Abstract
Objective
This is a study estimating diagnostic accuracy of CSF asialotransferrin to transferrin ratio measurement in eIF2B related disorders by using clinical evaluation and EIF2B mutation analysis as the reference standard. eIF2B-related disorder is a relatively common leukodystrophy with broad phenotypic variation that is caused by mutations in any of the five EIF2B genes. There is a need for a simple and clinically valid screening tool for physicians evaluating patients with an unclassified leukodystrophy.
Methods
CSF two-dimensional gel (2DG) electrophoresis analyses to measure asialotransferrin to transferrin ratios were performed in 60 subjects including 6 patients with documented EIF2B gene mutations, patients with other types of leukodystrophy, and patients with no leukodystrophy.
Results
All six patients with mutation proven eIF2B-related disease showed low to nearly undetectable amounts of asialotransferrin in their CSF when compared to 54 unaffected controls by CSF 2DG analyses in this study. eIF2B-like patients, with clinically similar presentations but no mutations in EIF2B1-5, were distinguished from patients with mutations in EIF2B1-5 by this biomarker. Patients with mutations in EIF2B1-5 had asialotransferrin/transferrin ratio levels significantly different from the group as a whole (p < 0.001). Using 8% asialotransferrin/transferrin ratio as a cutoff, this biomarker has a 100% sensitivity (95% CI = 52–100%) and 94% specificity (95% CI = 84–99%).
Conclusion
Decreased asialotransferrin/transferrin ratio in the CSF of patients with eIF2B-related disorder is highly sensitive and specific. This rapid (<48 hours) and inexpensive diagnostic tool for eIF2B-related disorders has the potential to identify patients with likely eIF2B-related disorder for mutation analysis.
eIF2B-related disorders were initially described as childhood onset ataxia with CNS hypomyelination (CACH),1 vanishing white matter disease (VWM),2 or myelinopathia centralis diffusa3 based on clinical and MRI criteria. eIF2B-related disorders are an autosomal recessive leukoencephalopathy currently diagnosed based on clinical phenotype and MRI pattern recognition confirmed by mutation analysis of the five EIF2B genes.4–6 In many cases, the clinical phenotype is readily recognizable, with childhood onset of a progressive spastic ataxia with variable seizures, optic atrophy, and late dementia. Episodic deterioration may be seen after fevers, mild head trauma, or fright,7 and may result in coma or even death. MRI typically demonstrates rarefaction and cystic degeneration of white matter best seen on fluid-attenuated inversion recovery images (FLAIR). White matter signal is replaced with CSF isointense signal, hence the appellation VWM.2,8
However, eIF2B-related disorders are proving to have a much broader phenotype than originally reported.9,10 The use of MRI or neuropathologic criteria to select patients with undetermined leukodystrophies for EIF2B1-5 gene analysis demonstrated the wide clinical spectrum of EIF2B1-5-mutated patients: from severe infantile cases such as Cree leukoencephalopathy11 and congenital forms with rapid death,12 to adult-onset forms with slow neurologic progression13–15 and ovarian failure such as ovarioleukodystrophy.16,17 Neonatal cases have been seen with severe encephalopathy and extraneurologic features such as cataracts, kidney hypoplasia, and ovarian dysgenesis.12,18 Rare patients with a peripheral neuropathy have been described.19,20 While MR images are often diagnostic,21 asymptomatic patients, early infantile patients, and patients early in the course of the disease2,15,22 may be hard to recognize. In addition, non-eIF2B related disorders can sometimes mimic the radiologic features of eIF2B-related disorders.23,24
eIF2B, composed of five subunits (eIF2Bα, eIF2Bβ, eIF2Bγ, eIF2Bδ, eIF2Bε), is encoded by five different genes (EIF2B1–5) with a total of 57 exons, making gene sequencing expensive and time consuming. Mutations have been found in all five genes and in multiple different exons.6,25 The great number of individual mutations has confounded efforts at simplifying mutation analysis in suspected eIF2B-related cases, requiring in many cases the sequencing of all five genes to exclude a diagnosis.
Efforts to establish a screening tool to streamline the diagnosis of eIF2B-related disorders have included CSF glycine levels,26 measuring guanine nucleotide exchange factor activity of eIF2B in lymphoblasts (GEF activity),27 and most recently, decreased CSF asialotransferrin to transferrin ratio.28 Detection of CSF asialotransferrin to transferrin ratio by 2DG analysis has the advantage of being technologically simple, rapid (<48 hours), inexpensive, and highly sensitive. In this study, we focus on establishing the diagnostic accuracy of 2 DG analysis of CSF asialotransferrin to transferrin ratio for the diagnosis of eIF2B-related disorders. Current diagnosis is based on clinical evaluation followed by confirmation of eIF2B mutation, and this approach will be used as the reference standard.
METHODS
Sample collection
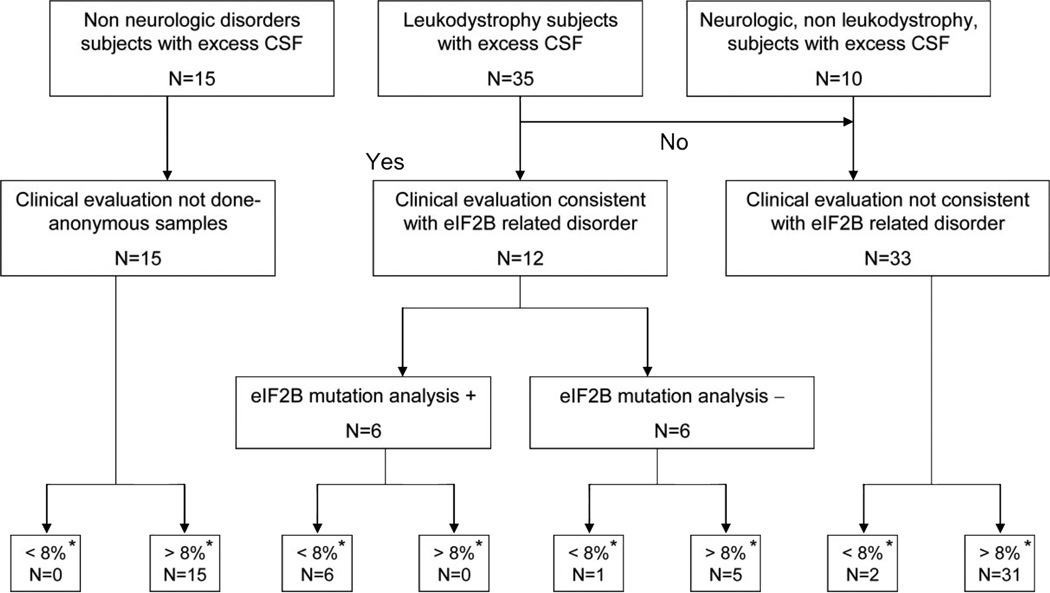
All CSF samples were collected in accordance with an Institutional Review Board approved protocol at Children’s National Medical Center and collaborating institutions. Affected and disease control samples were obtained at Children’s National Medical Center, the National Institute of Neurological Disorders and Stroke/NIH, and Clermont-Ferrand’s University Hospital. All available samples from patients with mutations in the genes EIF2B1–5 were included.6 All leukodystrophy control samples23 and neurologic disease control samples10 were obtained from patients referred to the above tertiary care centers for evaluation of a leukodystrophy of unknown cause. Non-neurologic disease control samples15 were obtained from Children’s National Medical Center. Only excess CSF drawn for other clinical or research purposes was used for these analyses. Patients, with the exception of the nonneurologic disease control samples, were clinically evaluated at INSERM UMR384/Clermont-Ferrand’s University Hospital or the National Institute of Neurological Disorders and Stroke/NIH and patients believed to have a leukodystrophy compatible with eIF2B-related disorder were selected for EIF2B mutation analysis.12 Confirmation of EIF2B1–5 mutation status was performed at Children’s National Medical Center and at INSERM UMR384/Clermont-Ferrand’s University Hospital prior to or concomitant with CSF studies. This approach to diagnosis has been the reference standard in eIF2B-related disorder. CSF samples were blinded at INSERM UMR384/Clermont-Ferrand’s University Hospital, Children’s National Medical Center, or the National Institute of Neurological Disorders and Stroke/NIH by a person not performing the CSF studies and received by A.V. for this analysis between April and November 2006. CSF samples were studied in 60 subjects (figure 1): 6 patients with documented EIF2B1–5 mutations; 6 patients with eIF2B-like presentation but no EIF2B mutations; 23 patients with other leukodystrophies (2 with Alexander disease, 3 with idiopathic hypomyelination, 7 with Pelizaeus-Merzbacher disease, 1 with X-linked adrenoleukodystrophy, 1 with Coats disease, 1 with a megalencephalic leukoencephalopathy with Cysts–like disorder, and 8 with an undetermined leukodystrophy); 10 patients with other neurologic abnormalities (1 with progressive cerebellar atrophy, 1 with spinal cord lesion, 1 with hereditary spastic paraplegia, one with Coffin Lowry syndrome, one with mitochondrial complex I deficiency, 1 with Gaucher disease type 3, 1 with X-linked mental retardation, 3 with encephalopathy of unknown origin); and 15 patients with non-neurologic reasons for lumbar puncture. In seven of these subjects, including two patients with mutations in EIF2B1–5, samples had been included in an unblinded fashion for the previous analysis,28 but were used in a blinded fashion in this study. Samples were collected in a standardized fashion, tested for blood contamination, and stored at −80°C until analysis.
Figure 1.
Flow diagram of CSF asialotransferrin/transferrin ratio diagnostic accuracy study.
* Asialotransferrin/transferrin ratio
Two-dimensional gel electrophoresis
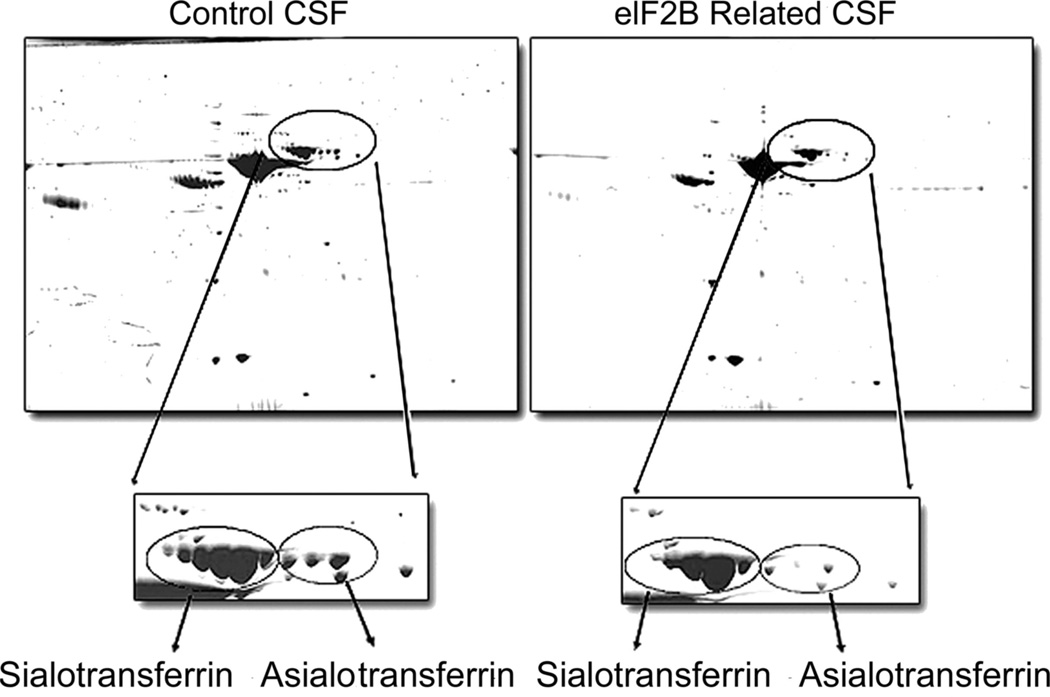
Protein concentration was measured in each CSF sample using the Bio- Rad protein assay reagent (Bio-Rad, Hercules, CA). Typically, CSF samples showed a protein concentration ranging from 0.16 to 0.4 mg/mL. Aliquots containing 100 µg of total protein were taken from each sample and processed for 2DG analysis as follows28,29: samples were desalted against 10 mM Tris HCl pH 7 using p6 Bio-Spin columns (Bio-Rad). Each solution was then dried by centrifugation under vacuum. A total of 180 µL of rehydration buffer (7 M urea, 2 M thiourea, 2% CHAPS, 50 mmol DTT, and 0.5% ampholyte pH 3–10) was added to the dry sample to solubilize and denature the proteins. First dimension electro-focusing was performed on IPG strips (11 cm, pH 3–10) using a Bio-Rad electro-focusing chamber operated as follows: 12 hours rehydration, 250 V for 15 minutes, 1,000 V for 1 hour, and 10,000 V for 4 hours. The second dimension SDS-PGE was performed on criterion Tris-HCl (8 to 16% or 10%) pre-cast gels (Bio-Rad). Protein spots were visualized using Bio-Safe Coomassie stain (Bio-Rad). The gel was then scanned on a GS800 densitometer (Bio-Rad) and archived as a TIFF file. Asialotransferrin and transferrin isoforms (figure 2) were identified based on previous mass spectrometry analyses.28 The resulting gels arrays were compared using a 2DG image analysis software, PDQuest (Bio-Rad), and the volume and intensity of spots of interest were determined using Quantity One software (Bio-Rad). All 2DG analyses were performed by J.M. and A.V. who were blinded to the results of EIF2B1-5 mutation sequencing and clinical characteristics of the patients studied.
Figure 2.
Scanned image of 2DG electrophoresis of control CSF (left) and EIF2B-mutated CSF (right)
Box corresponds to transferrin isoforms in each sample type. Note the decreased asialotransferrin isoforms relative to the total transferrin isoforms in EIF2B-mutated sample relative to the control sample. This patient (DMN 99.31) has mutations in EIF2B5 (Y343C/I385V).
Data analysis
Initially, 30 samples, including seven from patients with mutations in EIF2B1-5, had been analyzed with prior knowledge of EIF2B mutation status (Vanderver, Schiffmann et al., 2005). For the purposes of this validation study, an additional 60 samples were run with the investigator blinded to the EIF2B mutation status, including the number of samples with EIF2B mutations. This study was powered to detect a difference of greater than 20% between affected and unaffected individuals. In seven cases, blinded samples had also been analyzed in the initial data set, including two samples with EIF2B mutations. In addition, serial analyses were performed in 25 samples at different time points to establish intra-sample reproducibility. If multiple gels were performed on a single sample, the results were averaged. In no case of a patient with a mutation in EIF2B1–5 did individual results cross the cutoff boundary for reclassification prior to averaging. A ratio of 8% of CSF asialotransferrin relative to total transferrin has been identified as distinguishing between affected and unaffected samples based on the previous test data set (Vanderver, Schiffmann et al., 2005). Ratios were compared using a Fisher exact test. Sensitivity and specificity were calculated and 95% CIs were determined for each of them. Likelihood ratios were also calculated using STATA software. A log transformed Pearson’s correlation was calculated to determine the reproducibility of repeated gel analysis and to look for correlations between percentage of asialotransferrin and age at time of lumbar puncture and clinical features in affected patients.
RESULTS
An initial test data set of 30 samples (including 7 patients with documented mutations in EIF2B1–5, 3 patients with clinical pictures consistent with eIF2B-related disorder but no mutation, 5 patients with other leukoencephalopathies, and 15 patients with other reasons for lumbar puncture) identified a decrease in the ratio of asialotransferrin/transferrin isoforms seen in subjects with mutations in EIF2B1–5.28 Based on this data set, samples with asialotransferrin/transferrin ratio of less than or equal to 8% were considered to have possible mutations in EIF2B1–5, while samples with greater than 8% were considered to be unaffected (figure 2).
A subsequent validation data set of 60 blinded samples obtained from the National Institute of Neurological Disorders and Stroke/NIH36 and INSERM UMR38424 led to the correct identification of all six samples from patients with EIF2B1–5 mutations. Of importance, six patients with clinical phenotypes consistent with eIF2B-related disorders, but no documented mutations in EIF2B1–5, were also screened in a blinded fashion. In one of these eIF2B-like patients, the asialotransferrin/transferrin ratio was 7.98%; however, repeat measurements demonstrated higher ratios (mean 8.64, SD 0.704), and allowed for correct identification of the patient as a control. All the other eIF2B-like patients were appropriately classified as controls. In addition, only two other controls (one patient with an undetermined leukodystrophy, and one patient with a mutation in the GFAP gene) had percentages of asialotransferrin/ transferrin in the range of 5–8% and were classified as possibly affected.
Fisher exact test of the combined test and validation data sets was performed on the 60 blinded samples. Patients with EIF2B1–5 mutations had asialotransferrin/transferrin ratio levels significantly different from the group as a whole (Fisher exact test, p < 0.001) (table 1). Using 8% asialotransferrin/ transferrin ratio as a cutoff, this biomarker has a 100% sensitivity (95% CI = 52– 100%) and 94% specificity (95% CI = 84–99%). The likelihood ratio (LR) of mutation in EIF2B1–5 with a positive CSF test result as defined by less than 8% asialotransferrin/transferrin ratio was significant (LR = 18; 95% CI = 5.99– 54.06).
Table 1.
Percent of asialotransferrin to total transferrin in studied samples
| Sample type | |||||
|---|---|---|---|---|---|
| EIF2B mutated |
eIF2B-like, no mutation |
Other leukodystrophy |
Other neurologic |
Control non-neurologic |
|
| Range | 0.93–7.77 | 7.98–28.92 | 5.57–48.97 | 8.63–26.98 | 10.72–30.32 |
| Mean | 4.51 | 16.46 | 15.82 | 18.87 | 18.98 |
| SD | 2.18 | 8.14 | 9.01 | 8.30 | 6.66 |
Further analysis was performed on subgroups of patients defined as having mutations in EIF2B1-5 (6 patients), eIF2B-like without mutations (6 patients), and general controls (48 patients). The CSF asialotransferrin/transferrin ratio was significantly different in eIF2B-mutated and eIF2B-like patients (Fisher exact test, p ≤ 0.01). This subgroup was too small to identify valid sensitivity and specificity. CSF asialotransferrin/transferrin ratio was not significantly different in eIF2B-like patients without EIF2B mutations and the remainder of the controls (Fisher exact test, p = 0.302).
Internal validity was measured by analysis of CSF samples from the same patients at several days to several month intervals. Log transformed Pearson correlation calculations suggest high intra-sample reproducibility (Pearson r = 0.883). From a qualitative perspective, no patient with mutations in EIF2B1-5 had a pre-average asialotransferrin/ transferrin ratio of more than 8%. Asialotransferrin/transferrin ratio did not appear to correlate with age at onset, length of disease prior to lumbar puncture used for analysis, age at death, or constellation of symptoms in patients with eIF2B-related disorders. Gender and age at time of lumbar puncture did not correlate with asialotransferrin/transferrin ratio in the control patient population.
DISCUSSION
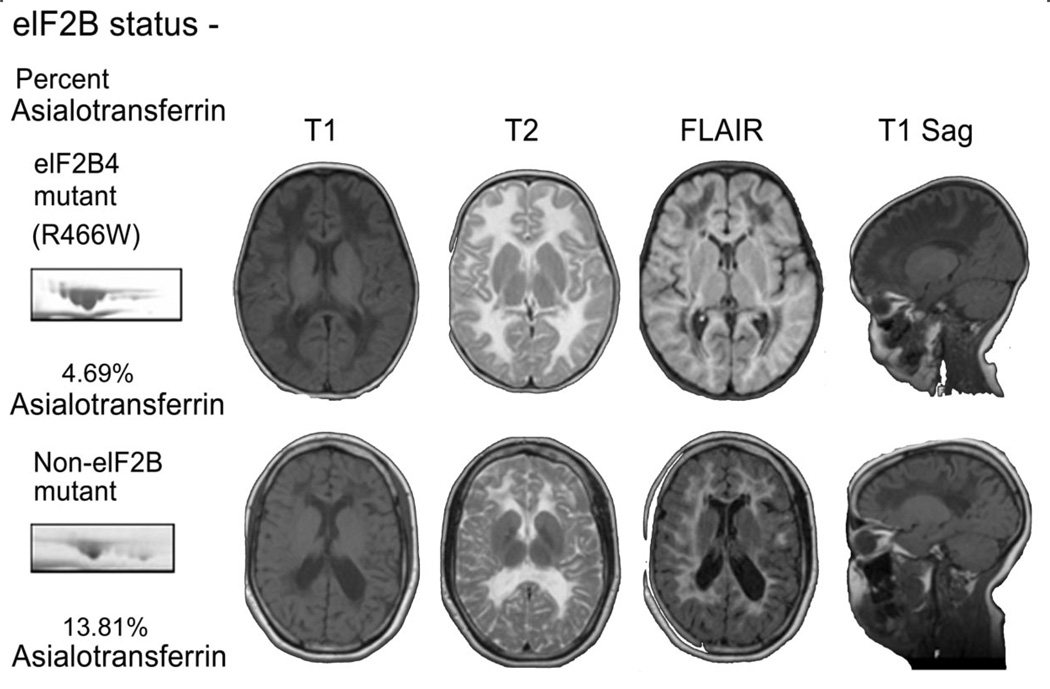
In our sample group, CSF asialotransferrin to transferrin ratios correctly identified all patients with mutations in EIF2B1–5. Six out of six patients with mutation proven eIF2Brelated disease showed low to nearly undetectable amounts of asialotransferrin in their CSF when compared to 54 unaffected controls by CSF 2DG analysis. Patients with nearly identical clinical and radiologic presentations (figure 3) but no mutations in EIF2B1–5 were identified as controls. Of note, the patient shown in figure 3, with eIF2B-like phenotype, was one of the original patients described with CACH1 (patient 4) who later was demonstrated not to have mutations in EIF2B1–5. The only two patients with asialotransferrin/transferrin ratios of 6–8% who were not affected with eIF2B-related disorder had clearly distinct radiologic and clinical presentations. In addition, serial 2DG analyses demonstrated good intra-sample reproducibility, even for samples stored for several months at −80°C.
Figure 3.
Patients with EIF2B4 (R466W homozygous) mutation and with eIF2B-like clinical and radiologic presentation, but without eIF2B mutations
Top: Patient with EIF2B4 (R466W homozygous) mutation. MRI reveals T2 signal intensity similar to CSF, fluid-attenuated inversion recovery (FLAIR) images consistent with rarefaction of involved white matter, and striae on T1 sagittal images. Asialotransferrin to transferrin ratio is less than 8%. Bottom: Patient with eIF2B-like clinical and radiologic presentation, but without eIF2B mutations. MRI reveals T2 signal intensity similar to CSF, FLAIR images consistent with rarefaction of involved white matter, and striae on T1 sagittal images. Asialotransferrin to transferrin ratio is more than 8%.
Samples from patients with eIF2B-related disorder included patients with missense and frame shift mutations in EIF2B2, EIF2B4, and EIF2B5, with the greatest number of patients with EIF2B5 mutations (table 2). Asialotransferrin to total transferrin ratio did not appear to correlate with age at onset, length of disease prior to lumbar puncture used for analysis, age at death, or constellation of symptoms in patients affected by eIF2B-related disorders. Specifically, patients with adult onset of symptoms had percentages of asialotransferrin similar to patients with early onset of symptoms. Of note, in this analysis, we did not have any patients with neonatal or very early onset disease. Further analyses will be necessary to confirm these findings in these subgroups.
Table 2.
Clinical characteristics and mutation analysis relative to percentage of asialotransferrin in CSF in eIF2B mutated patients and eIF2B-like patients
| Patient | % Asialotransferrin |
Nucleotide changes |
Amino acid change |
Gene | Age at disease onset |
Age ay death |
Symptoms | GEF activity |
|---|---|---|---|---|---|---|---|---|
| DMN 03.01 | 1.46 | 1069C>T/1069C>T | R357W/R357W | EIF2B4 | 2 y | NA | Motor decline with febrile illness, epilepsy | NA |
| DMN 91.46 | 2.11 | 338G>A/1884G>A | R113H/W628X | EIF2B5 | 18 mo | 6 y | Progressive ataxia after febrile illness | NA |
| DMN 96.115 | 3.71 | 512C>T/607–612delInsTG | S171F/M203fs | EIF2B2 | 10 y | NA | Scoliosis, gait disorder | NA |
| G648-2* | 4.11 | 638A>G/638A>G | E213G/E213G | EIF2B2 | 7 y | NA | Progressive ataxia, behavior disturbance | 64 ± 4 |
| DMN 99.31 | 4.36 | 1028A>G/1153A>G | Y343C/I385V | EIF2B5 | 13 mo | 5 y | Neurologic deterioration after febrile illnesses | NA |
| DMN 01.45* | 4.52 | 338G>A/splicing site exon 10 | R113H/splicing site exon 10 | EIF2B5 | 2 y | NA | Progressive ataxia after fall | 70 ± 1 |
| DMN 91.55 | 4.65 | 338G>A/805C>T | R113H/R269X | EIF2B5 | 18 mo | 5 y | Progressive ataxia, acute coma resulting in death | NA |
| DMN 05.06 | 4.69 | 1399C>T/1399C>T | R466W/R466W | EIF2B4 | 15 mo | NA | Neurologic deterioration after febrile illness | NA |
| DMN 01.11* | 5.50 | 338G>A/338G>A | R113H/R113H | EIF2B5 | 4 y | NA | Hemiparesis after fall | 77.5 ± 2.5 |
| DMN 02.02 | 7.77 | 338G>A/806G>T | R113H/R269L | EIF2B5 | 2 y | NA | Progressive ataxia w/o provoking event | NA |
| DMNL 99.001 | 4.8 | 47C>A/338G>A | A16D/R113H | EIF2B5 | 3 y | NA | Progressive ataxia after mild head trauma | NA |
| NA | 13.81 | None | None | None | 5 y | NA | Progressive ataxia w/o provoking event | NA |
| NA | 20.78 | None | None | None | 3 y | NA | Progressive ataxia w/o provoking event | NA |
| NA | 14.69 | None | None | None | 12 y | NA | Progressive ataxia w/o provoking event | NA |
| NA | 8.64 | None | None | None | 4 y | NA | Progressive ataxia w/o provoking event | 103.8 ± 2.8 |
| NA | 8.60 | None | None | None | 37 y | NA | Progressive ataxia w/o provoking event | 99 ± 3 |
| NA | 22.42 | None | None | None | 3 y | NA | Progressive ataxia w/o provoking event | 111.3 ± 3.7 |
Patients previously included in Fogli et al., 2004.27
GEF = guanine nucleotide exchange activity, measured in lymphoblasts from affected patients; NA = not available or still alive; fs = frameshift.
This decrease in CSF asialotransferrin to transferrin ratio has, to our knowledge, not been described in any other disorders. Of note, an increase in CSF asialotransferrin to transferrin ratio has been reported in patients with congenital disorders of glycosylation since these disorders have been recognized.30 There is increasing evidence that transferrin plays a role in oligodendrocyte maturation and homeostasis,31–37 in addition to its known role in iron metabolism and oxidative stress.38 Thus, decreased asialotransferrin to transferrin ratio in the CSF may simply be a manifestation of disturbed protein homeostasis within the CNS as a result of EIF2B mutations, or it may play a role in the pathophysiology of the disorder.
In serum, transferrin originates from the liver and exists as sialotransferrin, unless a pathologic condition occurs such as congenital disorders of glycosylation or alcoholism.39 In the CSF, both asialotransferrin and sialotransferrin are found.28,40 It is presumed that sialotransferrin may be found in CSF due to a small transfer of serum-derived transferrin across the blood–brain–CSF barriers. It has been suggested that asialo-forms could result from partial metabolic degradation of the serum sialotransferrin transferred to the brain compartment.41 Others have suggested de novo synthesis of asialotransferrin by oligodendrocytes and astrocytes.40,42 In the brain of patients with EIF2B mutations, oligodendrocytes have been reported with a foamy aspect consistent with the presence of material rich in glycoproteins.43 Increasing evidence supports an exaggerated ER stress response with abnormal activation of the unfolded protein response in EIF2B-mutated cells with abnormal protein synthesis and cellular homeostasis20,44–48 in particular in oligodendrocytes and astrocytes of the white matter from affected patients.46 Hence, we hypothesize that the decrease of CSF asialotransferrin to transferrin ratio in patients with eIF2B-related disorders may be due to reduced de novo secretion from these cells.
The expanding phenotype and hope for possible therapeutic strategies makes novel diagnostic approaches valuable in eIF2B-related disorders. Although clinical characteristics and radiologic features may be suggestive, the difficulty and cost of diagnosis by DNA sequencing make a rapid, inexpensive screening tool attractive for clinical practice. Decreased asialotransferrin/transferrin ratio is a novel, clinically useful biomarker for eIF2B-related disorder. Its high sensitivity and specificity makes it useful as a screening tool prior to EIF2B1–5 mutation analysis in selected cases. In addition, its technological simplicity, rapidity (less than 48 hours for CSF asialotransferrin/ transferrin ratio determination vs several weeks to months for EIF2B1-5 mutation analysis), and low cost (less than $30 for supplies and reagents) make it an excellent candidate for clinical use. Although testing of CSF is an invasive procedure, precedent exists for using CSF as a screening tool for more specific analysis, such as in the neurotransmitter disorders.49 CSF asialotransferrin/transferring ratio determination could be used as a screening tool in cases with suggestive MRI manifestations prior to EIF2B mutation analysis.
Supplementary Material
Acknowledgments
Supported in part by Children’s Health Research Center, NIH supported K12HD001399, by grants from the NIH (HD-P30-40677 Child Health Research Career Development Award; 1P30HD40677-01 Mental Retardation and Developmental Disabilities Research Center), by the Parson family foundation, and the intramural program of the National Institute of Neurological Disorders and Stroke. The participation of O.B.-T. and A. F. was supported by a grant from the European Leukodystrophy Association.
GLOSSARY
- 2DG
two-dimensional gel
- CACH
hood onset ataxia with CNS hypomyelination
- FLAIR
fluid-attenuated inversion recovery
- LR
likelihood ratio
- VWM
vanishing white matter
Footnotes
Disclosure: Drs. Vanderver and Hathout have a patent pending for asialotransferrin as a biomarker in eIF2B related disorders. The other authors report no disclosures.
REFERENCES
- 1.Schiffmann RMJ, Trapp BD, Shih HH, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol. 1994;35:331–340. doi: 10.1002/ana.410350314. [DOI] [PubMed] [Google Scholar]
- 2.van der Knaap MS, Barth PG, Gabreels FJ, et al. A new leukoencephalopathy with vanishing white matter. Neurology. 1997;48:845–855. doi: 10.1212/wnl.48.4.845. [DOI] [PubMed] [Google Scholar]
- 3.Hanefeld F, Holzbach U, Kruse B, et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics. 1993;24:244–248. doi: 10.1055/s-2008-1071551. [DOI] [PubMed] [Google Scholar]
- 4.Leegwater PA, Könst AA, Kuyt B, et al. The gene for leukoencephalopathy with vanishing white matter is located on chromosome 3q27. Am J Hum Genet. 1999;65:728–734. doi: 10.1086/302548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leegwater PA, Vermeulen G, Könst AA, et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet. 2001;29:383–388. doi: 10.1038/ng764. [DOI] [PubMed] [Google Scholar]
- 6.van der Knaap MS, Leegwater PA, Könst AA, et al. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264–270. doi: 10.1002/ana.10112. [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen G, Seidl R, Mercimek-Mahmutoglu S, et al. Fright is a provoking factor in vanishing white matter disease. Ann Neurol. 2005;57:560–563. doi: 10.1002/ana.20418. [DOI] [PubMed] [Google Scholar]
- 8.van der Knaap MS, Kamphorst W, Barth PG, et al. Phenotypic variation in leukoencephalopathy with vanishing white matter. Neurology. 1998;51:540–547. doi: 10.1212/wnl.51.2.540. [DOI] [PubMed] [Google Scholar]
- 9.Fogli A, Boespflug-Tanguy O. The large spectrum of eIF2B-related diseases. Biochem Soc Trans. 2006;34(Pt 1):22–29. doi: 10.1042/BST20060022. [DOI] [PubMed] [Google Scholar]
- 10.Ramaswamy V, Chan AK, Kolski HK. Vanishing white matter disease with periodic (paroxysmal) hemiparesis. Pediatr Neurol. 2006;35:65–68. doi: 10.1016/j.pediatrneurol.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Fogli A, Wong K, Eymard-Pierre E, et al. Cree leukoencephalopathy and CACH/VWM disease are allelic at the EIF2B5 locus. Ann Neurol. 2002;52:506–510. doi: 10.1002/ana.10339. [DOI] [PubMed] [Google Scholar]
- 12.van der Knaap MS, van Berkel CG, Herms J, et al. eIF2B-related disorders: antenatal onset and involvement of multiple organs. Am J Hum Genet. 2003;73:1199–1207. doi: 10.1086/379524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtake H, Shimohata T, Terajima K, et al. Adult-onset leukoencephalopathy with vanishing white matter with a missense mutation in EIF2B5. Neurology. 2004;62:1601–1603. doi: 10.1212/01.wnl.0000123117.11264.0e. [DOI] [PubMed] [Google Scholar]
- 14.Biancheri R, Rossi A, Di Rocco M, et al. Leukoencephalopathy with vanishing white matter: an adult onset case. Neurology. 2003;61:1818–1819. doi: 10.1212/01.wnl.0000098994.35677.3c. [DOI] [PubMed] [Google Scholar]
- 15.van der Knaap MS, Leegwater PA, van Berkel CGM, et al. Arg113His mutation in eIF2Bepsilon as cause of leukoencephalopathy in adults. Neurology. 2004;62:1598–1600. doi: 10.1212/01.wnl.0000123118.86746.fc. [DOI] [PubMed] [Google Scholar]
- 16.Schiffmann RTG, Kinkel RP, Trapp BD, et al. Leukodystrophy in patients with ovarian dysgenesis. Ann Neurol. 1997;41:654–661. doi: 10.1002/ana.410410515. [DOI] [PubMed] [Google Scholar]
- 17.Fogli ARD, Eymard-Pierre E, Bouhour F, et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am J Hum Genet. 2003;72:1544–1550. doi: 10.1086/375404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boltshauser E, Barth PG, Troost D, et al. “Vanishing white matter” and ovarian dysgenesis in an infant with cerebro-oculo-facio-skeletal phenotype. Neuropediatrics. 2002;33:57–62. doi: 10.1055/s-2002-32363. [DOI] [PubMed] [Google Scholar]
- 19.Federico A, Scali O, Stromillo ML, et al. Peripheral neuropathy in vanishing white matter disease with a novel EIF2B5 mutation. Neurology. 2006;67:353–355. doi: 10.1212/01.wnl.0000225077.40532.a5. [DOI] [PubMed] [Google Scholar]
- 20.Schiffmann R, Elroy-Stein O. Childhood ataxia with CNS hypomyelination/vanishing white matter disease–a common leukodystrophy caused by abnormal control of protein synthesis. Mol Genet Metab. 2006;88:7–15. doi: 10.1016/j.ymgme.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 21.van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- 22.Mascalchi M, De Grandis D, Ginestroni A, et al. Early MR imaging and spectroscopy appearance of eIF2B-related leukoencephalopathy. Neurology. 2006;67:537–538. doi: 10.1212/01.wnl.0000227920.57400.69. [DOI] [PubMed] [Google Scholar]
- 23.van der Knaap MS, Scheper GC. Non-eIF2B-related cystic leukoencephalopathy of unknown origin. Ann Neurol. 2006;59:724. doi: 10.1002/ana.20802. [DOI] [PubMed] [Google Scholar]
- 24.Labauge P, Fogli A, Castelnovo G, et al. Dominant form of vanishing white matter-like leukoencephalopathy. Ann Neurol. 2005;58:634–639. doi: 10.1002/ana.20573. [DOI] [PubMed] [Google Scholar]
- 25.Ohlenbusch A, Henneke M, Brockmann K, et al. Identification of ten novel mutations in patients with eIF2B-related disorders. Hum Mutat. 2005;25:411. doi: 10.1002/humu.9325. [DOI] [PubMed] [Google Scholar]
- 26.van der Knaap MS, Wevers RA, Kure S, et al. Increased cerebrospinal fluid glycine: a biochemical marker for a leukoencephalopathy with vanishing white matter. J Child Neurol. 1999;14:728–731. doi: 10.1177/088307389901401108. [DOI] [PubMed] [Google Scholar]
- 27.Fogli A, Schiffmann R, Hugendubler L, et al. Decreased guanine nucleotide exchange factor activity in eIF2B-mutated patients. Eur J Hum Genet. 2004;12:561–566. doi: 10.1038/sj.ejhg.5201189. [DOI] [PubMed] [Google Scholar]
- 28.Vanderver A, Schiffmann R, Timmons M, et al. Decreased asialotransferrin in cerebrospinal fluid of patients with childhood-onset ataxia and central nervous system hypomyelination/vanishing white matter disease. Clin Chem. 2005;51:2031–2042. doi: 10.1373/clinchem.2005.055053. [DOI] [PubMed] [Google Scholar]
- 29.Davidsson PPL, Hesse C, Blennow K, Nilsson C. Proteome studies of human cerebrospinal fluid and brain tissue using a preparative two dimensional electrophoresis approach prior to mass spectrometry. Proteomics. 2001;1:444–452. doi: 10.1002/1615-9861(200103)1:3<444::AID-PROT444>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Jaeken J, van Eijk HG, van der Heul C, et al. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin Chim Acta. 1984;144:245–247. doi: 10.1016/0009-8981(84)90059-7. [DOI] [PubMed] [Google Scholar]
- 31.Sow A, Lamant M, Bonny JM, et al. Oligodendrocyte differentiation is increased in transferrin transgenic mice. J Neurosci Res. 2006;83:403–414. doi: 10.1002/jnr.20741. [DOI] [PubMed] [Google Scholar]
- 32.de Arriba Zerpa G, Saleh MC, Fernandez PM, et al. Alternative splicing prevents transferrin secretion during differentiation of a human oligodendrocyte cell line. J Neurosci Res. 2000;61:388–395. doi: 10.1002/1097-4547(20000815)61:4<388::AID-JNR5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa de los Monteros AKS, Zhao P, Huang CJ, et al. Transferrin is an essential factor for myelination. Neurochem Res. 1999;24:235–248. doi: 10.1007/s11064-004-1826-2. [DOI] [PubMed] [Google Scholar]
- 34.Saleh MC, Espinosa de los Monteros A, de Arriba Zerpa GA, et al. Myelination and motor coordination are increased in transferrin transgenic mice. J Neurosci Res. 2003;72:587–594. doi: 10.1002/jnr.10619. [DOI] [PubMed] [Google Scholar]
- 35.Espinosa-Jeffrey A, Kumar S, Zhao PM, et al. Transferrin regulates transcription of the MBP gene and its action synergizes with IGF-1 to enhance myelinogenesis in the md rat. Dev Neurosci. 2002;24:227–241. doi: 10.1159/000065698. [DOI] [PubMed] [Google Scholar]
- 36.Escobar Cabrera OE, Zakin MM, Soto EF, et al. Single intracranial injection of apotransferrin in young rats increases the expression of specific myelin protein mRNA. J Neurosci Res. 1997;47:603–608. [PubMed] [Google Scholar]
- 37.Espinosa de los Monteros A, Kumar S, Zhao PM, et al. Transferrin is an essential factor for myelination. Neurochem Res. 1999;24:235–248. doi: 10.1007/s11064-004-1826-2. [DOI] [PubMed] [Google Scholar]
- 38.Bradbury M. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- 39.Butler M, Quelhas D, Critchley AJ, et al. Detailed glycan analysis of serum glycoproteins of patients with disorders of glycosylation indicate the specific defects and provides an insight into pathogenesis. Glycobiology. 2003;13:601–622. doi: 10.1093/glycob/cwg079. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann ANM, Getzlaff R, Conradt HS. Brain type N-glycosylation of asialo-transferrin from human cerebrospinal fluid. FEBS Lett. 1995;359:164–168. doi: 10.1016/0014-5793(95)00034-7. [DOI] [PubMed] [Google Scholar]
- 41.Gallo P, Bracco F, Morara S, et al. The cerebrospinal fluid transferrin/tau proteins A study by two-dimensional polyacrylamide gel electrophoresis (2D) and agarose isoelectrofocusing (IEF) followed by double-antibody peroxidase labeling and avidin-biotin amplification. J Neurol Sci. 1985;70:81–92. doi: 10.1016/0022-510x(85)90190-x. [DOI] [PubMed] [Google Scholar]
- 42.Aldred A, Black C, Schreiber G. The cerebral expression of plasma proteins in different species. Comp Biochem Physiol. 1995;111B:1–15. doi: 10.1016/0305-0491(94)00229-n. [DOI] [PubMed] [Google Scholar]
- 43.Wong K, Armstrong RC, Gyure KA, et al. Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol (Berl) 2000;100:635–646. doi: 10.1007/s004010000234. [DOI] [PubMed] [Google Scholar]
- 44.Scheper GC, Proud CG, van der Knaap MS. Defective translation initiation causes vanishing of cerebral white matter. Trends Mol Med. 2006;12:159–166. doi: 10.1016/j.molmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 45.van Kollenburg B, Thomas AA, Vermeulen G, et al. Regulation of protein synthesis in lymphoblasts from vanishing white matter patients. Neurobiol Dis. 2006;21:496–504. doi: 10.1016/j.nbd.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 46.van Kollenburg B, van Dijk J, Garbern J, et al. Glia-specific activation of all pathways of the unfolded protein response in vanishing white matter disease. J Neuropathol Exp Neurol. 2006;65:707–715. doi: 10.1097/01.jnen.0000228201.27539.50. [DOI] [PubMed] [Google Scholar]
- 47.van der Voorn JP, van Kollenburg B, Bertrand G, et al. The unfolded protein response in vanishing white matter disease. J Neuropathol Exp Neurol. 2005;64:770–775. doi: 10.1097/01.jnen.0000178446.41595.3a. [DOI] [PubMed] [Google Scholar]
- 48.Kantor L, Harding HP, Ron D, et al. Heightened stress response in primary fibroblasts expressing mutant eIF2B genes from CACH/VWM leukodystrophy patients. Hum Genet. 2005;118:99–106. doi: 10.1007/s00439-005-0024-x. [DOI] [PubMed] [Google Scholar]
- 49.Hyland K. The lumbar puncture for diagnosis of pediatric neurotransmitter diseases. Ann Neurol. 2003;54(suppl 6):7. doi: 10.1002/ana.10627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.