Abstract
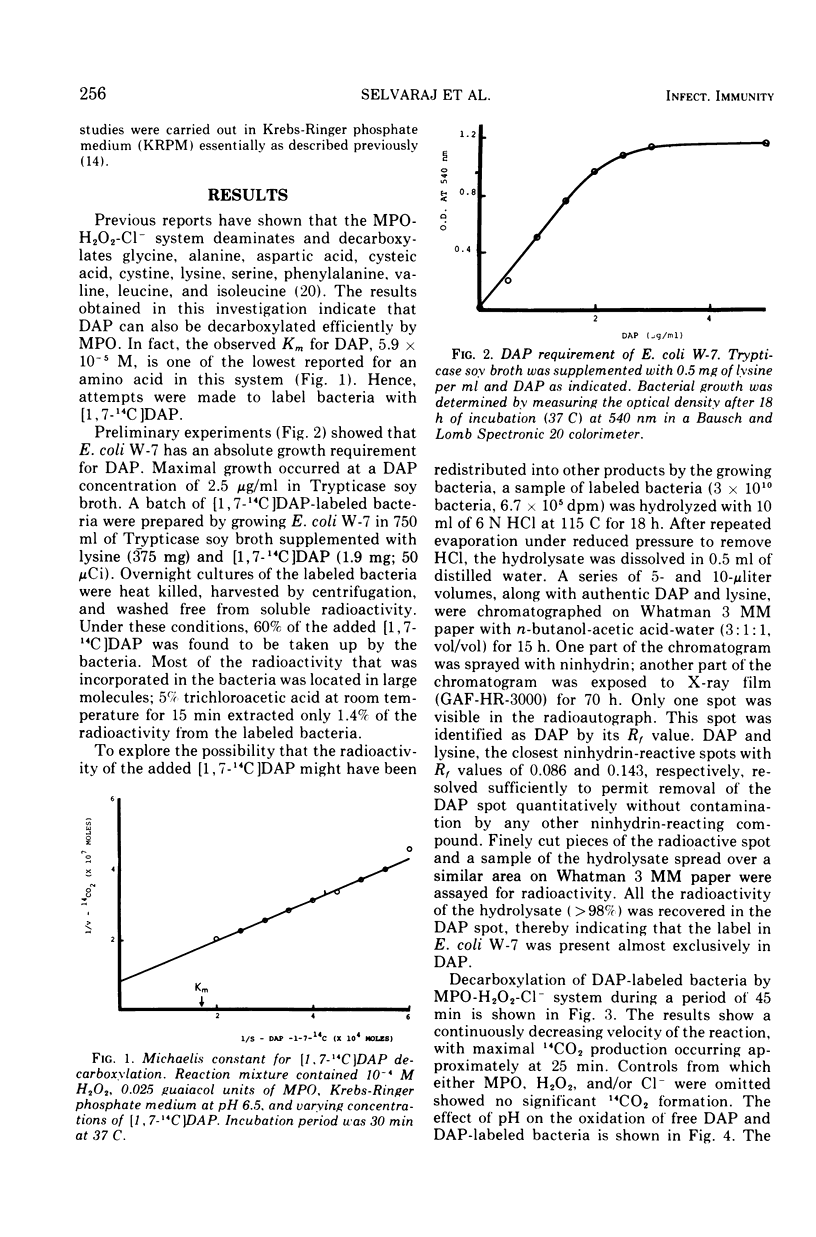
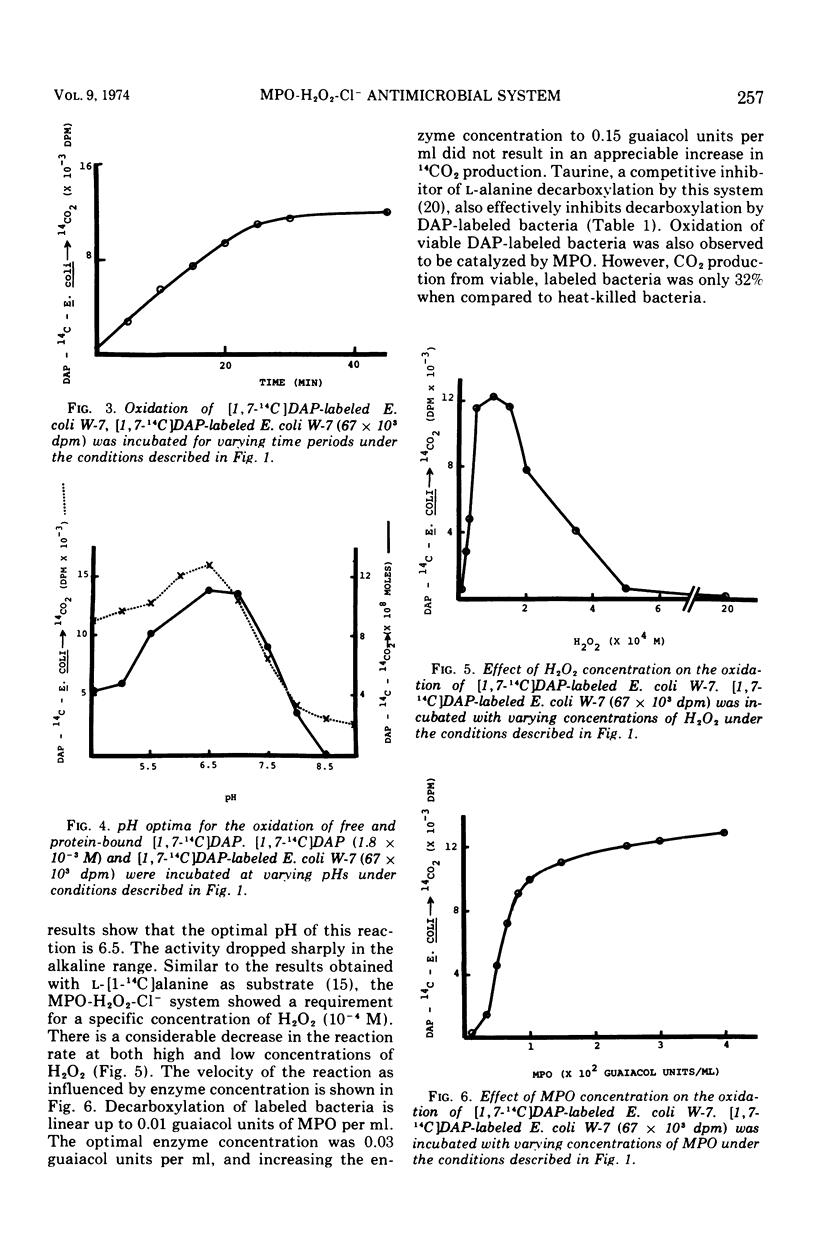
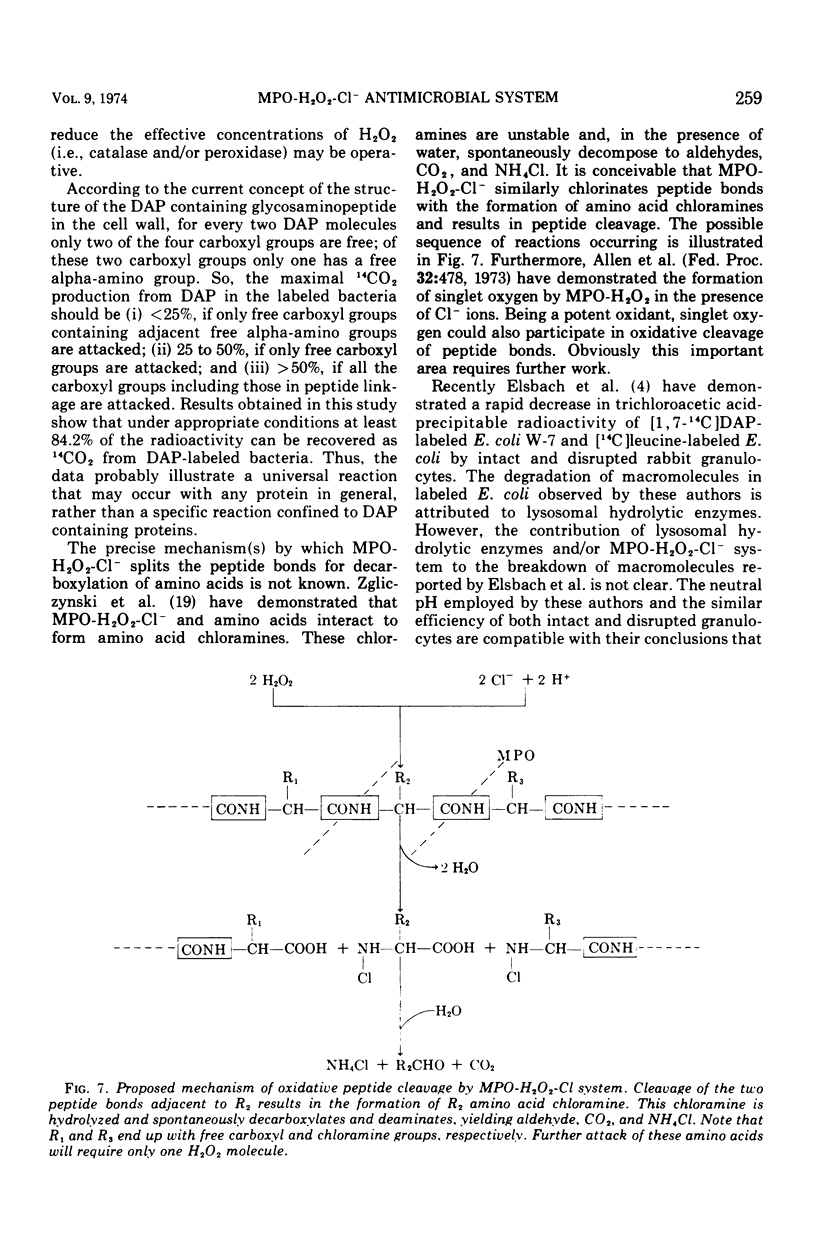
The antimicrobial activities of the myeloperoxidase-H2O2-halide system have received considerable attention recently. The precise mechanism by which this system exerts its lethal activity is presently not clear. In an effort to learn more regarding a possible mechanism of action, the susceptibility of protein-bound amino acids to enzymatic attack by myeloperoxidase (MPO) in the presence of chloride ions was investigated. [1, 7-14C]diaminopimelic acid (DAP) was incorporated into Escherichia coli W-7 proteins with little randomization of the radioactivity. Under appropriate conditions, it was observed that the MPO-H2O2-halide system released approximately 94% of the radioactivity from labeled bacteria. This would indicate that, in addition to decarboxylation, peptide bonds are also split during this reaction. The oxidative decarboxylation of DAP-labeled bacteria by MPO (i) is Cl− dependent, (ii) has an acid pH optimum, (iii) requires a specific concentration of H2O2 for activity, (iv) reaches a plateau by 25 min, and (v) is markedly inhibited by taurine. These properties are similar to those observed with free amino acids. It appears from these data that MPO can not only decarboxylate free and bound amino acids, yielding aldehydes, but also it can actively participate in oxidative peptide cleavage. Both of those activities may play a critical role in the microbicidal action of the leukocyte.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belding M. E., Klebanoff S. J., Ray C. G. Peroxidase-mediated virucidal systems. Science. 1970 Jan 9;167(3915):195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. D-amino acid oxidase in leukocytes: a possible D-amino-acid-linked antimicrobial system. Proc Natl Acad Sci U S A. 1969 Mar;62(3):756–763. doi: 10.1073/pnas.62.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. A., Quie P. G., Windhorst D. B., Page A. R., Rodey G. E., White J., Wolfson J. J., Holmes B. H. Fatal (chronic) granulomatous disease of childhood: a hereditary defect of leukocyte function. Semin Hematol. 1968 Jul;5(3):215–254. [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Low I. E., Paul B. B., Strauss R. R., Sbarra A. J. Mycoplasmacidal activity of peroxidase-H2O2-halide systems. Infect Immun. 1972 Jan;5(1):127–131. doi: 10.1128/iai.5.1.127-131.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. Leukin, a bactericidal agent from rabbit polymorphonuclear leucocytes. Nature. 1967 Nov 25;216(5117):806–808. doi: 10.1038/216806a0. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Mouse splenic peroxidase and its role in bactericidal activity. Infect Immun. 1972 Jan;5(1):120–126. doi: 10.1128/iai.5.1.120-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XXII. H2O2-dependent decarbosylation and deamination by myeloperoxidase and its relationship to antimicrobial activity. J Reticuloendothel Soc. 1970 Jun;7(6):754–761. [PubMed] [Google Scholar]
- ZEYA H. I., SPITZNAGEL J. K. ANTIBACTERIAL AND ENZYMIC BASIC PROTEINS FROM LEUKOCYTE LYSOSOMES: SEPARATION AND IDENTIFICATION. Science. 1963 Nov 22;142(3595):1085–1087. doi: 10.1126/science.142.3595.1085. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]



