area).46,47 Thus although subjects from this study were all classified as ‘non-obese’ based on BMI, variability in IMAT may have misrepresented data for whole muscle volume and modulated the association between muscle mass and strength. Nevertheless, without a direct measure of IMAT, we can only speculate on this as one potential underlying mechanism. Therefore, additional research is warranted to examine the potential attenuation of muscle among non-obese, young adults with varying degrees of adiposity, as well as to delineate the extent to which fat infiltration and SAT independently compromise MQ.
Certainly, it stands to reason that adipocyte proliferation and accumulation occurs long before an individual meets the criterion for ‘obesity,’ or is considered ‘at risk’ for cardiometabolic disease. Therefore, in conjunction with pronounced changes in the hormonal/metabolic milieu,41,48 greater adiposity could yield a chronic inflammatory state49 and general, inhospitable physiological environment that contributes to the degradation of contractile properties and diminished MQ. Although no subjects in this study presented with clinical risk factors for cardiometabolic disease, we were able to identify a significant association between SAT and various sub-clinical levels of cardiometabolic risk, and low-grade inflammation. Stratifying into equal tertiles for SAT provided further evidence of this trend (Supplementary Table 4), and seems to reflect proportional mean differences for serum markers between the low- and medium-SAT groups, as compared with the medium- and high-SAT groups. We were also able to examine the isolated association between muscle mass and strength across tertiles. By doing so, data revealed a significant statistical trend of diminished covariance and standardized regression coefficients across each incrementally higher SAT grouping (see Supplementary Figure 2), providing further evidence that adiposity modulates the relationship between muscle mass and function.
As adipose tissue is considered to be a dynamic organ with pleiotropic endocrine properties,49 it is conceivable that even sub-clinical elevation of cardiometabolic risk factors, including low grade inflammation, could induce early-onset contractile dysfunction. Previous research among non-diabetic, obese adults have revealed significantly higher levels of adipocyte-derived hormones and cytokines (that is, adipokines), which are specific contributors to elevated insulin resistance,50 as well as decreased muscle mass and strength capacity.51 The chronic and gradual confluence of adipogenesis, low-grade inflammation and metabolic disturbance may perpetuate a steady decline of muscular function before clinical presentation of disease risk, and thus, additional research to delineate this multidimensional phenomenon is warranted.
Certainly, identifying appropriate preventive strategies is a vital directive to reduce risk of latent cardiometabolic cormorbidity and functional strength decline. In this investigation, no attenuation of the adaptive response to RE was evidenced by hypertrophic changes. Data indicated a weak reciprocal association between SAT and strength adaptation for males, but no such attenuation among females. This finding is consistent with a previous study that demonstrated similar improvements in muscular strength and endurance between obese versus normal weight women, after a 14-week multi-modality fitness program.25 Nevertheless, in this investigation, post-intervention data revealed that after 12-weeks of progressive RE, the reciprocal association between SAT and MQ for both males and females was not ameliorated. Therefore, despite significant and well-documented11 increases in muscle mass and strength for all participants, the attenuation of MQ for subjects with elevated SAT was still present after training. This suggests that among individuals with larger SAT depot, a RE program without concurrent decreases in adiposity may not be a sufficient stimulus to correct attenuated muscle and/or impaired MQ. However, although the use of a unilateral RE protocol is necessary to control for various potential confounding variables, this model may have less generalizability as compared with full-body RE protocols. Indeed more research is needed to investigate the utility of full-body RE to target impaired MQ.
Previous investigations attempting to distinguish the association between obesity and functional capacity have yielded conflicting results. With respect to strength outcomes, various studies have demonstrated greater absolute strength among obese versus non-obese subjects.23,52,53 Conversely, when adjusting for body mass (for example, allometric scaled strength), most data confirm a significant ‘impairment’ of strength capacity among obese individuals.23,53 It has been proposed that a relative, diminished muscle functional capacity in obese subjects may be due to metabolic disturbances within the muscle, such as reduced oxidative capacity54,55 and/or decreased capillary density,56 as well as an altered motor unit activation57 and nerve conduction.58 Indeed, changes in the oxidative capacity and blood flow may reflect negative consequences for fatigue resistance.53 However, a reduction in motor unit recruitment or changes in nerve conduction are more likely to impose a definitive attenuation of strength capacity and muscular power. Previous research to examine motor unit activation in obese and non-obese subjects have reported lower relative maximal isokinetic and isometric knee extension strength among obese subjects, as well as significantly reduced motor unit activation.57 Such results indicate that the handicap of ‘dead weight’ adiposity57 in conjunction with reduced neuromuscular activation may account for the commonly observed strength ‘impairment’ in obese subjects.
Considering the exaggerated risk of functional deficit that occurs with obesity6,19 it is plausible that the affect of adiposity on MQ may therefore, independently contribute to mobility disability risk and decreased fine motor skill performance.21,59 However, previous studies have generally used stratified body mass characteristics (for example, lean (< 24 kg m−2), normal weight (24–29 kg m−2) and high (>29 kg m−2) BMI)26 as surrogate indicators of adiposity, which does not entirely characterize the discrete influence of adiposity on respective acute muscle function. Muscle mass is generally considered to be the primary determinant of the sex- and age-related variability in strength capacity, and therefore it stands to reason that measures should instead be corrected for muscle mass or fat-free mass (that is, MQ). Moreover, it is conceivable that depot-specific genetic, biochemical and metabolic features are responsible for variation in adipocyte proliferation and differentiation, as well as the potential influence on muscle function.60 Although current data are reflective of a reciprocal association between SAT and adaptive response to muscle strength and quality for males, subsequent research is needed to clarify the sex-specific contribution of distinct adipose tissue compartments on MQ and morphological/architectural characteristics among non-obese subjects.
Conclusions
Among healthy, non-obese (BMI < 30 kg m−2), young adults, localized subcutaneous adiposity is an independent negative predictor of MQ and despite significant increases in muscle mass and strength following 12 weeks of RE, this association was not ameliorated at post intervention. Further, data suggest that adiposity exerts a weak, yet reciprocal influence on the adaptive response to strength and MQ among males. These findings bear clinical significance considering the exaggerated risk of functional decline that coincides with disuse and weight gain. Thus, although previous research has demonstrated a general, robust association between muscle mass and strength capacity, current data indicate that SAT modulates the covariation between muscle mass and function. It is certainly plausible that this negative association may predispose non-obese individuals to greater risk of latent functional deficit through the span of adulthood. As such declines precipitate diminished autonomy and mobility disability, simultaneous preservation/increases in MQ and preservation/decrease of SAT may serve as a powerful preventive or treatment strategy.
Supplementary Material
Acknowledgments
Dr Mark Peterson is supported by the NIH, NICHD, NCMRR Grant no. 5 T32-HD007422. The Functional Polymorphisms Associated with Muscle Size and Strength Study is funded by a grant from the National Institute Health (5RO1NS040606-03).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 4.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerletti M, Shadrach JL, Jurga S, Sherwood R, Wagers AJ. Regulation and function of skeletal muscle stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:317–322. doi: 10.1101/sqb.2008.73.054. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 7.Rodeheffer MS. Tipping the scale: muscle versus fat. Nat Cell Biol. 2010;12:102–104. doi: 10.1038/ncb0210-102. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001;172:249–255. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones EJ, Bishop PA, Woods AK, Green JM. Cross-sectional area and muscular strength: a brief review. Sports Med. 2008;38:987–994. doi: 10.2165/00007256-200838120-00003. [DOI] [PubMed] [Google Scholar]
- 10.Moss BM, Refsnes PE, Abildgaard A, Nicolaysen K, Jensen J. Effects of maximal effort strength training with different loads on dynamic strength, cross-sectional area, load-power and load-velocity relationships. Eur J Appl Physiol Occup Physiol. 1997;75:193–199. doi: 10.1007/s004210050147. [DOI] [PubMed] [Google Scholar]
- 11.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. [PubMed] [Google Scholar]
- 12.Schantz P, Randall-Fox E, Hutchison W, Tyden A, Astrand PO. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol Scand. 1983;117:219–226. doi: 10.1111/j.1748-1716.1983.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 13.Castro MJ, McCann DJ, Shaffrath JD, Adams WC. Peak torque per unit cross-sectional area differs between strength-trained and untrained young adults. Med Sci Sports Exerc. 1995;27:397–403. [PubMed] [Google Scholar]
- 14.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch. 1997;434:246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- 15.Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol. 1992;47:M204–M210. doi: 10.1093/geronj/47.6.m204. [DOI] [PubMed] [Google Scholar]
- 16.Lemmer JT, Hurlbut DE, Martel GF, Tracy BL, Ivey FM, Metter EJ, et al. Age and gender responses to strength training and detraining. Med Sci Sports Exerc. 2000;32:1505–1512. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Peterson M, Rhea M, Sen A, Gordon P. Progressive resistance training for strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson M, Sen A, Gordon P. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011 Jun; doi: 10.1249/MSS.0b013e3181eb6265. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter S, Kunst A, Mackenbach J, Hofman A, Tiemeier H. Mortality and disability: the effect of overweight and obesity. Int J Obes (Lond) 2009;33:1410–1418. doi: 10.1038/ijo.2009.176. [DOI] [PubMed] [Google Scholar]
- 20.Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- 21.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92:834–840. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duche P, Ducher G, Lazzer S, Dore E, Tailhardat M, Bedu M. Peak power in obese and nonobese adolescents: effects of gender and braking force. Med Sci Sports Exerc. 2002;34:2072–2078. doi: 10.1097/00005768-200212000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25:676–681. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- 24.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E. Predictors of 6-min walk test results in lean, obese and morbidly obese women. Scand J Med Sci Sports. 2003;13:98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 25.Blake A, Miller WC, Brown DA. Adiposity does not hinder the fitness response to exercise training in obese women. J Sports Med Phys Fitness. 2000;40:170–177. [PubMed] [Google Scholar]
- 26.Rolland Y, Lauwers-Cances V, Pahor M, Fillaux J, Grandjean H, Vellas B. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am J Clin Nutr. 2004;79:552–557. doi: 10.1093/ajcn/79.4.552. [DOI] [PubMed] [Google Scholar]
- 27.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson PD, Moyna N, Seip R, Price T, Clarkson P, Angelopoulos T, et al. Functional polymorphisms associated with human muscle size and strength. Med Sci Sports Exerc. 2004;36:1132–1139. doi: 10.1249/01.mss.0000132274.26612.23. [DOI] [PubMed] [Google Scholar]
- 30.Pistilli EE, Devaney JM, Gordish-Dressman H, Bradbury MK, Seip RL, Thompson PD, et al. Interleukin-15 and interleukin-15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine. 2008;43:45–53. doi: 10.1016/j.cyto.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanton L, Visich P, Zoeller R, Angelopoulos T, Price T, Moyna N, et al. Strength, size and muscle quality in the upper arm following unilateral training in young and older males and females. Clin Med Arth and Muscoskel Disorders. 2009;2:9–18. [Google Scholar]
- 34.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–B218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 35.Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- 36.Baechle T, Earle R, Wathen D. Resistance training. In: Baechle T, Earle R, editors. Essentials of Strength Training and Conditioning. 3rd. Human Kinetics; Champaign, IL: 2008. pp. 381–412. [Google Scholar]
- 37.Twisk J, Proper K. Evaluation of the results of a randomized controlled trial: how to define changes between baseline and follow-up. J Clin Epidemiol. 2004;57:223–228. doi: 10.1016/j.jclinepi.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Rossi A, Zoico E, Goodpaster BH, Sepe A, Di Francesco V, Fantin F, et al. Quantification of intermuscular adipose tissue in the erector spinae muscle by MRI: agreement with histological evaluation. Obesity (Silver Spring) 2010;18:2379–2384. doi: 10.1038/oby.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leroy-Willig A, Willig TN, Henry-Feugeas MC, Frouin V, Marinier E, Boulier A, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15:737–744. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 42.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 43.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–278. [PubMed] [Google Scholar]
- 44.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 45.Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, et al. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E560–E565. doi: 10.1152/ajpendo.00202.2005. [DOI] [PubMed] [Google Scholar]
- 46.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes. 2004;5:219–226. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, You T, Yang R, Lyles MF, Demons J, Gong DW, et al. Muscle strength is associated with adipose tissue gene expression of inflammatory adipokines in postmenopausal women. Age Ageing. 2010;39:656–659. doi: 10.1093/ageing/afq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 51.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 52.Pescatello LS, Kelsey BK, Price TB, Seip RL, Angelopoulos TJ, Clarkson PM, et al. The muscle strength and size response to upper arm, unilateral resistance training among adults who are overweight and obese. J Strength Cond Res. 2007;21:307–313. doi: 10.1519/R-22236.1. [DOI] [PubMed] [Google Scholar]
- 53.Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A, et al. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101:51–59. doi: 10.1007/s00421-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 54.Newcomer BR, Larson-Meyer DE, Hunter GR, Weinsier RL. Skeletal muscle metabolism in overweight and post-overweight women: an isometric exercise study using (31)P magnetic resonance spectroscopy. Int J Obes Relat Metab Disord. 2001;25:1309–1315. doi: 10.1038/sj.ijo.0801673. [DOI] [PubMed] [Google Scholar]
- 55.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. The biomechanics of restricted movement in adult obesity. Obes Rev. 2006;7:13–24. doi: 10.1111/j.1467-789X.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 56.Mandroukas K, Krotkiewski M, Hedberg M, Wroblewski Z, Bjorntorp P, Grimby G. Physical training in obese women. Effects of muscle morphology, biochemistry and function. Eur J Appl Physiol Occup Physiol. 1984;52:355–361. doi: 10.1007/BF00943363. [DOI] [PubMed] [Google Scholar]
- 57.Blimkie CJ, Sale DG, Bar-Or O. Voluntary strength, evoked twitch contractile properties and motor unit activation of knee extensors in obese and non-obese adolescent males. Eur J Appl Physiol Occup Physiol. 1990;61:313–318. doi: 10.1007/BF00357619. [DOI] [PubMed] [Google Scholar]
- 58.Buschbacher RM. Body mass index effect on common nerve conduction study measurements. Muscle Nerve. 1998;21:1398–1404. doi: 10.1002/(sici)1097-4598(199811)21:11<1398::aid-mus6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 59.D'Hondt E, Deforche B, De Bourdeaudhuij I, Lenoir M. Childhood obesity affects fine motor skill performance under different postural constraints. Neurosci Lett. 2008;440:72–75. doi: 10.1016/j.neulet.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 60.Giorgino F. Adipose tissue function and dysfunction: organ cross-talk and metabolic risk. Am J Physiol Endocrinol Metab. 2009;297:E975–E976. doi: 10.1152/ajpendo.00488.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.