Abstract
CKD is associated with a complex state of immune dysfunction characterized by immune depression, predisposing patients to infections, and immune activation, resulting in inflammation that associates with higher risk of cardiovascular disease. Physical exercise may enhance immune function and exert anti-inflammatory effects, but such effects are unclear in CKD. We investigated the separate effects of acute and regular moderate-intensity aerobic exercise on neutrophil degranulation (elastase release), activation of T lymphocytes (CD69 expression) and monocytes (CD86 and HLA-DR expression), and plasma inflammatory markers (IL-6, IL-10, soluble TNF-receptors, and C-reactive protein) in patients with predialysis CKD. A single 30-minute (acute) bout of walking induced a normal pattern of leukocyte mobilization and had no effect on T-lymphocyte and monocyte activation but improved neutrophil responsiveness to a bacterial challenge in the postexercise period. Furthermore, acute exercise induced a systemic anti-inflammatory environment, evidenced by a marked increase in plasma IL-10 levels (peaked at 1 hour postexercise), that was most likely mediated by increased plasma IL-6 levels (peaked immediately postexercise). Six months of regular walking exercise (30 min/d for 5 times/wk) exerted anti-inflammatory effects (reduction in the ratio of plasma IL-6 to IL-10 levels) and a downregulation of T-lymphocyte and monocyte activation, but it had no effect on circulating immune cell numbers or neutrophil degranulation responses. Renal function, proteinuria, and BP were also unaffected. These findings provide compelling evidence that walking exercise is safe with regard to immune and inflammatory responses and has the potential to be an effective anti-inflammatory therapy in predialysis CKD.
CKD is associated with profound alterations in innate and adaptive immunity, leading to a complex state of immune dysfunction, in which signs of immune depression and immune activation paradoxically coexist.1–3 Although functional immune cell deficiencies predispose CKD patients to infectious complications, persistent immune cell activation contributes to a state of chronic inflammation that is associated with increased cardiovascular disease (CVD) risk. CVD and infection are the leading causes of morbidity and mortality in CKD.1–7 Hence, the immune dysfunction that accompanies CKD represents a major target for therapeutic interventions aiming to improve outcome.
Physical exercise has the potential to be one such therapy. It has been suggested that regular participation in moderate-intensity exercise may enhance certain aspects of immune function and exert anti-inflammatory effects.8–13 These effects are believed to contribute, at least in part, to the lower risk of infection and CVD that is observed in physically active individuals compared with their sedentary counterparts.12,13
Although the potential anti-inflammatory effects of regular exercise in CKD patients have been recognized, research in this area is lacking, particularly in predialysis patients.14,15 Apart from the potential benefits, given that exercise is currently advocated in the routine clinical care of CKD patients,16,17 it is also paramount to determine if exercise is safe to their underlying compromised immunity and inflammatory status. Although an acute bout of moderate exercise usually exerts little influence on immune and inflammatory responses in healthy people,18,19 it is not clear what effect it may have in CKD patients. Here, we report the separate effects of acute and regular moderate-intensity aerobic exercise on measures of immunity and systemic inflammation in predialysis CKD.
Results
Effects of Acute Exercise
Fifteen predialysis patients (Table 1) walked for 30 minutes on a motorized treadmill at a 1% gradient and a speed that elicited a rating of perceived exertion (RPE) in the range of 12–14 (somewhat hard). The effects of this acute bout of exercise on immune and inflammatory parameters are summarized in Table 2.
Table 1.
Effect of acute exercise: patients’ characteristics
| Characteristic | Group Mean |
|---|---|
| Sex (n men/n women) | 12/3 |
| Age (yr) | 59±10 |
| Weight (kg) | 80±15 |
| Height (cm) | 172±10 |
| Body mass index (kg/m2) | 27.1±5.1 |
| eGFR (ml/min per 1.73 m2) | 18.3±7.3 |
Data are mean±SD.
Table 2.
Effects of acute exercise on immune and inflammatory parameters
| Parameter | Pre-Exercise | Postexercise | 1 h Postexercise | P Valuea |
|---|---|---|---|---|
| Total and differential blood leukocyte counts | ||||
| Leukocytes (×109/L) | 6.6±1.9 | 7.5±1.9b | 7.2±2.2b | 0.001 |
| Neutrophils (×109/L) | 4.1±1.4 | 4.6±1.4b | 4.9±1.7b | 0.002 |
| Lymphocytes (×109/L) | 1.6±0.6 | 1.9±0.6b | 1.5±0.6 | <0.001 |
| Monocytes (×109/L) | 0.6±0.2 | 0.6±0.1 | 0.5±0.2 | 0.11 |
| Neutrophil degranulation (elastase release) | ||||
| Plasma elastase (µg/L) | 54±19 | 74±34b | 52±16 | 0.02 |
| Total bacterially-stimulated elastase release (µg/L) | 3398±1489 | 3765±1867 | 4359±2033b | 0.003 |
| Bacterially-stimulated elastase release per neutrophil (fg/cell) | 510±180 | 516±206 | 598±245b | 0.003 |
| SEB-stimulated CD4+ and CD8++ lymphocytes | ||||
| CD69+ cells (%) | ||||
| CD4+ | 23±10 | 23±11 | 23±11 | 0.81 |
| CD8++ | 24±11 | 26±14 | 24±11 | 0.71 |
| CD69 (GMFI) | ||||
| CD4+CD69+ | 141±49 | 132±42 | 143±53 | 0.10 |
| CD8++CD69+ | 106±51 | 101±55 | 109±60 | 0.24 |
| SEB-stimulated CD14+CD86+HLA-DR+ monocytes | ||||
| CD86 (GMFI ratio to unstimulated cells) | 1.9±0.9 | 1.8±0.8 | 1.9±0.8 | 0.98 |
| HLA-DR (GMFI ratio to unstimulated cells) | 2.2±1.0 | 2.2±0.9 | 2.2±0.9 | 0.99 |
| Plasma markers of systemic inflammation | ||||
| IL-6 (pg/ml) | 7.7±4.7 | 9.4±6.0b | 8.9±5.5b | <0.001 |
| IL-10 (pg/ml) | 3.2±0.8 | 3.8±1.1b | 4.3±1.1b | <0.001 |
| sTNF-RI (ng/ml) | 6.9±1.8 | 7.0±1.8 | 7.0±1.7 | 0.43 |
| sTNF-RII (ng/ml) | 16.8±4.0 | 17.5±3.9 | 18.0±4.2b | 0.02 |
| CRP (µg/ml) | 2.5±2.5 | 2.4±2.3 | 2.5±2.4 | 0.38 |
Data are mean±SD (n=15). GMFI, geometric mean of fluorescence intensity.
Effect of acute exercise (one-factor ANOVA).
P<0.05 versus pre-exercise (paired t tests).
Exercise induced an increase in total leukocyte concentration, which was more pronounced postexercise (P<0.001, effect size [ES]=0.44) but still observable at 1 hour postexercise (P=0.01, ES=0.27). These effects were mainly attributable to circulating neutrophils and lymphocytes, because exercise had no effect on monocyte count. Neutrophil concentration was elevated above pre-exercise levels postexercise (P<0.001, ES=0.35) and to a greater extent, 1 hour postexercise (P=0.002, ES=0.47). Lymphocyte concentration was also elevated postexercise (P<0.01, ES=0.53) but returned to pre-exercise levels by 1 hour postexercise.
Plasma elastase concentration (unstimulated neutrophil elastase release) was elevated above pre-exercise levels postexercise (P=0.03, ES=0.73) but returned to pre-exercise levels by 1 hour postexercise. Total neutrophil elastase release after 1 hour of in vitro stimulation with bacterial extract showed a trend to elevation postexercise (P=0.05, ES=0.22), which was evident at 1 hour postexercise (P<0.01, ES=0.54). When these data were expressed as bacterially-stimulated elastase release per neutrophil, values were also elevated in response to exercise; however, this result was only observed at 1 hour postexercise (P=0.04, ES=0.41).
There were no effects of exercise on the percentages of CD4+ or CD8++ lymphocytes expressing CD69 after 20 hours of in vitro staphylococcal enterotoxin B (SEB) stimulation or the SEB-stimulated CD69 expression by either CD4+CD69+ or CD8++CD69+ lymphocytes. Likewise, CD86 and HLA-DR expressions by SEB-stimulated CD14+CD86+HLA-DR+ monocytes were also unaffected.
Exercise induced an increase in plasma IL-6 levels, which was more evident postexercise (P<0.001, ES=0.33) but still noticeable at 1 hour postexercise (P=0.004, ES=0.25). Plasma IL-10 concentration was also elevated in response to acute exercise, and although an increase was already apparent postexercise (P<0.01, ES=0.58), it was further increased 1 hour postexercise (P=0.001, ES=1.12). Exercise also induced an elevation in plasma soluble TNF-receptor II (sTNF-RII) levels, which was only evident 1 hour postexercise (P=0.03, ES=0.30). Exercise had no effect on plasma soluble TNF-receptor I (sTNF-RI) and C-reactive protein (CRP) concentrations. The exercise-induced relative changes in these plasma markers of systemic inflammation are further illustrated in Supplemental Material (Figure 1).
Figure 1.
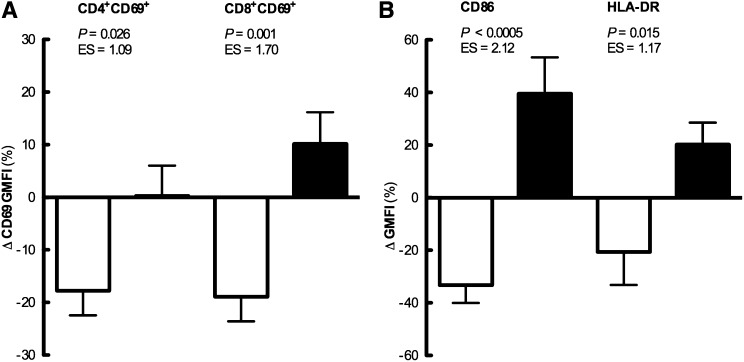
Effects of regular exercise on expression of SEB-stimulated immune cell activation markers. Relative changes over 6 months in SEB-stimulated CD69 expression by CD4+CD69+ or CD8+CD69+ lymphocytes (left panel) and SEB-stimulated CD86 or HLA-DR expression by CD14+CD86+HLA-DR+ monocytes (right panel) for the exercise (white bars) and control (black bars) groups. Data are mean±SEM (n=13 exercise group; n=11 control group). GMFI, geometric mean of fluorescence intensity.
Effects of Regular Exercise
The effects of regular exercise were investigated in a previously documented study.20 Briefly, 20 patients were assigned to a 6-month home-based walking exercise program, whereas 20 other patients continued with their habitual physical activity (control group); of these patients, 18 exercisers and 14 controls completed the study. However, given the outcomes addressed here, we have excluded patients on immunosuppressive therapy, which left data from 13 exercisers and 11 controls; their baseline characteristics are shown in Table 3.
Table 3.
Effects of regular exercise: patients’ baseline characteristics
| Characteristic | Exercise Group (n=13) | Control Group (n=11) | P Valuea |
|---|---|---|---|
| Sex (n men/n women) | 8/5 | 7/4 | |
| Age (yr) | 61±8 | 56±16 | 0.35 |
| Weight (kg) | 76.5±13.2 | 88.4±21.9 | 0.13 |
| Height (cm) | 170±11 | 174±11 | 0.35 |
| Body mass index (kg/m2) | 26.6±4.7 | 29.0±5.9 | 0.28 |
| eGFR (ml/min per 1.73 m2) | 23.2±8.2 | 26.7±8.8 | 0.31 |
Data are mean±SD.
Baseline comparison between groups (independent t tests).
We have previously reported that the exercise intervention improved exercise tolerance in the exercise group only.20 Data from the immune and inflammatory parameters investigated at baseline and 6 months for each group are summarized in Table 4. Most of the variables assessed did not differ between groups at baseline. Exceptions were CD86 expression by SEB-stimulated CD14+CD86+HLA-DR+ monocytes and plasma sTNF-RI levels (higher in exercisers) and CD69 expression by SEB-stimulated CD4+CD69+ and CD8++CD69+ lymphocytes (tendency to be higher in exercisers).
Table 4.
Effects of regular exercise on immune and inflammatory parameters
| Parameter | Exercise Group | Control Group | P Value | |||
|---|---|---|---|---|---|---|
| Baseline | 6 mo | Baseline | 6 mo | Baselinea | Effectb | |
| Total and differential blood leukocyte counts | ||||||
| Leukocytes (×109/L) | 6.7±2.4 | 6.6±2.1 | 6.2±1.5 | 5.9±1.6 | 0.56 | 0.63 |
| Neutrophils (×109/L) | 4.3±1.8 | 4.2±1.6 | 3.9±1.3 | 3.6±1.2 | 0.56 | 0.53 |
| Lymphocytes (×109/L) | 1.5±0.6 | 1.6±0.6 | 1.5±0.3 | 1.6±0.5 | 0.97 | 0.96 |
| Monocytes (×109/L) | 0.6±0.2 | 0.5±0.2 | 0.5±0.2 | 0.4±0.2 | 0.66 | 0.37 |
| Neutrophil degranulation (elastase release) | ||||||
| Plasma elastase (µg/L) | 68±22 | 63±22 | 70±18 | 62±9 | 0.83 | 0.71 |
| Total bacterially-stimulated elastase release (µg/L) | 3703±1892 | 3586±2107 | 3150±1636 | 2837±1134 | 0.67 | 0.71 |
| Bacterially-stimulated elastase release per neutrophil (fg/cell) | 522±121 | 539±174 | 530±144 | 535±141 | 0.99 | 0.98 |
| SEB-stimulated CD4+ and CD8++ lymphocytes | ||||||
| CD69+ cells (%) | ||||||
| CD4+ | 24±10 | 20±8 | 26±8 | 22±8 | 0.65 | 0.59 |
| CD8++ | 30±15 | 24±10 | 31±10 | 28±8 | 0.79 | 0.41 |
| CD69 (GMFI) | ||||||
| CD4+CD69+ | 175±55 | 143±53c | 131±41 | 130±42 | 0.06 | 0.02 |
| CD8++CD69+ | 110±44 | 85±32c | 77±23 | 82±23 | 0.05 | 0.002 |
| SEB-stimulated CD14+CD86+HLA-DR+ monocytes | ||||||
| CD86 (GMFI ratio to unstimulated cells) | 2.1±0.8 | 1.3±0.3c | 1.3±0.5 | 1.7±0.6c | 0.02 | <0.001 |
| HLA-DR (GMFI ratio to unstimulated cells) | 3.0±1.7 | 2.0±0.7c | 1.9±0.5 | 2.3±0.9c | 0.11 | <0.01 |
| Plasma markers of systemic inflammation | ||||||
| IL-6 (pg/ml) | 10.3±5.2 | 8.1±4.3 | 9.0±4.5 | 9.4±4.1 | 0.63 | 0.05 |
| IL-10 (pg/ml) | 3.1±1.1 | 4.6±2.7 | 4.4±1.9 | 3.9±1.5 | 0.15 | 0.04 |
| sTNF-RI (ng/ml) | 6.2±2.5 | 5.7±2.4c | 3.6±2.0 | 3.9±2.2 | <0.01 | 0.01 |
| sTNF-RII (ng/ml) | 15.6±6.5 | 13.9±5.0 | 14.1±7.0 | 15.2±9.4 | 0.56 | 0.08 |
| CRP (µg/ml) | 3.5±3.3 | 2.6±2.5 | 2.3±1.9 | 2.9±2.4 | 0.90 | 0.37 |
Data are mean±SD (n=13 exercise group; n=11 control group).
Baseline comparison between groups (independent t tests).
Effect of regular exercise (two-factor ANOVA: time×group interaction).
P<0.05 versus baseline (paired t tests).
There were no effects of the exercise intervention on total and differential blood leukocyte counts or measures of neutrophil degranulation. The percentages of SEB-stimulated CD4+ and CD8++ lymphocytes expressing CD69 were also unaffected by the exercise intervention. However, CD69 expression by SEB-stimulated CD4+CD69+ and CD8++CD69+ lymphocytes was downregulated after 6 months in exercisers (P=0.02, ES=0.59 and P=0.01, ES=0.65, respectively), whereas it remained unaltered in controls (P=0.91 and P=0.27, respectively). Likewise, the expression of CD86 and HLA-DR by SEB-stimulated CD14+CD86+HLA-DR+ monocytes was also downregulated after 6 months in exercisers (P=0.004, ES=1.27 and P=0.04, ES=0.72) but upregulated in controls (P=0.02, ES=0.76 and P=0.04, ES=0.51). Comparisons of the relative changes over 6 months between groups confirmed these effects (Figure 1).
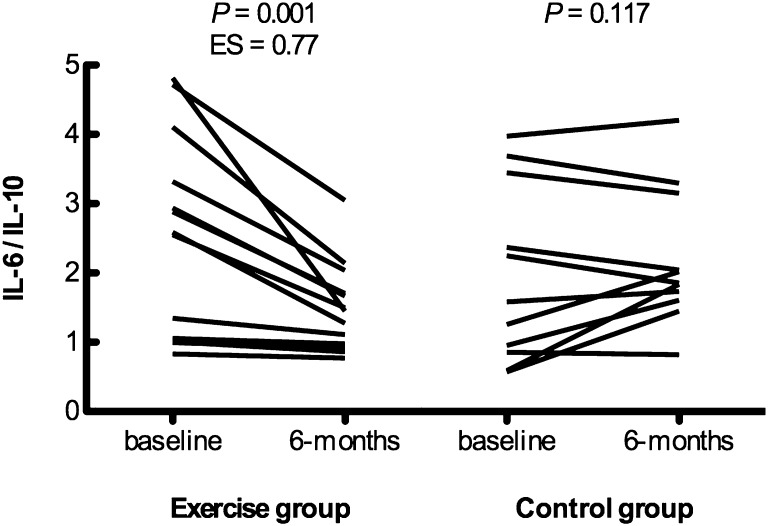
Circulating IL-6 levels tended to be reduced (P=0.06, ES=0.40), and IL-10 levels tended to be increased (P=0.07, ES=0.66) in exercisers but remained unchanged in controls (P=0.43 and P=0.33, respectively). Consequently, the plasma IL-6/IL-10 ratio, reflecting overall inflammatory status, was reduced in exercisers, whereas it did not change in controls (Figure 2). Plasma sTNF-RI levels were also reduced (P=0.04, ES=0.20), and plasma sTNF-RII showed a tendency to reduction (P=0.06, ES=0.25) in exercisers but not controls (P=0.13 and P=0.50, respectively). In contrast, plasma CRP levels were unaffected by the exercise intervention.
Figure 2.
Effects of regular exercise on plasma IL-6 to IL-10 ratio at baseline and 6 months for each group. Data are individual values (n=13 exercise group; n=11 control group). Time×group interaction: P=0.001.
There were no effects of the exercise intervention on body weight, renal function, proteinuria, or BP (Table 5).
Table 5.
Effects of regular exercise on clinical parameters
| Parameter | Exercise Group | Control Group | P Value | |||
|---|---|---|---|---|---|---|
| Baseline | 6 mo | Baseline | 6 mo | Baselinea | Effectb | |
| Weight (kg) | 76.5±13.2 | 74.9±13.5 | 88.4±21.9 | 86.8±19.5 | 0.13 | 0.93 |
| eGFR (ml/min per 1.73 m2) | 23.2±8.2 | 23.7±10.0 | 26.7±8.8 | 26.5±9.2 | 0.31 | 0.59 |
| uPCR (mg/mmol) | 59±97 | 59±72 | 85±116 | 75±107 | 0.64 | 0.71 |
| Systolic BP (mmHg) | 127±17 | 126±13 | 136±20 | 128±18 | 0.23 | 0.24 |
| Diastolic BP (mmHg) | 73±10 | 72±6 | 77±9 | 77±4 | 0.40 | 0.62 |
Data are mean±SD (n=13 exercise group; n=11 control group). uPCR, urine protein-to-creatinine ratio.
Baseline comparison between groups (independent t tests).
Effect of regular exercise (two-factor ANOVA: time×group interaction).
Discussion
The impact of physical exercise on immunity and inflammation in predialysis CKD patients is unresolved. Previous research in this area is limited and has produced conflicting results, with one study reporting favorable effects of resistance exercise training21 and two other studies showing no effects of aerobic exercise training22,23 on plasma IL-6 and/or CRP levels. Here, we describe a more detailed examination of the impact of both acute and regular walking exercise on immunity and inflammation in CKD.
Effects of Acute Exercise
The main findings were that exercise enhanced neutrophil responsiveness to a bacterial challenge without affecting T-lymphocyte and monocyte activation and induced a systemic anti-inflammatory environment.
The observed effects of exercise on blood leukocyte counts were comparable with the effects usually reported in healthy individuals.19,24–26 As consistently reported,19,27 plasma elastase concentration was elevated immediately after exercise, indicating spontaneous neutrophil activation (degranulation in vivo) with return to resting levels within 1 hour. Considering that neutrophils are primed in CKD,28 this observation is particularly important, because it suggests that the exercise-induced neutrophil activation was only transient. Bacterially-stimulated elastase release per neutrophil was unaltered immediately after exercise but increased 1 hour later, indicating enhanced neutrophil responsiveness to a bacterial challenge in the postexercise period. This finding is in contrast to the reported reductions in stimulated neutrophil degranulation after numerous exercise protocols.19,27 Given that neutrophil dysfunction is believed to increase susceptibility to infection in CKD,29,30 a potential protective effect in the postexercise period is encouraging.
T lymphocytes and monocytes exhibit signs of both functional depression and activation in CKD.2,3 Importantly, our study suggests that acute exercise does not hinder T-lymphocyte and monocyte immune competence, and it does not aggravate their preactivated state.
It has been consistently reported that plasma IL-6 levels increase during exercise, with a magnitude that is determined by the combination of mode, intensity, and duration of the exercise performed.31,32 In CKD, circulating IL-6 levels are chronically elevated,33 raising a question about the safety of another exercise-induced increase. However, it is now clear that contracting skeletal muscle is the main source of the IL-6 in circulation in response to exercise,34,35 and muscle-derived IL-6, the first so-called myokine described,36 seems to mediate metabolic and anti-inflammatory effects both locally and systemically in an hormone-like fashion.8,32 In contrast to the cytokine cascade in sepsis, where IL-6 appearance is preceded by proinflammatory TNF-α and IL-1β, in response to exercise, IL-6 is typically the first cytokine present in the circulation, and then, it is followed by the cytokine inhibitors IL-1ra and sTNF-R as well as the anti-inflammatory IL-10.8,32 The kinetics of the cytokine response to exercise reported here are consistent with this finding. The greatest increase in IL-6 observed immediately after exercise was followed by a marked increase in IL-10 1 hour later, with a small increase in sTNF-RII also detected at this time. However, CRP was unaffected by exercise, which is expected, because alterations in CRP are often not seen until a few hours or even the day after exercise.37,38
In summary, a single bout of walking exercise in predialysis CKD patients seems to be safe from an immune and inflammatory perspective, improves immunosurveillance, and exerts anti-inflammatory effects.
Effects of Regular Exercise
The main findings of this study were that regular exercise downregulated T-lymphocyte and monocyte activation and that these effects were accompanied by improvements in systemic inflammation.
The exercise intervention had no effect on in vivo or in vitro neutrophil degranulation responses. In CKD, plasma elastase levels are elevated,39–41 and primed neutrophils are associated with inflammation and oxidative stress.28 Our findings indicate that regular exercise does not exacerbate the activation state of resting neutrophils (although transient spontaneous neutrophil activation occurs during each exercise bout). In addition, although the improved neutrophil responses observed after acute exercise do not translate into a chronic adaptation, given that neutrophil deficiencies may contribute to the high incidence of infections in CKD,30 it is reassuring that regular exercise does not hinder neutrophil immune competence. Furthermore, it is still possible that there is a window of protection after each exercise bout.
The observation that regular exercise downregulated T-lymphocyte activation is a very promising effect considering that it has been consistently shown that T lymphocytes are chronically activated in CKD.42–45 Given that T-lymphocyte preactivation in CKD patients is associated with premature apoptosis and impaired proliferation of these cells,43,44,46–48 it may be speculated that the observed decrease in T-lymphocyte activation might lead to enhanced function of these cells. Interestingly, previous studies in CKD have used sTNF-RI levels as an indicator of the susceptibility of T cells to undergo increased activation-induced cell death.44,48 Here, plasma sTNF-RI levels were reduced in exercisers but unaltered in controls, suggesting, albeit indirectly, that a downregulation of T-lymphocyte activation might have been accompanied by reduced T-lymphocyte apoptosis.
The exercise intervention also downregulated monocyte activation, and it is likely that it has contributed, at least in part, to the observed effects on T-lymphocyte activation, because it occurs through interactions between the monocyte and the T cell. However, given that exercise training downregulates monocyte Toll-like receptor 4 (TLR4) expression49,50 and that activation of TLRs upregulates monocyte expression of HLA-DR and CD86,51 it is plausible that the downregulation of CD86 and HLA-DR observed here was mediated by a downregulation of TLR4. Another explanation might be a reduced proportion of circulating CD16+ monocytes, which are known to express higher levels of CD86 and HLA-DR compared with classic monocytes51 and have been shown to be reduced after regular exercise.52 Interestingly, IL-10 production is low to absent in CD16+ monocytes.53 Although we did not perform direct measurements of monocyte IL-10 production, we did observe a trend for increased systemic IL-10 levels, which provides indirect support for a possible shift into a subset of circulating monocytes that are able to produce IL-10.
It may be argued that the observed reductions in T-lymphocyte and monocyte activation are signs of a detrimental effect of regular exercise on the orchestration of the adaptive immune response. However, it is important to recall that the complex state of immune dysfunction that accompanies CKD is characterized by immune depression as well as immune activation. Monocytes from CKD patients are preactivated and overproduce proinflammatory cytokines.54–58 Likewise, T lymphocytes from CKD patients show increased expression of early activation markers and heightened apoptotic turnover.43–48,59–61 Of note, data from our control patients indicates a progressive state of monocyte activation over the study period. Thus, it seems that the apparent exercise-induced downregulation of the immune response might be a small price to pay for the anti-inflammatory effects. Other than contributing to reduced inflammation, a downregulation of the preactivated state of immune cells might actually contribute to improved immune response in the longer term.3
The potential systemic anti-inflammatory effects of regular exercise were confirmed. In exercisers, plasma IL-6 concentration tended to be reduced after 6 months, whereas plasma IL-10 concentration tended to be increased, resulting in a significantly decreased ratio of IL-6 to IL-10 in the circulation. Plasma IL-6 levels are elevated and have consistently been shown to predict all-cause and cardiovascular mortality in CKD.33,62,63 Plasma levels of IL-10, one of the most important anti-inflammatory immune-regulating cytokines,64 are usually elevated in CKD because of persistent underlying inflammation.44,56,58 Hemodialysis patients with lower plasma IL-10 levels seem to be at greater risk for atherosclerosis,65 whereas those patients genetically predisposed to produce higher IL-10 levels show better immune balance66 and are at lower risk of cardiovascular events.67 It can be argued that the balance between IL-6 and IL-10 is a more valid parameter to draw conclusions about the overall inflammatory status. sTNF-Rs are the extracellular forms of the naturally occurring inhibitors of TNF but also, footprints of its activity68 and thus, not surprisingly elevated in CKD.42,48,69 Plasma sTNF-RI and sTNF-RII levels were or tended to be reduced after exercise, again suggesting an anti-inflammatory role of regular exercise in predialysis CKD patients.
In contrast to the favorable effects on plasma cytokines, we have failed to detect an effect of regular exercise on plasma CRP. This finding might be partly explained by the lack of an effect of the exercise intervention on body weight, because exercise without weight loss does not seem to reduce CRP.70 However, although it is also well established that CRP levels are elevated and predict all-cause and cardiovascular mortality in CKD,71,72 CRP is a rather nonspecific marker, and therefore, it might be difficult to detect changes in small patient cohorts. It is noteworthy that IL-6 has been consistently reported to be a stronger predictor of all-cause and cardiovascular outcomes in CKD than any other inflammatory marker, including CRP.63,73–77
In summary, our findings suggest that regular walking exercise in predialysis CKD patients is safe from immune and inflammatory perspectives and that it exerts anti-inflammatory effects at both systemic and cellular levels. Obviously, this study is limited by its small sample size, and such effects need to be confirmed in larger cohorts of predialysis CKD patients. In addition, given the close links between wasting, inflammation, and atherosclerosis in CKD,62,78 future studies should also address the impact of resistance exercise. There is initial evidence that regular resistance exercise can also induce anti-inflammatory effects in predialysis CKD patients,21 but a more in-depth study of such effects is warranted. Ideally, investigating both exercise modes alone and combined in representative groups of patients at different CKD stages would help tailor future exercise guidelines for this population.
In conclusion, this report provides convincing evidence that exercise has the potential to be an effective anti-inflammatory therapy in predialysis CKD patients and in this way, may reduce the high risk of CVD in these very vulnerable patients. Additional research is, however, needed to fully elucidate the impact of exercise on the complex state of immune dysfunction that accompanies CKD.
Concise Methods
Patients
Patients included in this report are subgroups of those patients described previously.20 All patients were recruited from nephrology outpatient clinics at Leicester General Hospital. Exclusion criteria were age<18 years, pregnancy, and orthopedic or cardiovascular disability that severely limited exercise capacity. The study received approval from the UK National Research Ethics Committee, and all patients gave written informed consent to participate.
The effects of acute exercise were investigated in a subgroup of patients that completed the baseline exercise test but were not receiving immunosuppressive therapy. The effects of regular exercise were investigated in a subgroup of patients that completed the 6-month study period but were not receiving immunosuppressive therapy. Patient characteristics are presented in Results (Tables 1 and 3).
Exercise Testing
All patients completed an exercise test at baseline and 6 months. At baseline, the exercise test consisted of 30 minutes of walking on a motorized treadmill at a 1% gradient and a speed that elicited an RPE in the range of 12–14 (somewhat hard).79 RPE was recorded every 2 minutes, and the treadmill speed was adjusted to maintain the RPE in the target range. The treadmill speed was also recorded every 2 minutes. At 6 months, the exercise test was repeated using exactly the same treadmill speed profile (i.e., same absolute exercise intensity), and the RPE response was recorded every 2 minutes. The effects of the exercise intervention on exercise tolerance have been previously reported.20
Exercise Program
Patients in the exercise group were prescribed a home-based exercise program, which consisted of at least 30 minutes of walking five times per week at an RPE in the range of 12–14 (somewhat hard) for a total duration of 6 months. Because most CKD patients receive β-blocker therapy, an overall heart rate (HR) range could not be used to prescribe the exercise intensity. Nevertheless, an individual HR range was established during the baseline exercise test by recording the HR response at the required RPE target range, and it was also provided to each patient in conjunction with an HR monitor. For monitoring purposes, patients were asked to keep exercise diaries, where they recorded the duration and the overall RPE of each exercise session. In addition, patients were requested to attend the hospital gym one time per month for a supervised exercise session to ensure compliance and make any necessary adjustments to the exercise program. Patients in the control group continued with their usual physical activity.
Blood Sampling, Handling, and Analysis
To investigate the effects of acute exercise, venous blood samples were obtained at rest (pre-exercise) and immediately after (postexercise) and 1 hour after (1 hour postexercise) the baseline exercise test. To investigate the effects of regular exercise, resting venous blood samples were obtained at baseline and 6 months. On both occasions, samples were collected at the same time of day, and patients were asked to refrain from exercise during the preceding 24 hours. All samples, approximately 20 ml each, were collected by venepuncture from an antecubital vein using a 21-g butterfly needle cannula and a dry syringe and immediately dispensed into two separate tubes as follows: approximately 7.5 ml into one S-Monovette tube (Sarstedt) containing K3EDTA (1.6 mg/ml) and approximately 12.5 ml into one universal tube containing heparin (16 units/ml).
Hematology
For the following analysis, K3EDTA-treated whole blood was used. Hemoglobin concentration was determined in duplicate using the cyanmethemoglobin method. Hematocrit was determined by measuring packed cell volumes in triplicate on a microhematocrit centrifuge. Plasma volume of resting blood samples was estimated from the hematocrit values. Plasma volume changes in postexercise blood samples were estimated from the hemoglobin and hematocrit values,80 and all cell counts and plasma measurements were corrected for these changes relative to the resting blood sample in the acute study only. In the exercise training study, there was no effect of time (baseline versus 6 months) on plasma volume (data not shown), and therefore, no adjustments were made. Total and differential leukocyte counts were determined using an automated hematology analyzer (Coulter Ac·T 5diff OV; Beckman Coulter).
Plasma Markers of Systemic Inflammation
The remaining K3EDTA-treated whole blood was centrifuged at 1500×g for 10 minutes in a refrigerated centrifuge at 4°C. The plasma obtained was aliquoted into Eppendorf tubes at 0.5 ml/tube and stored at −80°C for later determination of plasma concentrations of IL-6, IL-10, sTNF-RI, sTNF-RII, and CRP by ELISA as detailed in Supplemental Material.
Neutrophil Degranulation
One milliliter heparinized whole blood was immediately added to an Eppendorf tube containing 50 µl 10 mg/ml bacterial extract solution (stimulant, 84015; Sigma-Aldrich). The tube was sealed; blood and stimulant were mixed by gentle inversion, incubated for 1 hour at 37°C, and gently mixed again after 30 minutes. After incubation, the mixture was centrifuged for 2 minutes at 12,400×g, and the resulting supernatant was stored at −80°C before analysis. Another 1 ml heparinized whole blood was centrifuged at 1500×g for 10 minutes in a refrigerated centrifuge at 4°C, and the plasma obtained was stored at −80°C before analysis. Polymorphonuclear cell elastase concentration was determined in both bacterially stimulated and unstimulated (plasma elastase) samples using a commercially available ELISA kit (RD191021100; BioVendor GmbH) according to the manufacturer’s instructions. Bacterially stimulated and unstimulated samples were prediluted 1:1000 and 1:50 in the dilution buffer provided, respectively. All samples were assayed in duplicate, and all samples from the same patient were assayed in the same plate. The inter- and intra-assay coefficients of variation for all elastase ELISAs were 2.3% and 2.9%, respectively. Total bacterially-stimulated elastase release was calculated by subtracting plasma elastase concentration from the bacterially-stimulated elastase concentration and then, dividing by the neutrophil count to obtain the bacterially-stimulated elastase release per neutrophil.
T-Lymphocyte and Monocyte Activation
Two hundred-microliter aliquots of heparinized whole blood were cultured in 12×75-mm polystyrene round-bottomed tubes with caps (BD Biosciences) with no additive (unstimulated condition) or 1 µg/ml SEB (S4881; Sigma-Aldrich). Triplicate tubes were set up for each condition and incubated at 37°C in a humid 5% CO2 atmosphere for 20 hours. After incubation, the whole-blood aliquots were labeled in the respective culture tubes with cocktails of fluorochrome-conjugated mouse mAbs against human cell surface markers (BD Biosciences) as follows (one per condition): (1) lymphocyte surface markers: FITC-conjugated anti-CD4 (555346), phycoerythrin (PE)-conjugated anti-CD69 (555531), and PE-Cy5–conjugated anti-CD8 (555368); and (2) monocyte surface markers: FITC-conjugated anti-CD14 (555397), PE-conjugated anti-CD86 (555658), and peridinin–chlorophyll protein complex–conjugated anti–HLA-DR (347402). The remaining tube was left unstained. Labeling was carried out on ice for 20 minutes, which was followed by erythrocyte lysis and leukocyte fixation achieved by incubating samples for 10 minutes in the dark with FACS Lysing solution (BD Biosciences). Leukocytes were subsequently washed two times in PBS containing 0.1% BSA and 2 mM EDTA and resuspended again in the same buffer for immediate flow cytometer acquisition. Samples were acquired on a flow cytometer (BD FACSCalibur) equipped with the CellQuest software package (BD Biosciences). For samples labeled with lymphocyte markers, side scatter versus forward scatter plots were used to gate on the lymphocyte population by morphology, and 30,000 lymphocytes were acquired per sample. For samples labeled with monocyte markers, 100,000 total cells were acquired per sample. Negative unstained control samples were also acquired. Software-generated data files were stored for later analyses using FlowJo version 9 for Macintosh (Treestar) as detailed in Supplemental Material.
Statistical Analyses
Data are presented as mean±SD unless otherwise stated. All data were inspected for normality using the Shapiro–Wilk test. If a dataset was not normally distributed, statistical analysis was performed on the logarithmic transformation of the data, but data were back-transformed for presentation.
Data from the acute effects of exercise were examined using a one-factor (time: pre-exercise, postexercise, or 1 hour postexercise) ANOVA with repeated measures design. For any significant unadjusted F values subsequently shown, sphericity of the data was determined using the Mauchly’s test, and appropriate adjustments in the degrees of freedom were made81 (i.e., Greenhouse–Geisser correction was applied when Greenhouse–Geisser ε was <0.75 or Huynh–Feldt correction was used for less severe asphericity). A priori planned paired t tests with Holm–Bonferroni correction for multiple comparisons applied to the unadjusted P value were then performed between specific time points (postexercise versus pre-exercise and 1 hour postexercise versus pre-exercise).
To investigate the effects of regular exercise, baseline data were initially screened using independent t tests (exercise versus control). All data were then examined using an ANOVA with mixed design: within subjects (time: baseline versus 6 months)×between subjects (group: exercise versus control). Assumption of homogeneity was checked using Levene’s test. Where significant time×group interactions were observed, data were further examined using a priori planned t tests with Holm–Bonferroni correction as follows: paired (baseline versus 6 months) for each group and independent (exercise versus control) for 6-month data. T-lymphocyte and monocyte activation data were further examined by computing the relative (percentage of difference from baseline) changes over 6 months for each group and using independent t tests (exercise versus control).
Effect sizes were calculated according to Cohen82 with correction for sample size.83 Statistical significance was accepted at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Stephen John of Royal Derby Hospital for his assistance with the BP measurements in this study.
This work was partly supported by Kidney Research UK Grant RP33/1/2007 and Portuguese Foundation for Science and Technology Grant SFRH/BD/27838/2006. At the time of writing, J.L.V. was supported by the National Institute for Health Research Diet, Lifestyle & Physical Activity Biomedical Research Unit based at University Hospitals of Leicester and Loughborough University.
The views expressed are those of the authors and not necessarily the views of the NHS, the National Institute for Health Research, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013070702/-/DCSupplemental.
References
- 1.Descamps-Latscha B, Jungers P, Witko-Sarsat V: Immune system dysregulation in uremia: Role of oxidative stress. Blood Purif 20: 481–484, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I: Disturbances of acquired immunity in hemodialysis patients. Semin Dial 20: 440–451, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B: Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naqvi SB, Collins AJ: Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis 13: 199–204, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Saran AM, DuBose TD, Jr.: Cardiovascular disease in chronic kidney disease. Ther Adv Cardiovasc Dis 2: 425–434, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Go AS: Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1487–1493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenvinkel P: Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456–467, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Petersen AMW, Pedersen BK: The anti-inflammatory effect of exercise. J Appl Physiol (1985) 98: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Senchina DS, Kohut ML: Immunological outcomes of exercise in older adults. Clin Interv Aging 2: 3–16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haaland DA, Sabljic TF, Baribeau DA, Mukovozov IM, Hart LE: Is regular exercise a friend or foe of the aging immune system? A systematic review. Clin J Sport Med 18: 539–548, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Beavers KM, Brinkley TE, Nicklas BJ: Effect of exercise training on chronic inflammation. Clin Chim Acta 411: 785–793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieman D: Moderate exercise improves immunity and decreases illnesss rates. Am J Lifestyle Med 5: 338–345, 2011 [Google Scholar]
- 13.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA: The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11: 607–615, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Bronas UG: Exercise training and reduction of cardiovascular disease risk factors in patients with chronic kidney disease. Adv Chronic Kidney Dis 16: 449–458, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Heiwe S, Jacobson SH: Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 10: CD003236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmadakis GC, Bevington A, Smith AC, Clapp EL, Viana JL, Bishop NC, Feehally J: Physical exercise in patients with severe kidney disease. Nephron Clin Pract 115: c7–c16, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Johansen KL, Painter P: Exercise in individuals with CKD. Am J Kidney Dis 59: 126–134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleeson M: Immune function in sport and exercise. J Appl Physiol (1985) 103: 693–699, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P: Position statement. Part one: Immune function and exercise. Exerc Immunol Rev 17: 6–63, 2011 [PubMed] [Google Scholar]
- 20.Kosmadakis GC, John SG, Clapp EL, Viana JL, Smith AC, Bishop NC, Bevington A, Owen PJ, McIntyre CW, Feehally J: Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant 27: 997–1004, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS: Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 43: 607–616, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Leehey DJ, Moinuddin I, Bast JP, Qureshi S, Jelinek CS, Cooper C, Edwards LC, Smith BM, Collins EG: Aerobic exercise in obese diabetic patients with chronic kidney disease: A randomized and controlled pilot study. Cardiovasc Diabetol 8: 62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Headley S, Germain M, Milch C, Pescatello L, Coughlin MA, Nindl BC, Cornelius A, Sullivan S, Gregory S, Wood R: Exercise training improves HR responses and V˙O2peak in predialysis kidney patients. Med Sci Sports Exerc 44: 2392–2399, 2012 [DOI] [PubMed] [Google Scholar]
- 24.McCarthy DA, Grant M, Marbut M, Watling M, Wade AJ, Macdonald I, Nicholson S, Melsom RD, Perry JD: Brief exercise induces an immediate and a delayed leucocytosis. Br J Sports Med 25: 191–195, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, Davis JM, Butterworth DE, Herring JL, Nehlsen-Cannarella SL: Effect of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med 15: 199–206, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Nieman DC, Henson DA, Austin MD, Brown VA: Immune response to a 30-minute walk. Med Sci Sports Exerc 37: 57–62, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Peake JM: Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: Possible mechanisms of action. Exerc Immunol Rev 8: 49–100, 2002 [PubMed] [Google Scholar]
- 28.Sela S, Shurtz-Swirski R, Cohen-Mazor M, Mazor R, Chezar J, Shapiro G, Hassan K, Shkolnik G, Geron R, Kristal B: Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol 16: 2431–2438, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Cohen G, Haag-Weber M, Hörl WH: Immune dysfunction in uremia. Kidney Int Suppl 62: S79–S82, 1997 [PubMed] [Google Scholar]
- 30.Chonchol M: Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial 19: 291–296, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Fischer CP: Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc Immunol Rev 12: 6–33, 2006 [PubMed] [Google Scholar]
- 32.Pedersen BK, Febbraio MA: Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B: Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529: 237–242, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toft AD, Falahati A, Steensberg A: Source and kinetics of interleukin-6 in humans during exercise demonstrated by a minimally invasive model. Eur J Appl Physiol 111: 1351–1359, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B: Searching for the exercise factor: Is IL-6 a candidate? J Muscle Res Cell Motil 24: 113–119, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kasapis C, Thompson PD: The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol 45: 1563–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Plaisance EP, Grandjean PW: Physical activity and high-sensitivity C-reactive protein. Sports Med 36: 443–458, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Costa E, Rocha S, Rocha-Pereira P, Nascimento H, Castro E, Miranda V, Faria MS, Loureiro A, Quintanilha A, Belo L, Santos-Silva A: Neutrophil activation and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Am J Nephrol 28: 935–940, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Caimi G, Carollo C, Montana M, Vaccaro F, Lo Presti R: Elastase, myeloperoxidase, nitric oxide metabolites and oxidative status in subjects with clinical stable chronic renal failure on conservative treatment. Clin Hemorheol Microcirc 43: 253–258, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Polańska B, Augustyniak D, Makulska I, Niemczuk M, Zwolińska D, Jankowski A: Elastase, α1-proteinase inhibitor, and interleukin-8 in pre-dialyzed and hemodialyzed patients with chronic kidney disease. Pediatr Int 52: 735–743, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, Verger C, Dahmane D, de Groote D, Jungers P,: Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol 154: 882–892, 1995 [PubMed] [Google Scholar]
- 43.Meier P, Dayer E, Blanc E, Wauters J-P: Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol 13: 204–212, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Moser B, Roth G, Brunner M, Lilaj T, Deicher R, Wolner E, Kovarik J, Boltz-Nitulescu G, Vychytil A, Ankersmit HJ: Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: A comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem Biophys Res Commun 308: 581–585, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Litjens NHR, van Druningen CJ, Betjes MGH: Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol 118: 83–91, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Meier P, Dayer E, Ronco P, Blanc E: Dysregulation of IL-2/IL-2R system alters proliferation of early activated CD4+ T cell subset in patients with end-stage renal failure. Clin Nephrol 63: 8–21, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Meier P, Spertini F, Blanc E, Burnier M: Oxidized low-density lipoproteins activate CD4+ T cell apoptosis in patients with end-stage renal disease through Fas engagement. J Am Soc Nephrol 18: 331–342, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ankersmit HJ, Deicher R, Moser B, Teufel I, Roth G, Gerlitz S, Itescu S, Wolner E, Boltz-Nitulescu G, Kovarik J: Impaired T cell proliferation, increased soluble death-inducing receptors and activation-induced T cell death in patients undergoing haemodialysis. Clin Exp Immunol 125: 142–148, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn MG, McFarlin BK: Toll-like receptor 4: Link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev 34: 176–181, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Gleeson M, McFarlin B, Flynn M: Exercise and Toll-like receptors. Exerc Immunol Rev 12: 34–53, 2006 [PubMed] [Google Scholar]
- 51.Takeda K, Kaisho T, Akira S: Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD: Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J Leukoc Biol 84: 1271–1278, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Ziegler-Heitbrock L: The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol 81: 584–592, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Girndt M, Köhler H, Schiedhelm-Weick E, Schlaak JF, Meyer zum Büschenfelde KH, Fleischer B: Production of interleukin-6, tumor necrosis factor alpha and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney Int 47: 559–565, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Girndt M, Sester U, Kaul H, Köhler H: Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J Am Soc Nephrol 9: 1689–1696, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Morita Y, Yamamura M, Kashihara N, Makino H: Increased production of interleukin-10 and inflammatory cytokines in blood monocytes of hemodialysis patients. Res Commun Mol Pathol Pharmacol 98: 19–33, 1997 [PubMed] [Google Scholar]
- 57.Higuchi T, Yamamoto C, Kuno T, Mizuno M, Takahashi S, Kanmatsuse K: Increased production of interleukin-1beta and interleukin-1 receptor antagonist by peripheral blood mononuclear cells in undialyzed chronic renal failure. Nephron 76: 26–31, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Sardenberg C, Suassuna P, Watanabe R, Cruz Andreoli MC, Aparecida Dalboni M, Faria Seabra V, Draibe SA, Cendoroglo Neto M, Jaber B: Balance between cytokine production by peripheral blood mononuclear cells and reactive oxygen species production by monocytes in patients with chronic kidney disease. Ren Fail 26: 673–681, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Meier P, Meier R, Blanc E: Influence of CD4+/CD25+ regulatory T cells on atherogenesis in patients with end-stage kidney disease. Expert Rev Cardiovasc Ther 6: 987–997, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Meier P, von Fliedner V, Markert M, van Melle G, Deppisch R, Wauters JP: One-year immunological evaluation of chronic hemodialysis in end-stage renal disease patients. Blood Purif 18: 128–137, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Stachowski J, Pollok M, Burrichter H, Baldamus CA: Immunodeficiency in ESRD-patients is linked to altered IL-2 receptor density on T cell subsets. J Clin Lab Immunol 34: 171–177, 1991 [PubMed] [Google Scholar]
- 62.Stenvinkel P, Bárány P, Heimbürger O, Pecoits-Filho R, Lindholm B: Mortality, malnutrition, and atherosclerosis in ESRD: What is the role of interleukin-6? Kidney Int Suppl 80: 103–108, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke H-D, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 77: 550–556, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Seyrek N, Karayaylali I, Balal M, Paydas S, Aikimbaev K, Cetiner S, Seydaoglu G: Is there any relationship between serum levels of interleukin-10 and atherosclerosis in hemodialysis patients? Scand J Urol Nephrol 39: 405–409, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Girndt M, Sester U, Sester M, Deman E, Ulrich C, Kaul H, Köhler H: The interleukin-10 promoter genotype determines clinical immune function in hemodialysis patients. Kidney Int 60: 2385–2391, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Girndt M, Kaul H, Sester U, Ulrich C, Sester M, Georg T, Köhler H: Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 62: 949–955, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Brockhaus M: Soluble TNF receptor: What is the significance? Intensive Care Med 23: 808–809, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA: Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int 45: 890–896, 1994 [DOI] [PubMed] [Google Scholar]
- 70.Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, Ross R, Blair SN: Exercise without weight loss does not reduce C-reactive protein: The INFLAME study. Med Sci Sports Exerc 42: 708–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lacson E, Jr, Levin NW: C-reactive protein and end-stage renal disease. Semin Dial 17: 438–448, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Wanner C, Drechsler C, Krane V: C-reactive protein and uremia. Semin Dial 22: 438–441, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Wetmore JB, Lovett DH, Hung AM, Cook-Wiens G, Mahnken JD, Sen S, Johansen KL: Associations of interleukin-6, C-reactive protein and serum amyloid A with mortality in haemodialysis patients. Nephrology (Carlton) 13: 593–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pachaly MA, do Nascimento MM, Suliman ME, Hayashi SY, Riella MC, Manfro RC, Stenvinkel P, Lindholm B: Interleukin-6 is a better predictor of mortality as compared to C-reactive protein, homocysteine, pentosidine and advanced oxidation protein products in hemodialysis patients. Blood Purif 26: 204–210, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Zoccali C, Tripepi G, Mallamaci F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Honda H, Qureshi AR, Heimbürger O, Bárány P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16[Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Borg GA: Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 80.Dill DB, Costill DL: Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974 [DOI] [PubMed] [Google Scholar]
- 81.Atkinson G: Analysis of repeated measurements in physical therapy research. Phys Ther Sport 2: 194–208, 2001 [Google Scholar]
- 82.Cohen JW: Statistical Power Analysis for the Behavioral Sciences, 2nd Ed., Hillsdale, NJ, Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 83.Hedges LV: Distributional theory for Glass’s estimator of effect size and related estimators. J Educ Stat 6: 107–128, 1981 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.