Abstract
A focus of health care reform has been on reducing 30-day hospital readmissions. Patients with ESRD are at high risk for hospital readmission. It is unknown whether more monitoring by outpatient providers can reduce hospital readmissions in patients receiving hemodialysis. In nationally representative cohorts of patients in the United States receiving in-center hemodialysis between 2004 and 2009, we used a quasi-experimental (instrumental variable) approach to assess the relationship between frequency of visits to patients receiving hemodialysis following hospital discharge and the probability of rehospitalization. We then used a multivariable regression model and published hospitalization data to estimate the cost savings and number of hospitalizations that could be prevented annually with additional provider visits to patients in the month following hospitalization. In the main cohort (n=26,613), one additional provider visit in the month following hospital discharge was estimated to reduce the absolute probability of 30-day hospital readmission by 3.5% (95% confidence interval, 1.6% to 5.3%). The reduction in 30-day hospital readmission ranged from 0.5% to 4.9% in an additional four cohorts tested, depending on population density around facilities, facility profit status, and patient Medicaid eligibility. At current Medicare reimbursement rates, the effort to visit patients one additional time in the month following hospital discharge could lead to 31,370 fewer hospitalizations per year, and $240 million per year saved. In conclusion, more frequent physician visits following hospital discharge are estimated to reduce rehospitalizations in patients undergoing hemodialysis. Incentives for closer outpatient monitoring following hospital discharge could lead to substantial cost savings.
A major goal of health care reform is to reduce the rate of 30-day hospital readmission.1 Unplanned rehospitalizations cost the Medicare program $17.4 billion in 2004.2 While policy efforts to reduce rehospitalization have focused on changing hospital practices,3,4 outpatient providers also have a role in preventing rehospitalization. Several clinical trials and one retrospective cohort study on chronic diseases, such as chronic obstructive pulmonary disease and congestive heart failure, demonstrate reduced rates of rehospitalization when patients are monitored more closely by their outpatient providers.5,6 An observational study of patients on hemodialysis suggests that closer laboratory monitoring in the first week following hospitalization may prevent rehospitalization.7 In contrast, one randomized trial of primary care physician follow-up did not find reduced rehospitalizations in patients monitored more closely.8
Preventing rehospitalization in patients with ESRD who are undergoing hemodialysis could improve health outcomes and yield significant cost savings. Patients with ESRD are at a high risk for readmission to the hospital—in 2009, patients receiving hemodialysis were admitted to the hospital nearly two times per year on average, 36% of whom were rehospitalized within 30 days.9 Frequent hospital readmissions may contribute to both high mortality rates and poor health-related quality of life for patients with ESRD.9,10 Patients with ESRD also consume a disproportionate share of the Medicare budget: they make up 1% of Medicare beneficiaries, but they consume >6% of Medicare expenditures.
We test the hypothesis that more frequent face-to-face outpatient nephrology provider visits in the month following hospital discharge reduces the rate of 30-day hospital readmission in patients receiving hemodialysis. Additionally, we examine the relation between provider visits and death in the 30 days following hospital discharge and estimate the change in health care costs if providers were to visit patients once more in the month following hospital discharge. Understanding whether outpatient provider practices influence rehospitalization and death could lead to substantial improvements in ESRD care.
Results
Baseline Characteristics
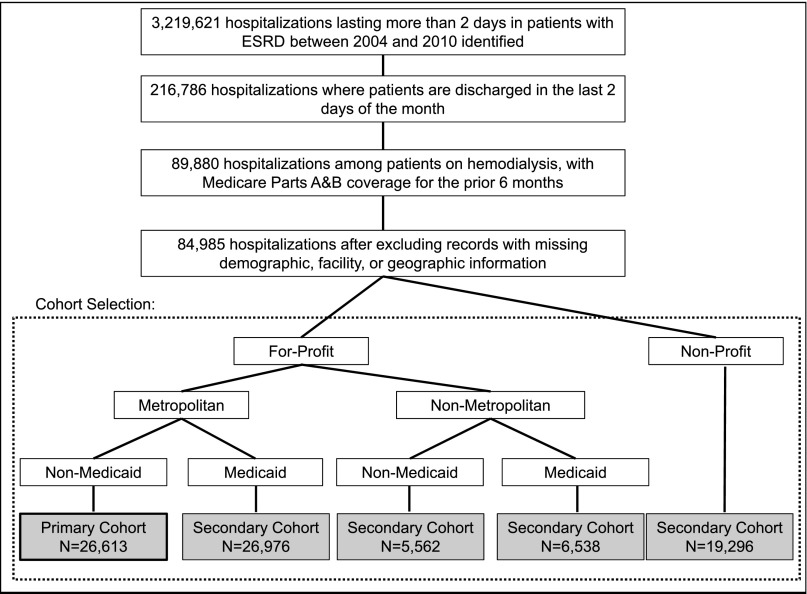
Patients in the United States receiving in-center hemodialysis who were discharged from the hospital in the last 2 days of a month between 2004 and 2009 were divided into five cohorts based on socioeconomic and dialysis facility characteristics. A total of 26,613 patient-months were in our main cohort, which included patients without Medicaid who reside in metropolitan areas and receive dialysis at for-profit facilities (Figure 1). Patients were rehospitalized in 36.2% of the months following hospital discharge and died in 8.1% of the months following hospital discharge. The mean number of face-to-face provider (physician and/or advanced practitioner) visits in the month following hospital discharge was (mean±SD) 2.8±1.5. Among patients who died or were rehospitalized in the month following hospital discharge, mean visit frequency was 2.1±1.6. Among patients who were not rehospitalized and did not die, mean visit frequency was 3.3±1.2. Patients who were rehospitalized were more likely to have the following comorbid conditions: drug use, cerebrovascular disease, peptic ulcer disease, coronary artery disease, heart failure, pulmonary disease, diabetes, liver disease, and peripheral vascular disease. In addition, the duration of hospitalization in the previous month was longer in patients who were rehospitalized (Table 1).
Figure 1.
Diagram of cohort selection. Individual patients were allowed to contribute more than one hospitalization. In our cohorts, 32.0% of the selected hospitalizations involved patients with more than one record. Population density based on census-based Rural Urban Commuting Area codes.35
Table 1.
Baseline characteristics in primary cohort
| Variable | Rehospitalized | Standardized Difference | |
|---|---|---|---|
| No (n=16,957) | Yes (n=9656) | ||
| Demographic | |||
| Men (%) | 45.4 | 46.6 | 2.5 |
| Age (yr) | 68.6 | 67.8 | 6.8 |
| American Indian (%) | 0.5 | 0.4 | 2.2 |
| Black (%) | 32.1 | 34.2 | 4.6 |
| White (%) | 65.1 | 63.5 | 3.5 |
| Other race (%)a | 2.3 | 1.9 | 2.5 |
| Hispanic ethnicity (%) | 8.3 | 7.9 | 1.5 |
| Socioeconomic and comorbid conditions (%) | |||
| Alcohol use | 1.9 | 2.6 | 4.9 |
| Drug use | 4.0 | 6.1 | 10.0b |
| Smoker | 7.8 | 10.4 | 9.2 |
| Cerebrovascular disease | 40.5 | 46.2 | 11.6b |
| Peptic ulcer disease | 25.4 | 31.4 | 13.4b |
| Coronary artery disease | 33.7 | 43.2 | 19.6b |
| Heart failure | 77.8 | 85.2 | 19.0b |
| Pulmonary disease | 47.2 | 54.0 | 13.6b |
| HIV infection | 0.9 | 1.2 | 2.5 |
| Cancer | 15.1 | 16.4 | 3.7 |
| Dementia | 15.0 | 17.9 | 7.9 |
| Diabetes | 68.3 | 73.1 | 10.5b |
| Liver disease | 11.8 | 15.3 | 10.2b |
| Paralysis | 5.2 | 6.1 | 3.8 |
| Periperal vascular disease | 47.7 | 54.5 | 13.5b |
| Rheumatic disease | 5.3 | 5.0 | 1.2 |
| Acuity of care and facility | |||
| First year of hemodialysis (%) | 13.3 | 14.1 | 2.4 |
| Duration of hospitalization (d) | 9.2 | 10.8 | 13.3b |
| Facility size (n patients) | 103.3 | 101.4 | 3.7 |
Sample includes patients not eligible for Medicaid who underwent dialysis in metropolitan areas at for-profit facilities. See Supplemental Tables 2, 6, 10, and 14 for baseline characteristics of secondary cohorts.
Includes Pacific Islander, Middle Eastern, Indian Subcontinent, Asian and other/multiracial.
>10% standardized difference between groups.
Assessing the Instrumental Variable
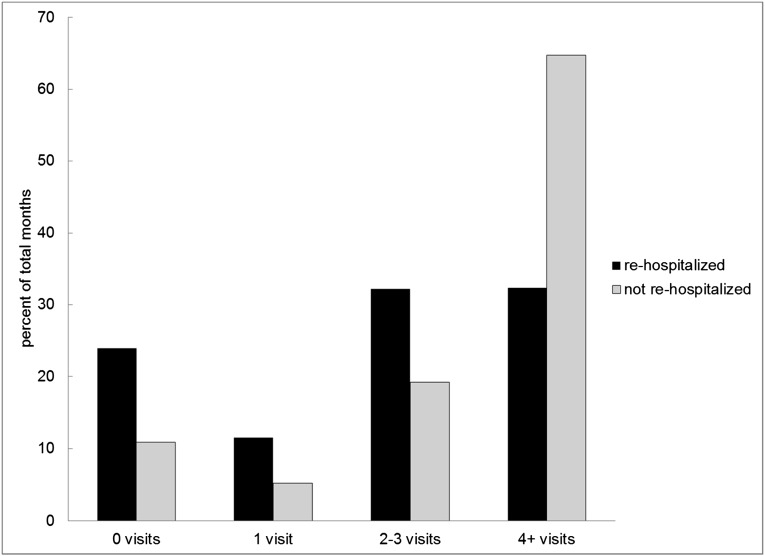
A regression analysis in which rehospitalization is simply modeled as a function of visit frequency would be substantially biased. This is because patients who are rehospitalized or die in the month following hospital discharge are available to be seen by their provider in the outpatient dialysis setting for fewer days, reducing the frequency with which they are seen. This bias is evident when one considers differences in the proportion of patients seen four or more times in the month following hospital discharge stratified by rehospitalization. Among patients not rehospitalized, 64.7% were seen four or more times in the month following hospital discharge, in contrast to 32.4% of patients who were rehospitalized (Figure 2). A multivariable regression analysis where rehospitalization is modeled as a function of visits suggests that one additional visit in the month following hospital discharge is associated with a 9.0% reduction in the probability of rehospitalization. This large treatment effect also likely reflects this source of bias, underscoring the need for an alternative analytic method, such as an instrumental variable analysis (Supplemental Appendix).
Figure 2.
Visit frequency in the month following hospital discharge stratified by rehospitalization.
We used the number of visits per month to prevalent hemodialysis patients at a given patient’s dialysis facility in an instrumental variable analysis to predict the number of provider visits to each patient that would have occurred if he or she were not rehospitalized and did not die. In the main cohort, the mean number of visits per month to prevalent hemodialysis patients (the instrumental variable) was 2.4±0.6. In the five study cohorts, the F-statistic for the first-stage least-squares regression—which characterizes the correlation between the exposure of interest (the number of times a patient is seen by his or her provider in the month following hospital discharge) and the instrument (the mean number of visits to prevalent patients receiving dialysis in a patient’s facility) among patients who were not hospitalized and did not die—ranged from 761 to 1172. The F-statistic testing the association between the instrument and visit frequency in the entire study sample (i.e., patients who died or were rehospitalized in addition to those who were not) ranged from 602 to 1039. This satisfies the first criterion for an instrumental variable analysis requiring a strong association between the instrumental variable and the actual exposure of interest. In our main cohort, patient, facility, and geographic characteristics were similar across quartiles of the instrumental variable. Only “other race” and facility size had a standardized difference of 10% or more between the first and fourth quartiles of the instrumental variable (Table 2).
Table 2.
Comparison of baseline characteristics across quartiles of the instrumental variable
| Variable | Physician Visits | Quartile of Facility Visits, Instrumental Variablea | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–3 Visits (n=12,291) | ≥4 Visits (n=14,332) | Standardized Difference | 1st | 2nd | 3rd | 4th | Standardized Difference (1st versus 4th) | |
| Demographic | ||||||||
| Men (%) | 44.1 | 46.0 | 3.7 | 45.6 | 45.8 | 45.8 | 46.2 | 1.2 |
| Age (yr) | 68.9 | 68.2 | 5.8 | 68.4 | 68.1 | 68.4 | 68.3 | 0.4 |
| American Indian (%) | 0.4 | 0.5 | 0.6 | 0.7 | 0.3 | 0.5 | 0.4 | 4.0 |
| Black (%) | 30.2 | 34.3 | 8.8 | 31.0 | 35.8 | 32.6 | 32.1 | 2.5 |
| White (%) | 67.0 | 63.1 | 8.1 | 64.3 | 62.0 | 65.3 | 66.5 | 4.7 |
| Other race (%)b | 2.4 | 2.1 | 1.7 | 4.1 | 1.9 | 1.5 | 1.0 | 19.7c |
| Hispanic ethnicity (%) | 7.2 | 8.4 | 4.6 | 9.1 | 7.4 | 7.8 | 8.1 | 3.7 |
| Socioeconomic and comorbid conditions (%) | ||||||||
| Alcohol use | 2.7 | 1.8 | 6.1 | 2.4 | 2.2 | 1.9 | 2.0 | 2.7 |
| Drug use | 5.7 | 4.1 | 7.0 | 5.1 | 4.7 | 4.7 | 4.6 | 2.7 |
| Smoker | 9.1 | 8.0 | 3.8 | 8.0 | 9.0 | 9.1 | 8.9 | 3.2 |
| Cerebrovascular disease | 45.4 | 41.2 | 8.6 | 42.1 | 43.4 | 43.6 | 41.1 | 2.1 |
| Peptic ulcer disease | 29.1 | 26.8 | 5.1 | 28.0 | 28.4 | 26.8 | 27.2 | 1.7 |
| Coronary artery disease | 39.0 | 36.2 | 5.9 | 38.9 | 37.6 | 36.7 | 35.4 | 7.2 |
| Heart failure | 82.4 | 78.8 | 9.2 | 81.2 | 81.2 | 80.3 | 79.2 | 4.9 |
| Pulmonary disease | 52.1 | 48.2 | 7.7 | 50.1 | 50.3 | 50.0 | 48.1 | 3.9 |
| HIV infection | 1.1 | 0.9 | 1.5 | 1.4 | 1.2 | 0.8 | 0.7 | 6.8 |
| Cancer | 17.6 | 14.7 | 7.8 | 15.6 | 16.2 | 15.7 | 14.7 | 2.6 |
| Dementia | 19.3 | 14.3 | 13.2c | 15.7 | 17.2 | 16.6 | 14.7 | 2.9 |
| Diabetes | 70.7 | 69.3 | 3.1 | 69.4 | 71.4 | 70.2 | 69.2 | 0.4 |
| Liver disease | 13.6 | 12.6 | 3.0 | 13.3 | 13.5 | 13.0 | 12.5 | 2.3 |
| Paralysis | 6.4 | 5.2 | 5.1 | 5.3 | 5.8 | 6.2 | 5.0 | 1.0 |
| Periperal vascular disease | 52.0 | 48.8 | 6.5 | 49.6 | 51.5 | 49.7 | 49.9 | 0.4 |
| Rheumatic disease | 5.4 | 5.0 | 2.1 | 5.0 | 5.5 | 5.0 | 5.1 | 0.5 |
| Acuity of care and facility | ||||||||
| First year of hemodialysis (%) | 14.7 | 13.1 | 4.7 | 14.9 | 13.5 | 13.0 | 12.7 | 6.4 |
| Duration of hospitalization (d) | 12.0 | 8.9 | 22.5c | 9.9 | 9.6 | 9.9 | 9.8 | 0.6 |
| Facility size (n patients) | 98.2 | 105.7 | 14.3c | 100.1 | 99.9 | 104.3 | 106.2 | 11.4c |
See Supplemental Tables 3, 7, 11, and 15 for assessment of the instrumental variable in secondary cohorts.
n=6653 for each quartile.
Includes Pacific Islander, Middle Eastern, Indian Subcontinent, Asian and other/multiracial.
Greater than 10% standardized difference between comparison groups.
Results from Instrumental Variable Regression Analyses
In our main cohort, the unadjusted correlation between mean visits to prevalent dialysis patients at their facility (i.e., the instrumental variable) and rehospitalization was −0.028, indicating that facilities where patients are seen more frequently have slightly reduced rates of rehospitalization. In the multivariable analysis, one additional provider visit was estimated to reduce the probability of rehospitalization by 3.5% (95% confidence interval [95% CI], 1.6% to 5.3%) (Table 3). For a patient with the mean probability of rehospitalization (36.2%), the probability of rehospitalization with one additional provider visit would be 32.7%, corresponding to a relative risk reduction of 9.7%. In the main cohort, there was no significant relation between visit frequency following hospital discharge and death in the following month; the change in probability of death from one additional predicted visit was −0.5% (95% CI, −1.5% to 0.5%). More frequent visits were not estimated to reduce the probability of death in any of the additional patient cohorts.
Table 3.
Instrumental variable regression of the probability of rehospitalization
| Variable | Change in Probability of Rehospitalization (95% CI) |
|---|---|
| Physician visits (per 1 additional) | −0.04 (−0.05 to −0.02) |
| Demographic and socioeconomic characteristics: | |
| Male sex | 0.02 (0.01 to 0.03) |
| Age (10 yr) | −0.02 (−0.021 to −0.01) |
| White | Reference |
| Native American | −0.06 (−0.14 to 0.03) |
| Black | 0.01 (−0.002 to 0.03) |
| Other race | −0.02 (−0.06 to 0.02) |
| Hispanic ethnicity | −0.01 (−0.04 to 0.01) |
| Alcohol use | 0.02 (−0.02 to 0.06) |
| Drug use | 0.06 (0.03 to 0.09) |
| Smoking history | 0.05 (0.03 to 0.07) |
| Comorbid condition | |
| Cerebrovascular disease | 0.02 (0.01 to 0.04) |
| Peptic ulcer disease | 0.04 (0.03 to 0.06) |
| Coronary artery disease | 0.06 (0.05 to 0.08) |
| Heart failure | 0.07 (0.05 to 0.08) |
| Pulmonary disease | 0.03 (0.02 to 0.05) |
| HIV infection | 0.03 (−0.03 to 0.09) |
| Cancer | 0.03 (0.01 to 0.05) |
| Dementia | 0.03 (0.01 to 0.05) |
| Diabetes | 0.03 (0.02 to 0.04) |
| Liver disease | 0.05 (0.03 to 0.06) |
| Paralysis | 0.001 (−0.03 to 0.03) |
| Peripheral vascular disease | 0.04 (0.02 to 0.05) |
| Rheumatic disease | −0.03 (−0.05 to 0.00) |
| Acuity of care and facility | |
| First year of dialysis | 0.003 (−0.01 to 0.02) |
| Duration of hospitalization (d) | 0.002 (0.001 to 0.002) |
| Facility size (25-patient difference) | −0.003 (−0.01 to 0.001) |
See Supplemental Tables 4, 8, 12, and 16 for regression results in secondary cohorts. See Supplemental Appendix for discussion of the inverse correlation between age and rehospitalization. 95% CI, 95% confidence interval.
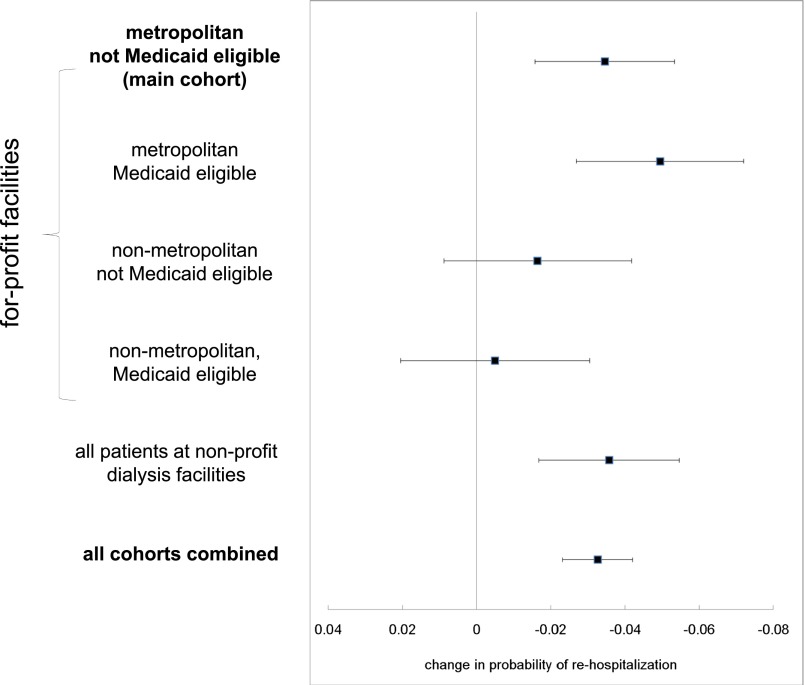
In all patient cohorts, the estimated effect of more frequent visits was to reduce the probability of rehospitalizations. The estimated reduction in rehospitalization ranged from 0.5% to 4.9%. This reduction was statistically significant in patients residing in metropolitan areas with and without Medicaid and in patients undergoing dialysis in nonprofit facilities, due in part to the larger sizes of these cohorts (Figure 3).
Figure 3.
Estimated change in probability of rehospitalization from one additional visit in different patient cohorts. A test for heterogeneity between visit frequency estimates based on the Q-statistic from a meta-analysis was unable to reject the hypothesis that the mean was the same (P=0.07).36 Error bars represent 95% confidence intervals for the estimated changes in probability of rehospitalization.
Cost Analyses
Total costs per patient in the month following hospitalization were $12,634 if patients were rehospitalized compared with $3700 if they were not. After adjustment for demographic, socioeconomic, and geographic and facility characteristics, rehospitalization was associated with an average increase of $8842 in health care costs in the month following hospitalization. After accounting for the cost of an additional provider visit,11 the reduction in rehospitalizations from one more visit would yield approximately $250 in savings in the month following hospital discharge.
In a model based on current rates of hospital admissions and rehospitalizations among all patients receiving in-center hemodialysis in the United States,9 we project that one extra visit per patient would translate into 31,370 fewer hospitalizations per annum. At current Medicare reimbursement rates, an attempt by providers to see patients one additional time in the month following hospital discharge could lead to $240 million in aggregate cost savings.
Discussion
One more visit by physicians (and/or advanced practitioners) to patients undergoing hemodialysis in the month following a hospitalization was estimated to reduce the absolute probability of 30-day hospital readmission by 3.5%. The rate of rehospitalization decreased in all cohorts studied, although the relationship was statistically significant only in the larger cohorts. Although a 3.5% reduction in the rate of rehospitalization may seem modest, it could lead to large aggregate reductions in the number of hospitalizations per year. One more visit per month to patients recently hospitalized could translate into 31,370 fewer hospitalizations per year and $240 million per year saved at current reimbursement rates.
A study using data from the Dialysis Outcomes and Practice Patterns Study found that patients experienced improved survival when their providers spent more time conducting face-to-face visits at the outpatient hemodialysis unit.12 This suggests that not all provider visits to patients have the same health benefit—longer, more comprehensive visits may yield improved health outcomes compared with shorter, more casual ones. Our findings suggest that the timing of provider visits may also be important. It is plausible that visits occurring in the days and early weeks following hospital discharge may be of particular benefit to patients.
Since January 1, 2004, the Centers for Medicare & Medicaid Services (CMS) has reimbursed physicians caring for patients on hemodialysis through a tiered fee-for-service system.13 Under the current system, physicians and group practices are paid according to how often they see their patients on hemodialysis, with larger payments when they see patients more frequently. Although the economic incentive to see patients more frequently under the current payment structure applies equally to all patients receiving hemodialysis—regardless of how sick they are—evidence suggests that recently hospitalized patients do not receive the same amount of attention from their providers as patients who have not been recently hospitalized. A recent study of variation in visit frequency found that patients who were recently hospitalized were 2% less likely to be seen four or more times than patients without a recent hospitalization.14 This inverse association highlights an area where health care delivery can be improved.
Section 3025 of the United States Affordable Care Act created a new pay-for-performance program wherein the CMS reduces Medicare payments to hospitals with excess standardized hospital readmission rates.15 Some critics of this new program argue that, rather than focusing only on hospitals, incentives to reduce hospital readmissions should include all health care providers.16 Our findings highlight the potential value of including outpatient health care providers in programs aimed at preventing 30-day hospital readmissions.
There are several ways that reimbursement policy aimed at reducing 30-day hospital readmissions could encourage additional provider visits in the month following hospital discharge. First, providers could be rewarded with additional payments for more frequent visits after a hospital discharge. This could be structured like the transitional care payments enacted by CMS in the 2013 physician-payment rule for primary care providers caring for patients following hospital discharge.17 Second, 30-day readmissions could be part of a pay-for-performance initiative directed toward physician groups caring for patients undergoing hemodialysis. Following the Hospital Readmissions Reduction Program pay-for-performance model recently enacted by CMS for hospital reimbursement, physician groups with excess standardized readmission rates could be penalized with lower reimbursement. As in pay-for-performance programs implemented in other areas of health care, the specific incentive size and structure chosen would be important determinants of the effectiveness of such a program.18 Finally, including physician and hospital services together as a part of a larger “bundled” payment for comprehensive care of patients on hemodialysis could also properly align incentives. This last solution would have the benefit of involving multiple stakeholders and would align incentives among patients, care providers, and hospitals.
There is unlikely to ever be a randomized prospective trial assessing frequency and duration of nephrologist visits to dialysis patients and health outcomes. Consequently, observational studies using techniques to minimize bias may provide the best evidence to inform important public policy questions. Nevertheless, this study has several limitations. First, it is based on observational data and, therefore, subject to bias, despite our use of instrumental variable analysis. Second, because we only observed visit frequency by monthly claims, we were unable to determine the precise timing following hospitalization when more frequent visits are beneficial, which visits involved physicians versus advance practitioners, and what care was provided during visits. Because we included only patients covered by Medicare parts A and B for 6 months, these findings may not be generalizable to patients within 6 months of starting dialysis. Finally, our cost analysis is restricted to the month following hospitalization, while the benefit of more frequent visits could be longer-lasting. However, restricting our cost analysis to the month following hospitalization was necessary to avoid including cost differences not associated with rehospitalizations.
Much of the policy discourse in the United States surrounding rehospitalizations has focused on modifying economic incentives faced by hospitals.3,4 We find that medical costs might also be reduced by modifying outpatient provider incentives. Our finding highlights an area where the current reimbursement system for hemodialysis that creates an economic incentive to visit all patients undergoing hemodialysis with equal frequency, rather than encouraging additional visits for patients who are most likely to benefit, could be improved. A policy designed to encourage “high-value” visits to dialysis patients recently discharged from the hospital could potentially lead to lower rates of rehospitalization and reduced health care costs.
Concise Methods
Patient Selection
We selected cohorts of patients receiving in-center hemodialysis in the United States between 2004 and 2009 who were discharged from the hospital in the last 2 days of a month. We obtained data on patients and dialysis facilities from the US States Renal Data System, a registry of almost all patients with treated ESRD in the United States. We chose patients discharged in the last 2 days of a month so that the provider visit frequency documented in the subsequent month’s physician reimbursement claim represents the number of face-to-face provider (physician or advanced practitioner) visits in the month following hospital discharge without having a portion of the month following hospital discharge overlap with the hospitalization itself. The claims data did not allow us to identify the specific days a patient was seen or whether the patient was seen by a nephrologist or advanced practitioner. We included only patients with hospitalizations lasting ≥2 days and required that patients have Medicare parts A and B as their primary payer for at least 6 months before hospital discharge to allow sufficient time to identify comorbid conditions from Medicare claims. We excluded from our analysis months following hospital discharge when patients changed dialysis provider, or modality, or ended Medicare coverage.
To minimize bias, we separated patients into five cohorts based on socioeconomic, geographic, and dialysis facility characteristics. Cohorts were selected before performing statistical analyses based on shared patient characteristics within each cohort. Patients who underwent dialysis in for-profit facilities were divided into four cohorts according to their Medicaid eligibility and whether their dialysis facility was located in a metropolitan area. Patients who received dialysis in nonprofit facilities formed a fifth cohort. For our primary analysis, we used the cohort of patients who (1) had dialysis in for-profit facilities, (2) resided in metropolitan areas, and (3) are not eligible for Medicaid. We selected these patients as our main cohort because of its large size and because observed patient demographic characteristics were most similar over a range of visit frequencies. The remaining four cohorts were included in secondary analyses.
Study Outcome
Each patient was followed for 1 month following hospital discharge. Our primary outcome was rehospitalization in the 30 days following hospital discharge. Our secondary outcomes were death in the 30 days following hospital discharge and costs of care. We report absolute probabilities of rehospitalization and death but also estimated the associated relative changes in probability of rehospitalization.
Study Exposure
The study exposure was the frequency with which outpatient providers (i.e., physicians and advanced practitioners) see patients face-to-face in the month following hospital discharge. For patients who were rehospitalized or who died, we use the predicted number of visits that would have occurred if patients were not hospitalized and did not die in the month following hospital discharge. Actual provider visit frequency for each month was obtained from medical claims. Because codes captured zero, one, two to three, or four or more visits, we assigned claims for two to three visits a value of two and a half visits, and claims for four or more visits a value of four visits. These assumptions were varied in sensitivity analyses (Supplemental Appendix).
Instrumental Variable Analysis
A naive regression model in which the probability of rehospitalization is modeled as a function of the actual number of visits following hospital discharge has two fundamental limitations: If patient health were not fully observed, results could be biased because unobserved patient health could be correlated with both the exposure (physician visits) and outcome (rehospitalization). Second, patients who die or are rehospitalized in the month following hospital discharge are at their dialysis unit for fewer days, making them less available for outpatient provider visits.
To overcome these limitations, we used an instrumental variable to predict provider visit frequency in the month following hospital discharge. Instrumental variable analysis is a quasi-experimental approach that has been used to reduce bias in observational studies.19–21 For each patient, we calculated the average frequency of physician visits to all prevalent Medicare patients who had dialysis at the patient’s facility in the calendar year (excluding visits to patients in our study cohorts). Patients with higher values for this instrumental variable undergo dialysis at facilities where patients on dialysis, in general, are seen more frequently by physicians or advanced practitioners. We used this measure of provider visit frequency practices at the dialysis facility level as an instrumental variable to predict how often a patient would be seen in the month following hospital discharge if he or she were not rehospitalized and did not die. Observed similarities among physician networks, observations that providers working in close proximity to one another share practice patterns, and previous findings that provider visit frequency in hemodialysis is closely linked to the dialysis location form a theoretical basis for our choice of instrument.14,22–25
For an instrumental variable to be valid, it must be correlated with the exposure of interest and not be correlated with the outcome other than through the exposure. The correlation between our instrument and visit frequency was assessed using the F-statistic from a first-stage regression model of visit frequency on the instrument.26 For the second criterion, we argue that providers visiting other patients in a dialysis facility should not affect a given patient’s chances of rehospitalization or death other than through more frequent visits with the given patient. The degree to which the second criterion may be violated because of differences in the type of patient undergoing dialysis in facilities with higher and lower values of the instrumental variable (i.e., facilities at which there was higher versus lower visit frequency) was explored by comparing observed characteristics among different levels of the instrument.27
Other Variables
We controlled for patient, facility, and geographic characteristics in our analyses. We determined whether comorbid conditions were present on the basis of 6 prior months of Medicare claims. We included all comorbid conditions contained in the Charlson comorbidity index adapted for ESRD, in addition to HIV and hemiplegia.28 Because of large population size, we used 10% standardized difference as a marker of heterogeneity when comparing differences in characteristics between groups.29
Statistical Analyses
We used a two-stage, two-sample least-squares instrumental variable regression analysis to estimate the effect of physician visit frequency on the probability of rehospitalization (Supplemental Appendix).30 In the first stage, we used linear regression to predict visit frequency as a function of the covariates listed in Table 1 and the instrumental variable—mean visits to prevalent hemodialysis patients in a patient’s facility during the calendar year. Only patients who were not hospitalized and did not die in the month following hospital discharge were included in the first stage. Next, using estimates from the first stage of the instrumental variable analysis, we calculated the predicted visit frequency for the entire cohort. Predicted visit frequency in the second stage thus represents the predicted visit frequency for recently discharged patients had they lived through the month and were not rehospitalized.
In the second stage of the instrumental variable analysis, we used a linear probability model to predict the probability of rehospitalization as a function of the predicted visit frequency, controlling for demographic characteristics, comorbid conditions, and facility characteristics listed in Table 1. Block bootstrap SEMs were used to account for repeated measures (i.e., hospitalizations) within patients, with 10,000 simulations. Previous analyses of instrumental variable methods demonstrate that a linear probability model produces asymptotically unbiased estimates of a single binary outcome compared with other methods, such as a bivariate probit model.31,32 We used SAS software to construct the dataset, and performed statistical analyses using STATA.
Cost Analyses
Using all patient cohorts, we tabulated all medical costs paid by Medicare in the following month for each hospital discharge. We excluded costs paid for nephrologist or advanced practitioner outpatient dialysis visits because these costs were modeled explicitly later in the cost analysis. We used a generalized linear model with a gamma distribution (due to a skewed cost distribution) and a log link function to estimate the change in medical costs in the month following hospitalization as a function of whether a patient was rehospitalized in the month, adjusting for demographic characteristics, comorbid conditions, and facility and geographic characteristics. From this estimate, we predicted average costs in the presence and absence of a rehospitalization to determine the average change in cost associated with rehospitalization. We combined this average with our results from the main instrumental variable analysis to estimate the net average change in cost per patient associated with an “attempt” to see patients one more time in the month following hospitalization if the additional visit were reimbursed at the current rate for a fourth visit. Because not all “attempted” visits result in an actual visit, we assumed that the cost of reimbursing an additional attempted visit was equal to a weighted average of the reimbursement for a fourth attempted visit in the following two groups: patients who were rehospitalized and patients who were not rehospitalized. Reimbursement for an attempted visit in each group equaled the cost of a fourth visit ($64) multiplied by the ratio of predicted to actual visits for that group (Supplemental Appendix).
Additionally, we used published reports on the total number of hospitalizations and rehospitalizations among patients undergoing hemodialysis in 2009 along with the proportion of total deaths occurring in the hospital in order to project the change in total hospitalizations and aggregate costs associated with one more attempted visit to all hemodialysis patients in the United States in the month following hospital discharge (Supplemental Appendix).9,33
Sensitivity Analyses
We tested several of our assumptions in sensitivity analyses. First, we tested the sensitivity to assumption that claims with 2–3 visits documented represented 2.5 visits. Second, we tested the sensitivity to our assumption that the relationship between the instrumental variable (visits to prevalent dialysis patients at a patient’s facility) and attempted visit frequency is identical in patients who were hospitalized or died and those who were not. We tested the sensitivity to clustering at the dialysis facility level, to additional potential confounders, and to the possibility of a differential benefit from physician versus advanced practitioner visits. We explored mean frequency of visits to prevalent hemodialysis patients residing in different geographic locations—characterized by health-service-area visit levels—as an instrument for visit frequency and obtained similar results. Finally, we tested the sensitivity to our choice of a linear probability model using a two-stage residual inclusion estimation probit model.34 Our main finding did not change substantially in these sensitivity analyses (see the Supplemental Appendix for sensitivity analysis results).
This project was approved by an institutional review board of Stanford University School of Medicine.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was conducted under a data use agreement between W.C.W. and the National Institutes for Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed the manuscript and approved it for submission. The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the United States Government.
The study was supported by grants F32 HS019178 from the Agency for Healthcare Research and Quality (K.F.E.) and DK085446 from the NIH/NIDDK (G.M.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Reducing Avoidable Rehospitalization in ESRD: A Shared Accountability,” on pages 1891–1893.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080879/-/DCSupplemental.
References
- 1.Medicare Payment Advisory Committee: A path to bundled payment around a rehospitalization. In: Report to the Congress: Reforming the Delivery System. Washington, DC, Medicare Payment Advisory Committee, June 2008, pp 83–106
- 2.Jencks SF, Williams MV, Coleman EA: Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 360: 1418–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Stone J, Hoffman GJ: Medicare hospital readmissions: issues, policy options and PPACA. Washington, DC, Congressional Research Service, 2010 [Google Scholar]
- 4.Medicare Payment Advisory Committee: Report to the Congress: Reforming the Delivery System. Washington, DC, Medicare Payment Advisory Committee, 2008 [Google Scholar]
- 5.Sharma G, Kuo Y-F, Freeman JL, Zhang DD, Goodwin JS: Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 170: 1664–1670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM: A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 333: 1190–1195, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Chan KE, Lazarus JM, Wingard RL, Hakim RM: Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int 76: 331–341, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Weinberger M, Oddone EZ, Henderson WG: Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med 334: 1441–1447, 1996 [DOI] [PubMed] [Google Scholar]
- 9.USRDS : Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 10.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services: Centers for Medicare & Medicaid Services. Physician Fee Schedule Search.Available at https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx Accessed July 18, 2012
- 12.Kawaguchi T, Karaboyas A, Robinson BM, Li Y, Fukuhara S, Bieber BA, Rayner HC, Andreucci VE, Pisoni RL, Port FK, Morgenstern H, Akizawa T, Saran R: Associations of frequency and duration of patient-doctor contact in hemodialysis facilities with mortality. J Am Soc Nephrol 24: 1493–1502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare program; revisions to payment policies under the physician fee schedule for calendar year 2004. Final rule with comment period. Fed Regist 68: 63195–63395, 2003 [PubMed] [Google Scholar]
- 14.Erickson KF, Tan KB, Winkelmayer WC, Chertow GM, Bhattacharya J: Variation in nephrologist visits to patients on hemodialysis across dialysis facilities and geographic locations. Clin J Am Soc Nephrol 8: 987–994, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patient Protection and Affordable Care Act, 42 U.S.C. § 18001 (2010).
- 16.McCarthy D, Johnson MB, Audet AM: Recasting readmissions by placing the hospital role in community context. JAMA 309: 351–352, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Bindman AB, Blum JD, Kronick R, Bindman AB, Blum JD, Kronick R: Medicare’s transitional care payment—a step toward the medical home. N Engl J Med 368: 692–694, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Jha AK, Jha AK: Time to get serious about pay for performance. JAMA 309: 347–348, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP: Long-term survival after first-ever stroke: The Oxfordshire Community Stroke Project. Stroke 24: 796–800, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA: Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 353: 2335–2341, 2005 [DOI] [PubMed] [Google Scholar]
- 21.McClellan M, McNeil BJ, Newhouse JP: Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA 272: 859–866, 1994 [PubMed] [Google Scholar]
- 22.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL: The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med 138: 273–287, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL: The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med 138: 288–298, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, Morris N, McLaughlin B, Gao X, Willison DJ, Asinger R, Gobel F: Effect of local medical opinion leaders on quality of care for acute myocardial infarction: A randomized controlled trial. JAMA 279: 1358–1363, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Landon BE, Keating NL, Barnett ML, Onnela J-P, Paul S, O’Malley AJ, Keegan T, Christakis NA: Variation in patient-sharing networks of physicians across the United States. JAMA 308: 265–273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staiger D, Stock JH: Instrumental variables regression with weak instruments. Econometrica 65: 29, 1997 [Google Scholar]
- 27.Altonji JG, Elder TE, Taber CR: Selection on observed and unobserved variables: Assessing the effectiveness of catholic schools. J Polit Econ 113: 151–184, 2005 [Google Scholar]
- 28.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28: 3083–3107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angrist JD, Krueger AB: The Effect of age at school entry on educational attainment: An application of instrumental variables with moments from two samples. J Am Stat Assoc 87: 328–336, 1992 [Google Scholar]
- 31.Bhattacharya J, Goldman D, McCaffrey D: Estimating probit models with self-selected treatments. Stat Med 25: 389–413, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Angrist JD: Instrumental variables estimation of average treatment effects in econometrics and epidemiology. National Bureau of Economic Research Technical Working Paper Series, 115, 1991
- 33.O’Hare AM, Rodriguez RA, Hailpern SM, Larson EB, Kurella Tamura M: Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA 304: 180–186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terza JV, Basu A, Rathouz PJ: Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J Health Econ 27: 531–543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WWAMI: Rural-Urban Commuting Area Codes (RUCA), Seattle, WA, WWAMI Rural Health Research Center, 2005 [Google Scholar]
- 36.Neyeloff JL, Fuchs SC, Moreira LB: Meta-analyses and Forest plots using a Microsoft Excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res Notes 5: 52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.