Abstract
Obesity is associated with higher mortality in the general population, but this association is reversed in patients on dialysis. The nature of the relationship of obesity with adverse clinical outcomes in nondialysis-dependent CKD and the putative interaction of the severity of disease with this association are unclear. We analyzed data from a nationally representative cohort of 453,946 United States veterans with eGFR<60 ml/min per 1.73 m2. The associations of body mass index categories (<20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, 40 to <45, 45 to <50, and ≥50 kg/m2) with all-cause mortality and disease progression (using multiple definitions, including incidence of ESRD, doubling of serum creatinine, and the slopes of eGFR) were examined in Cox proportional hazards models and logistic regression models. Multivariable adjustments were made for age, race, comorbidities and medications, and baseline eGFR. Body mass index showed a relatively consistent U-shaped association with clinical outcomes, with the best outcomes observed in overweight and mildly obese patients. Body mass index levels <25 kg/m2 were associated with worse outcomes in all patients, independent of severity of CKD. Body mass index levels ≥35 kg/m2 were associated with worse outcomes in patients with earlier stages of CKD, but this association was attenuated in those patients with eGFR<30 ml/min per 1.73 m2. Thus, until clinical trials establish the ideal body mass index, a cautious approach to weight management is warranted in this patient population.
Obesity defined by elevated body mass index (BMI) has been regarded as a cardiovascular risk factor in the general population.1–4 Obesity is also associated with increased risk of incident CKD5–9 and ESRD.10–13 Negative effects of obesity include those effects mediated by conditions caused or worsened by it, such as diabetes mellitus (DM) or hypertension, and direct adverse metabolic effects, such as inflammation, increased synthesis of apolipoprotein B and very LDLs, increased production of insulin, and insulin resistance.14 Obesity also induces glomerular hyperfiltration,15 and weight loss in morbidly obese patients attenuates proteinuria.16
However, even in relatively healthy populations, very low BMI levels have been consistently associated with higher all-cause mortality,17 and the optimal BMI for survival has varied from study to study.18,19 Contrasting the unequivocally higher risk associated with elevated BMI in the general population, studies that examined patient groups with various chronic diseases have either found no association20 or described paradoxically lower mortality associated with high BMI levels.21,22 The reversal of the obesity–mortality association has been very robust in patients with ESRD,23,24 but there are limited studies showing conflicting results20,25,26 in patients with nondialysis–dependent CKD (NDD-CKD). The heterogeneity of the NDD-CKD population, which encompasses patients with kidney function ranging from near-normal to near-nil, could make it difficult to determine the role that obesity plays as a risk factor in this group and the ideal therapeutic weight management goals.
We examined the association of BMI with all-cause mortality and progressive CKD in a large national cohort of United States veterans with eGFR<60 ml/min per 1.73 m2.
Results
The mean age of the cohort was 73.9±9.3 years, 87.0% (n=394,763) were white, and 9.1% (n=41,231) were African American. The mean BMI was 29.0±5.5 kg/m2, and the mean eGFR was 47.8±9.9 ml/min per 1.73 m2. Baseline characteristics of patients categorized by their baseline BMI are described in Table 1. Patients with higher BMI were younger, were more likely to be white, had higher BP and higher medication use, had higher prevalence of chronic heart failure (CHF), DM, and depression, and had lower prevalence of malignancies, dementia, and lung, liver, rheumatologic, and peptic ulcer diseases.
Table 1.
Baseline characteristics of 453,946 United States veterans with NDD-CKD categorized by their baseline BMI
| Variables | BMI (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| <20 (n=9916) | 20 to <25 (n=92,585) | 25 to <30 (n=185,116) | 30 to <35 (n=109,099) | 35 to <40 (n=39,186) | 40 to <45 (n=12,301) | 45 to <50 (n=3924) | ≥50 (n=1819) | |
| Age, yr | 77.5±9.5 | 77.3±8.7 | 74.9±8.7 | 72.0±9.1 | 69.3±9.1 | 66.9±8.9 | 64.9±8.5 | 63.3±8.3 |
| Sex, men | 93.9 | 96.9 | 97.9 | 97.3 | 96.3 | 94.6 | 91.9 | 90.5 |
| Race | ||||||||
| White | 79.4 | 86.9 | 89.1 | 88.2 | 87.5 | 86.7 | 85.7 | 86.6 |
| Black | 17.1 | 9.6 | 8.1 | 9.4 | 10.2 | 11.2 | 11.9 | 12.0 |
| Hispanic | 1.8 | 1.8 | 1.5 | 1.1 | 0.9 | 0.6 | 0.9 | 0.2 |
| Other | 1.8 | 1.7 | 1.4 | 1.3 | 1.5 | 1.5 | 1.6 | 1.3 |
| Systolic BP | 132±20 | 134±19 | 135±18 | 136±17 | 136±17 | 136±17 | 137±18 | 137±18 |
| Diastolic BP | 70±11 | 70±11 | 71±11 | 73±11 | 73±11 | 73±11 | 73±11 | 72±12 |
| CVD | 38.1 | 42.3 | 43.6 | 44.4 | 45.2 | 43.9 | 40.6 | 38.9 |
| CHF | 17.2 | 14.5 | 13.3 | 15.7 | 20.8 | 25.3 | 29.2 | 32.1 |
| Diabetes | 17.9 | 26.6 | 36.6 | 49.9 | 62.3 | 69.4 | 74.1 | 75.1 |
| Cancer | 23.1 | 20.8 | 19.0 | 17.0 | 15.1 | 13.5 | 12.1 | 10.7 |
| Lung disease | 42.2 | 27.0 | 22.1 | 23.3 | 26.4 | 29.5 | 32.5 | 34.0 |
| Rheumatologic | 2.8 | 2.5 | 2.1 | 2.0 | 1.8 | 1.7 | 2.0 | 1.9 |
| PUD | 5.1 | 3.5 | 2.8 | 2.5 | 2.3 | 2.2 | 2.0 | 2.0 |
| Liver disease | 1.1 | 0.8 | 0.7 | 0.8 | 0.9 | 0.8 | 0.8 | 0.7 |
| Depression | 5.6 | 4.9 | 5.3 | 6.6 | 8.4 | 10.0 | 10.7 | 11.1 |
| Dementia | 5.0 | 3.4 | 2.1 | 1.4 | 1.1 | 0.9 | 0.6 | 0.4 |
| eGFR (ml/min per 1.73 m2) | 46.6±10.6 | 47.1±10.2 | 47.9±9.8 | 48.2±9.7 | 48.1±9.8 | 48.1±9.9 | 48.0±10.1 | 48.0±9.9 |
| Loop diuretics | 19.0 | 19.6 | 20.9 | 28.3 | 39.5 | 49.4 | 56.8 | 61.7 |
| Thiazide diuretics | 21.0 | 22.9 | 26.2 | 29.1 | 30.9 | 32.2 | 32.6 | 31.2 |
| ACEI/ARB | 41.9 | 47.9 | 52.7 | 58.0 | 62.2 | 64.6 | 65.9 | 67.1 |
| α-Blockers | 20.6 | 23.4 | 25.7 | 26.9 | 27.3 | 26.7 | 25.4 | 21.2 |
| β-Blockers | 41.9 | 46.5 | 50.0 | 54.4 | 58.7 | 60.3 | 61.0 | 60.4 |
| Ca channel blockers | 32.8 | 33.7 | 34.5 | 37.0 | 38.6 | 38.7 | 39.7 | 38.3 |
| Statins | 45.8 | 61.6 | 70.0 | 74.3 | 76.6 | 77.1 | 76.4 | 73.7 |
Values expressed as percent or mean±SD. CVD, cardiovascular disease; PUD, peptic ulcer disease; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Mortality
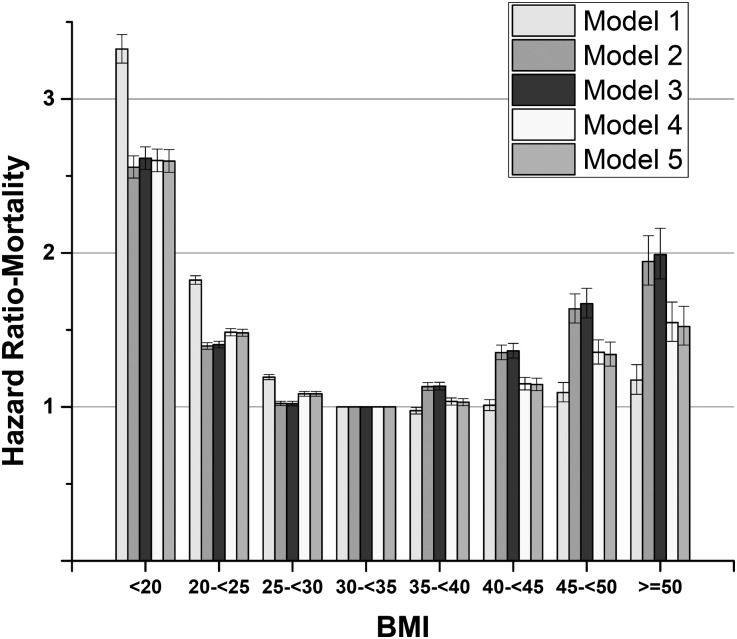
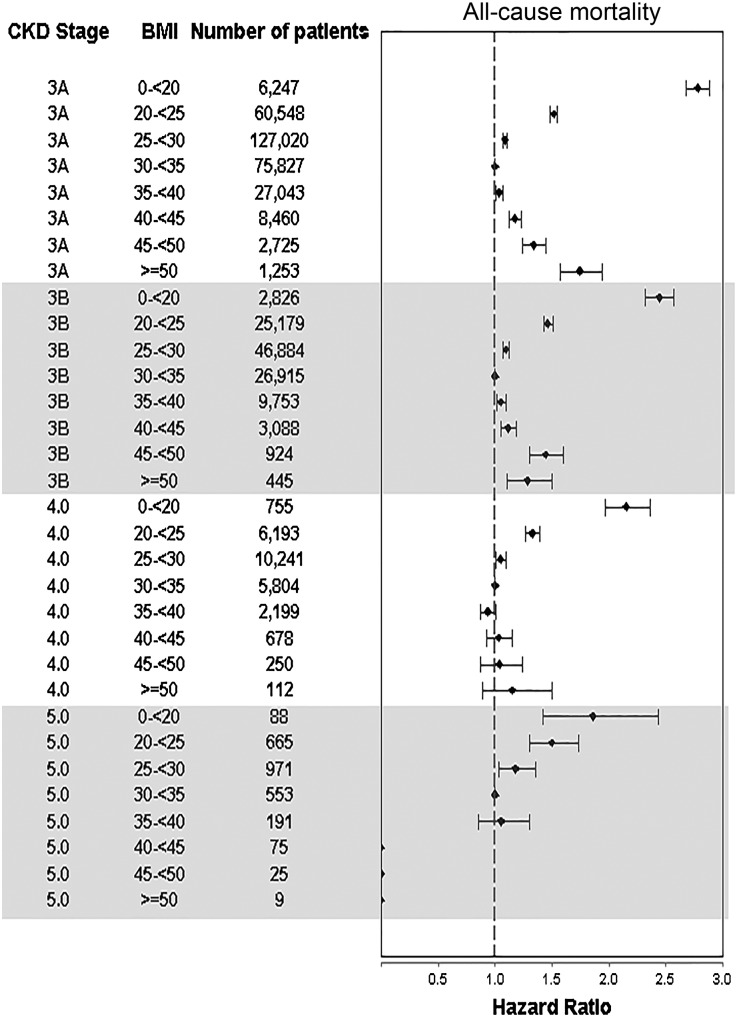
In total, 148,276 patients died (mortality rate=73.9/1000 patient-years [PYs]; 95% confidence interval [95% CI], 73.5 to 74.3). Figure 1 describes associations of BMI with mortality in the overall cohort. Both higher and lower BMIs were associated with higher mortality, with the lowest adjusted mortality observed in the 30 to <35 kg/m2 group. Similar U-shaped associations were present in all subgroups, except patients with eGFR<30 ml/min per 1.73 m2, in whom higher BMI levels were not associated with significant increases in mortality (Supplemental Figure 1). The lack of significant association between higher BMI levels and mortality in patients with advanced CKD (CKD stages 4 and 5) was also apparent in analyses using a more granular CKD stage-based categorization (Figure 2). Additional adjustment for DM and BP in multivariable models did not attenuate the U-shaped association between BMI and mortality (Supplemental Figure 2).
Figure 1.
BMI categories showing a U-shaped association with mortality in 453,946 United States veterans with eGFR<60 ml/min per 1.73 m2. Hazard ratios (95% CIs) of all-cause mortality associated with BMI categories in crude and multivariable-adjusted Cox models: crude (model 1), age-adjusted (model 2), model 2 plus race-adjusted (model 3), model 3 plus comorbidities- and medications-adjusted (model 4), and model 4 plus baseline eGFR-adjusted (model 5).
Figure 2.
BMI categories showing a U-shaped association with mortality in patients with CKD stages 3A and 3B, but an attenuation of this association in patients with CKD stages 4 and 5. Hazard ratios (95% CIs) of all-cause mortality associated with various levels of BMI in patients grouped by their baseline CKD stage. Results were obtained from Cox models adjusted for age, race, comorbidities, medications, and baseline eGFR.
Progression of CKD
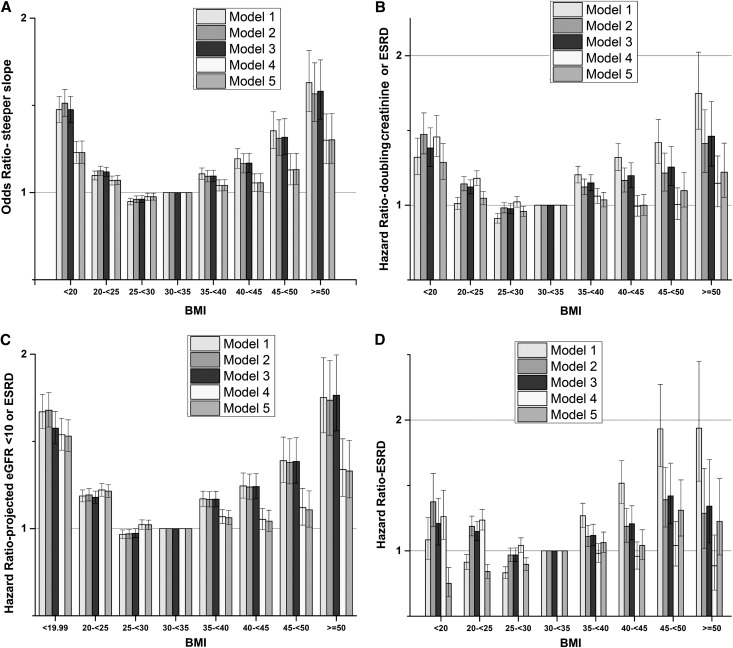
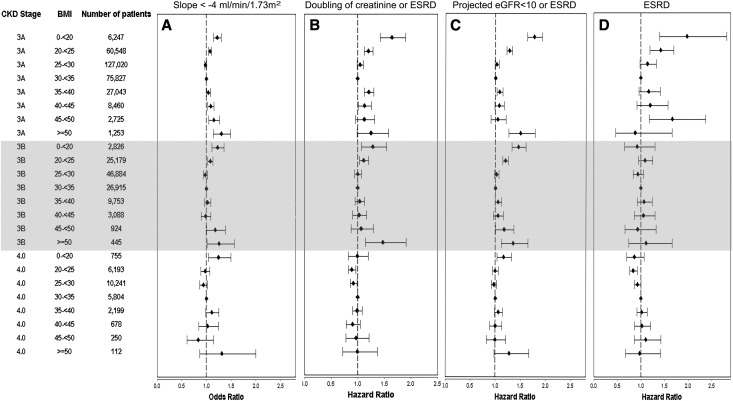
ESRD developed in 9117 patients (5.6 events/1000 PYs; 95% CI, 5.5 to 5.7), the combined end point of doubling of serum creatinine or ESRD developed in 21,688 patients (12.2/1000 PYs; 95% CI, 12.0 to 12.3), and 40,696 patients had a projected eGFR<10 ml/min per 1.73 m2 or ESRD assuming no deaths during the follow-up period (14.9/1000 PYs; 95% CI, 14.8 to 15.1). The median slope was −0.84 ml/min per 1.73 m2 per year (interquartile range=−3.14 to 1.25), and 18.8% of patients had a slope steeper than −4 ml/min per 1.73 m2 per year. The multivariable-adjusted associations between BMI and the various outcomes of CKD progression were U-shaped (Figure 3, A–C), except for the incidence of ESRD (Figure 3D), which showed a linearly incremental association. The lowest CKD progression for the end points showing U-shaped associations was observed with BMI corresponding to overweight/mild obesity (25–35 kg/m2). A U-shaped association of BMI with progressive CKD was also apparent in subgroup analyses, except for patients with more advanced CKD (Supplemental Figure 3). In analyses categorizing patients by CKD stages, the association of higher BMI with progression of CKD diminished in patients with CKD stage 4 (Figure 4). Adjustment for DM and hypertension significantly attenuated the association between BMI and progressive CKD (Supplemental Figure 4).
Figure 3.
BMI categories showing a U-shaped association with progression of chronic kidney disease in 453,946 United States veterans with eGFR<60 ml/min per 1.73 m2. Association of BMI categories with four different outcomes representing progression of CKD: (A) steeper slopes of eGFR versus time (defined as slopes<−4 ml/min per 1.73 m2 per year), (B) doubling of serum creatinine or ESRD, (C) projected eGFR<10 ml/min per 1.73 m2 or ESRD, and (D) ESRD. Estimates were calculated from a logistic regression model (for steeper slopes) and Cox models (for all other outcomes) adjusted for age, race, comorbidities, medications, and baseline eGFR.
Figure 4.
BMI categories showing a U-shaped association with progression of chronic kidney disease in patients with CKD stages 3A and 3B, and no association in patients with CKD stage 4. Association of BMI categories with four different outcomes representing progression of CKD in patients grouped by their baseline CKD stage: (A) steeper slopes of eGFR versus time (defined as slopes<−4 ml/min per 1.73 m2 per year), (B) doubling of serum creatinine or ESRD, (C) projected eGFR<10 ml/min per 1.73 m2 or ESRD, and (D) ESRD. Estimates were calculated from a logistic regression model (for steeper slopes) and Cox models (for all other outcomes) adjusted for age, race, comorbidities, medications, and baseline eGFR.
Discussion
We examined the association of BMI with mortality and progression of CKD in a large cohort of NDD-CKD patients and detected optimal outcomes associated with overweight/mild obesity status. BMI levels below 30 kg/m2 were linearly associated with higher mortality. Conversely, BMI levels above 35 kg/m2 were also associated with higher mortality, except in patients with advanced CKD (eGFR<30 ml/min per 1.73 m2), in whom the association was attenuated and nonsignificant. Our study adds to the findings of prior studies showing a paradoxical benefit of obesity in the setting of comorbid conditions, such as CHF,27,28 DM,22,29 chronic obstructive pulmonary disease,30 rheumatoid arthritis,31 NDD-CKD,26 and ESRD.14,24 Contrary to the discrepant and controversial association of high BMI levels with mortality in many studies, an association of low BMI with higher mortality has been described in numerous previous studies of various patient populations, including several studies performed in the general population.3,18,32,33 In the latter studies, the BMI levels associated with ideal survival varied significantly, including those levels corresponding to normal up to mild obesity status (BMI=30 to <35 kg/m2), with BMI levels above these nadirs typically showing associations with increased mortality.
Similar to associations with mortality, we also detected U-shaped associations of BMI with progression of CKD, except when we defined progression as the incidence of ESRD. In the latter case, we described a linear association of higher progression associated with higher BMI, which is similar to two previous studies using the same end point.12,13 This discrepancy may have been because of different population characteristics and much longer follow-up duration in general population-based studies or competing risk by the very high mortality experienced in patients with the lowest BMI levels, especially because deaths significantly outnumbered ESRD events in our cohort and alternative definitions of progressive CKD (which attenuated the competing risk related to deaths) uniformly indicated U-shaped associations. A U-shaped association and a trend to increased risk of progressive CKD associated with the lowest BMI levels were also recently described in a large population-based cohort of 1.2 million individuals from Israel,11 although the very low number of ESRD events seen in those individuals with low BMI precluded precise risk estimates. Similar to the mortality end point, we also observed effect modification by severity of kidney disease for the association of higher BMI with progressive CKD, with BMI losing predictive value at eGFR levels <30 ml/min per 1.73 m2.
Obesity can exert negative effects through various mechanisms, which include mediation by comorbidities caused by obesity (such as DM and hypertension) and also direct metabolic effects of increased adiposity.14,15 Indeed, in our study, the association of higher BMI with progressive CKD was attenuated by adjustment for DM and BP. In light of such well defined adverse effects of obesity, it seems controversial that, in our study and other studies, BMI levels in the overweight/mildly obese range were associated with the best outcomes and that, in patients with advanced CKD, even morbid obesity was not associated with adverse outcomes at all. Several potential explanations have been offered for this so-called obesity paradox. The short lifespan of patients with more advanced CKD and significant comorbid conditions may not allow the full fruition of the adverse metabolic effects of obesity, which could, in turn, have short-term protective effects in these patients by virtue of better nutritional reserves.34 Another possible explanation for the high adverse event rates seen in patients with low BMI could be the high prevalence of metabolically obese normal weight individuals in patient groups with high comorbidity burden,35 in whom adverse metabolic effects of obesity may be manifest despite low BMI levels. Other possible explanations for the obesity paradox include the presence of lower metabolic rates in obese individuals, leading to lower production of uremic waste products and hence, better tolerance of CKD-related morbidity, the presence of short-term protective cytokine profiles in obese individuals,36–38 and protective effects of either higher muscle mass39–42 and/or higher body fat43 typically present in patients with higher BMI. The latter two conditions could offer short-term advantages under conditions of duress, such as better nutritional reserves, enhanced antioxidant capacity,39 or lower circulating actin and higher plasma gelsolin levels,44 thus predisposing to better outcomes. These putative advantages could be magnified in patients with the most advanced stages of CKD (who also suffer from a higher comorbidity burden and have shorter life expectancy) and thus, result in a gradual attenuation of the negative effects seen in those patients with normal kidney function or milder stages of CKD.
The observed association of BMI with outcomes in our study could also have been affected by certain other characteristics of our cohort other than different levels of kidney function. The mean age in our cohort was >70 years, which is at least 20 years older than general population cohorts, and could be specifically related to certain characteristics, like functional decline, cognitive impairment, and frailty, which may be particularly relevant in underweight individuals.45 It is, thus, possible that the association of BMI with outcomes could be different in this older age group compared with younger people, which has suggested by other studies.46–48 Patients in our cohort also suffered from a high prevalence of comorbid conditions, which could themselves have had an impact on the observed associations as shown by previous studies.22–24,26–31 Our subgroup analyses did not detect significant effect modification by these various characteristics, but we cannot rule out potential effects by unmeasured conditions or some individual combinations of measured characteristics (e.g., associations may be different in young patients with no comorbidities even at more advanced stages of CKD). The main purpose of our study was to examine BMI as a risk factor in the overall CKD population, and a detailed assessment of the roles of various clinical characteristics will have to be performed in future dedicated studies.
Our study has limitations that need to be acknowledged. Our study was observational, and hence, its results cannot be used to infer causality. Our data do not allow us to glean the effects of interventions that intentionally lower or increase BMI, and it is, therefore, important to emphasize that our observations cannot be used to advocate weight gain in patients with a BMI<30 kg/m2 or suggest that therapeutic weight loss would worsen outcomes. Most of our patients were men and United States veterans; hence, the results may not apply to women or the general United States population. We examined a prevalent cohort of CKD patients, and thus, we could not examine effects that obesity might have had on incident CKD. The competing risk of death3,4 versus kidney disease1,2 caused by higher BMI in patients with no CKD may have affected the composition of our cohort, which was suggested by the markedly lower baseline age of those patients with the highest BMI levels. Studies in the general population would have to clarify how BMI affects clinical characteristics of patients with incident CKD and how they may impact on future outcomes, such as mortality and progression of kidney disease. BMI may not be an ideal marker of obesity, because high BMI does not differentiate patients with relatively high muscle mass or bone mass who are not truly obese, and other indices, such as waist circumference or waist-to-height ratio, have been suggested to be better markers of obesity.49–51 Nevertheless, BMI remains the predominant index to establish obesity in clinical practice; hence, our results have direct clinical relevance. We did not examine cause-specific mortality, which could have informed about different effects of low and high BMI on various outcomes. We adjusted for a number of potential confounders, but residual confounding remains possible, especially because comorbidities in our study were only identified by International Classification of Diseases, Ninth Revision codes and we did not have sufficient information on levels of proteinuria, another potentially important mediator of the adverse effects of obesity.
In conclusion, the association between BMI and adverse outcomes in patients with NDD-CKD is U-shaped, especially in those patients with earlier stages of CKD. The best outcomes were associated with BMI levels consistent with overweight/mild obesity status. Traditional weight management strategies may, thus, not be appropriate in patients with all stages of NDD-CKD. The ideal BMI and the best ways to achieve this BMI need to be established from randomized controlled clinical trials. Before such information becomes available, a cautious approach to BMI-based weight management in CKD is warranted.
Concise Methods
Study Population
The creation of this NDD-CKD cohort was previously described.52–54 Briefly, study subjects were identified by using measurements of serum creatinine in the Veterans Affairs (VA) Decision Support System National Data Extracts Laboratory Results file55 from October 1, 2004 to September 30, 2006. eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation.56 Patients with eGFR<60 ml/min per 1.73 m2 on at least two occasions no less than 3 months apart and available BMI measurements within this period were included in the study. Cohort entry was defined as the date of the first baseline eGFR used to define CKD status. Patients with ESRD, defined as either receiving maintenance dialysis during this period or the presence of a kidney transplant based on records obtained from the US Renal Data System (USRDS),57 were excluded from the cohort. The final cohort included 453,946 patients.
Patients’ age, sex, race, and BP were obtained from the VA Corporate Data Warehouse. Information on race was cross-referenced with data obtained from Medicare through the VA–Medicare data merge project.58 Information on prevalent comorbidities was extracted from the VA Inpatient and Outpatient Medical SAS Datasets59 using International Classification of Diseases, Ninth Revision Diagnostic and Procedure Codes and Current Procedural Terminology Codes recorded from October 1, 2004, to September 30, 2006. Cardiovascular disease was defined based on diagnostic codes for coronary artery disease, angina, or myocardial infarction or procedure codes for percutaneous coronary interventions or coronary artery bypass grafting. Data related to baseline (first 90 days after cohort entry) medication exposure were collected from VA Pharmacy dispensation records.60 To minimize random variation, we used the respective means of all the BMI, BP, and laboratory measurements from the first 90 days after cohort entry as baseline values for these variables in our analyses. Incident ESRD was defined as initiation of maintenance dialysis or preemptive renal transplantation occurring between the cohort entry date and September 30, 2009, as provided by the USRDS. Information about all-cause mortality was obtained from the VA Vital Status Files.58
We collected information on all weight and height measurements obtained on cohort participants and calculated BMI as the weight in kilograms divided by the height in meters squared. To detect nonlinear associations and better characterize the effects of very low and very high BMI categories, we divided BMI into eight a priori-defined categories: <20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, 40 to <45, 45 to <50, and ≥50 kg/m2; we used the 30 to <35 kg/m2 category as referent in all analyses based on the hypothesis that BMI has a U-shaped association with outcomes. We favored this categorization as opposed to the BMI categories advocated by the World Health Organization61 because of this large cohort population size allowing more precise effect estimates in all BMI ranges and recent studies suggesting a steady elevation in mean BMI levels in both men and women worldwide.62
Missingness for variables used in multivariable models was small, with 99% of patients contributing to the main fully adjusted models; therefore, missing data were not imputed.
Statistical Analyses
Descriptive analyses were performed using means±SDs, medians (interquartile ranges), and proportions as appropriate. Event rates were calculated using the PY approach. The association of baseline BMI with all-cause mortality was examined in Cox proportional hazards models. Patients were followed in survival analyses from the date of the baseline BMI measurement to death or April 1, 2012. The association of BMI with progression of CKD was examined in patients with baseline eGFR=15 to <60 ml/min per 1.73 m2 by using both slopes of eGFR in patients with at least three available serum creatinine measurements and time-to-event analyses with ESRD and the composite of doubling of serum creatinine or ESRD as the event of interest (censored for pre-ESRD deaths or on September 30, 2009). To mitigate the competing risk effect of pre-ESRD mortality in time-to-event analyses, we also examined the incidence of the composite outcome of ESRD or an eGFR<10 ml/min per 1.73 m2 generated using measured slopes of eGFR assuming that no deaths occurred until the end of the follow-up period (April 1, 2012). The association of BMI with the slopes of eGFR was examined in logistic regression models after categorizing eGFR slopes as progressive (decrease in eGFR by more than 4 ml/min per 1.73 m2 per year) and nonprogressive (decrease in eGFR by 4 ml/min per 1.73 m2 per year or less). Time-to-event analyses of CKD progression were performed using Cox proportional hazards models.
The effect of potential confounders was analyzed by constructing models with incremental adjustments: unadjusted (model 1); age (model 2); age, sex, and race (model 3); model 3 plus comorbid conditions (dementia, rheumatologic disease, malignancy, depression, cardiovascular disease, CHF, peptic ulcer disease, and chronic lung disease) and medications (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, α-blockers, β-blockers, calcium channel blockers, loop diuretics, thiazide diuretics, and statins; model 4); and model 4 plus baseline eGFR adjusted (model 5). Multivariable models analyzing slopes of eGFR and the incidence of an eGFR<10 ml/min per 1.73 m2 were also adjusted for the presence or absence of deaths. Our main multivariable models did not include DM and BP, because they are likely effect mediators of obesity rather than confounders. To examine whether DM and/or BP mediate the effects of obesity on outcomes, we constructed additional models adjusting for these two variables in addition to all other variables in the main fully adjusted model.
Effect modification by key patient characteristics was examined in subgroup analyses after categorizing patients according to prespecified cutoffs of various key characteristics. Baseline kidney function was categorized as both an eGFR<30 and ≥30 ml/min per 1.73 m2 and according to the Kidney Disease: Improving Global Outcomes classification (stages 3A–5 for analyses of mortality and stages 3A–4 for analyses of progressive CKD).63
Statistical analyses were performed using Stata MP version 11 (Stata Corporation, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
This study is supported by Grant 1R01-DK078106-01 from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (to K.K.-Z. and C.P.K.) and the Department of Veterans Affairs.
Parts of this material were presented at the XVI Conference on Renal Nutrition and Metabolism (June 26, 2012) in Honolulu, HI, and the American Society of Nephrology Kidney Week 2013 (November 6–10, 2013) in Atlanta, GA.
C.P.K. is an employee of the Department of Veterans Affairs. Opinions expressed in this paper are the opinions of the authors and do not necessarily represent the opinions of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013070754/-/DCSupplemental.
References
- 1.Dawber TR, Meadors GF, Moore FE, Jr.: Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health Nations Health 41: 279–281, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP: An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110: 281–290, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW, Jr.: Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 341: 1097–1105, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Cornoni-Huntley JC, Harris TB, Everett DF, Albanes D, Micozzi MS, Miles TP, Feldman JJ: An overview of body weight of older persons, including the impact on mortality. The National Health and Nutrition Examination Survey I—Epidemiologic Follow-up Study. J Clin Epidemiol 44: 743–753, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS: Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am J Kidney Dis 52: 39–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D: Obesity and prevalent and incident CKD: The Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O: Obesity and risk for chronic renal failure. J Am Soc Nephrol 17: 1695–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE, PREVEND Study Group : A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41: 733–741, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Perry HM, Jr., Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, Carmody SE: Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension 25: 587–594, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, Calderon-Margalit R: Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med 172: 1644–1650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S: Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65: 1870–1876, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Haslam DW, James WP: Obesity. Lancet 366: 1197–1209, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE: Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12: 1211–1217, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Taylor DH, Jr., Ostbye T: The effect of middle- and old-age body mass index on short-term mortality in older people. J Am Geriatr Soc 49: 1319–1326, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Janssen I, Mark AE: Elevated body mass index and mortality risk in the elderly. Obes Rev 8: 41–59, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ: Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211–2219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madero M, Sarnak MJ, Wang X, Sceppa CC, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Body mass index and mortality in CKD. Am J Kidney Dis 50: 404–411, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF: Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355: 763–778, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Zoppini G, Verlato G, Leuzinger C, Zamboni C, Brun E, Bonora E, Muggeo M: Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes Relat Metab Disord 27: 281–285, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC: Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol 13: 136–143, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49: 581–591, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kwan BC, Murtaugh MA, Beddhu S: Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2: 992–998, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Clark AL, Chyu J, Horwich TB: The obesity paradox in men versus women with systolic heart failure. Am J Cardiol 110: 77–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM: The obesity paradox: Body mass index and outcomes in patients with heart failure. Arch Intern Med 165: 55–61, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tseng CH: Obesity paradox: Differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis 226: 186–192, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Wilson DO, Rogers RM, Wright EC, Anthonisen NR, The National Institutes of Health Intermittent Positive-Pressure Breathing Trial : Body weight in chronic obstructive pulmonary disease. Am Rev Respir Dis 139: 1435–1438, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Escalante A, Haas RW, del Rincón I: Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med 165: 1624–1629, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Flegal KM, Graubard BI, Williamson DF, Gail MH: Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298: 2028–2037, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Kit BK, Orpana H, Graubard BI: Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309: 71–82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE: Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 10: 433–442, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Conus F, Rabasa-Lhoret R, Péronnet F: Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 32: 4–12, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Stenvinkel P, Marchlewska A, Pecoits-Filho R, Heimbürger O, Zhang Z, Hoff C, Holmes C, Axelsson J, Arvidsson S, Schalling M, Barany P, Lindholm B, Nordfors L: Adiponectin in renal disease: Relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int 65: 274–281, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Rauchhaus M, Koloczek V, Volk H, Kemp M, Niebauer J, Francis DP, Coats AJ, Anker SD: Inflammatory cytokines and the possible immunological role for lipoproteins in chronic heart failure. Int J Cardiol 76: 125–133, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW: Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 277: E971–E975, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Beddhu S, Pappas LM, Ramkumar N, Samore M: Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14: 2366–2372, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ: Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 207: 534–540, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K: Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 5: 2258–2268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD: Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 83: 202–210, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lee PS, Sampath K, Karumanchi SA, Tamez H, Bhan I, Isakova T, Gutierrez OM, Wolf M, Chang Y, Stossel TP, Thadhani R: Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J Am Soc Nephrol 20: 1140–1148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowling CB, Muntner P: Epidemiology of chronic kidney disease among older adults: A focus on the oldest old. J Gerontol A Biol Sci Med Sci 67: 1379–1386, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL: The effect of age on the association between body-mass index and mortality. N Engl J Med 338: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Seidell JC, Visscher TL: Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr 54[Suppl 3]: S33–S39, 2000 [DOI] [PubMed] [Google Scholar]
- 48.de Mutsert R, Snijder MB, van der Sman-de Beer F, Seidell JC, Boeschoten EW, Krediet RT, Dekker JM, Vandenbroucke JP, Dekker FW: Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol 18: 967–974, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Kovesdy CP, Czira ME, Rudas A, Ujszaszi A, Rosivall L, Novak M, Kalantar-Zadeh K, Molnar MZ, Mucsi I: Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant 10: 2644–2651, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Guallar-Castillón P, Balboa-Castillo T, López-García E, León-Muñoz LM, Gutiérrez-Fisac JL, Banegas JR, Rodríguez-Artalejo F: BMI, waist circumference, and mortality according to health status in the older adult population of Spain. Obesity (Silver Spring) 17: 2232–2238, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Stenvinkel P, Zoccali C, Ikizler TA: Obesity in CKD—what should nephrologists know? J Am Soc Nephrol 24: 1727–1736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 125: 677–684, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Survival advantage in black versus white men with CKD: Effect of estimated GFR and case mix. Am J Kidney Dis 62: 228–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balogun RA, Abdel-Rahman EM, Balogun SA, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Kalantar-Zadeh K, Kovesdy CP: Association of depression and antidepressant use with mortality in a large cohort of patients with nondialysis-dependent CKD. Clin J Am Soc Nephrol 7: 1793–1800, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Department of Veterans Affairs HSRADS VA Information Resource Center : VIReC Research User Guide: Veterans Health Administration Decision Support System Clinical National Data Extracts, 2nd Ed., Hines, IL, VA Information Resource Center, 2009 [Google Scholar]
- 56.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute of Diabetes and Digestive and Kidney Diseases. USRDS Coordinating Center : US Renal Data System Annual Data Report, Researcher's Guide, Reference Tables, ADR Slides, Ann Arbor, MI, National Institute of Diabetes and Digestive and Kidney Diseases, US Renal Data System Coordinating Center, 2013 [Google Scholar]
- 58.Arnold N, Sohn M, Maynard C, Hynes DM: VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. Edward Hines, Jr.VA Hospital, Hines, IL, VA Information Resource Center, 2006 [Google Scholar]
- 59.US Department of Veterans Affairs : VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006, Hines, IL, VA Information Resource Center, 2007 [Google Scholar]
- 60.VA Information Resource Center (VIReC) : VIReC Research User Guide: VHA Pharmacy Prescription Data, 2nd Ed., Hines, IL, VA Information Resource Center, 2008 [Google Scholar]
- 61.WHO: Global Database on Body Mass Index, 2006. Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html Accessed March 1, 2013
- 62.Kuczmarski RJ, Flegal KM: Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr 72: 1074–1081, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.