Abstract
Correction of anemia with erythropoietin (EPO) is associated with improved kidney transplant outcomes. Emerging evidence, predominantly from animal models, indicates that these observations may be erythropoiesis-independent and that EPO exhibits immunosuppressive properties. We examined the effects of EPO on human T-cell alloimmunity by first documenting that CD4+ and CD8+ T cells express EPO receptor (EPO-R) on their surfaces. In mixed lymphocyte reactions, EPO induced a dose-dependent decrease in allogeneic CD4+ T-cell proliferation (EPO 1000 U/ml: 44.6%±22.9% of vehicle, P<0.05; 2000 U/ml: 11.1%±4% of vehicle, P<0.001) without inducing cell death. The effects required signals transmitted directly through the EPO-R expressed on T cells, resulting in diminished Th1 differentiation without effects on regulatory T-cell induction. Mechanistic studies revealed that EPO prevented IL-2–induced proliferation by uncoupling IL-2 receptor signaling, inhibiting phosphorylation of the intracellular intermediaries AKT and extracellular signal-regulated kinase that are known to mediate T-cell expansion. EPO treatment reduced expansion of human naïve CD4+ T cells after adoptive transfer into NOD scid γcnull mouse recipients, verifying the effects in vivo. Although activated T cells expressed CD131, an alternative EPO receptor, addition of a specific CD131 agonist peptide, ARA290, did not alter T-cell proliferation or cytokine production. Our findings link EPO-R signaling on T cells to inhibition of T-cell immunity, providing one mechanism that could explain the observed protective effects of EPO in kidney transplant recipients.
The anemia that commonly occurs in kidney transplant recipients has been hypothesized to contribute to chronic allograft injury, in part by limiting oxygen delivery to the tubulointersitium and as a consequence, enabling fibrogenesis.1 In support of a link between anemia and kidney allograft injury, results of a 2012 randomized controlled study of erythropoietin (EPO) therapy after transplantation showed that targeting hemoglobin values to a normal range of ≥13 g/dl slowed progression of chronic allograft nephropathy and prolonged graft survival compared with partial correction of anemia.2
Although the above-cited clinical study and selected studies in rodents indicate correlations between EPO-induced increases in hematocrit and reduced tubulointerstitial damage as well as preserved renal tubular cells and improved kidney function,3 direct evidence linking EPO-induced erythropoiesis to better transplant outcomes is lacking. In fact, experiments performed in a fully mismatched rat kidney transplant model showed that EPO, but not the correction of anemia by blood transusion, limits chronic allograft injury,4,5 supporting the conclusion that the transplant-protective effects of EPO do not require an EPO-induced increase in hematocrit. Among potential alternative mechanisms, emerging evidence from animal models suggests that EPO has immune-suppressive properties. EPO therapy reduced the clinical expression of murine experimental autoimmune encephalomyelitis,6 with associated reduction in effector T-cell (Teff) proliferation, expansion of regulatory T cells (Tregs), and inhibition of dendritic cell (DC) function.7 A detailed understanding of how EPO alters murine T-cell function remains obscure, and whether and how EPO impacts human alloreactive T-cell immunity have not been carefully studied.
To address effects of EPO on human T cells, we performed a series of experiments that, together, show that EPO directly inhibits alloreactive human T-cell immunity through ligating T cell–expressed EPO receptors (EPO-Rs) and as a consequence, inhibits biochemical signaling pathways known to be downstream of the T-cell receptor and the IL-2 receptor. These unique functional and biochemical results linking EPO-R signaling on T cells to inhibition of T-cell proliferation and cytokine production in vitro and in vivo provide one mechanism that could explain the observed protective effects of EPO in kidney transplant recipients.
Results
EPO Reduces Naïve CD4+ T-Cell Expansion in Mixed Lymphocyte Reactions
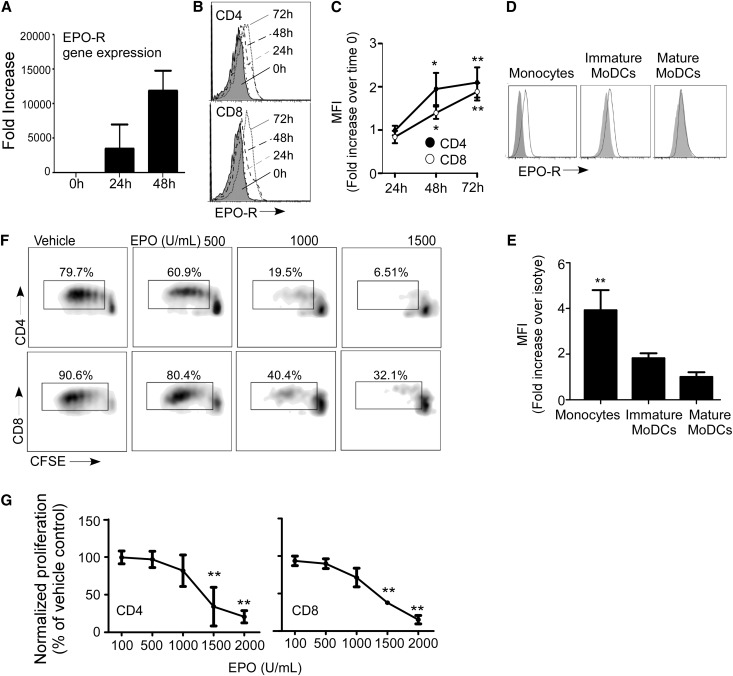
We initially examined human T cells for EPO-R RNA and surface protein expression by quantitative PCR (Figure 1A) and flow cytometry (Figure 1, B and C), respectively. These experiments revealed that resting CD4+ and CD8+ T cells produce low levels of EPO-R RNA and express EPO-R protein on their surfaces. Anti-CD3/anti-CD28 mAb stimulation induced upregulation of both RNA and surface protein over 1–3 days (Figure 1, A–C). Monocytes also express EPO-R on their surfaces (Figure 1, D and E), but we did not detect EPO-R on in vitro–generated monocyte-derived DCs (moDCs) after maturation with LPS.
Figure 1.
EPO inhibits T-cell expansion. (A) Kinetics of EPO-R gene expression in total T lymphocytes after anti-CD3/anti-CD28 stimulation as assessed by quantitative RT-PCR (means+SEMs; two experiments in triplicate). (B) Representative flow cytometry histograms and (C) quantified changes in mean fluorescence intensities (MFIs) versus baseline of EPO-R expression on the cell surface of CD4+ and CD8+ T lymphocytes after 24, 48, or 72 hours of anti-CD3/anti-CD28 stimulation (means+SEMs; eight experiments). (D) Representative flow cytometry histograms and (E) MFI (fold increase over isotype) of EPO-R on monocytes and immature and mature moDCs (means+SEMs; four experiments). (F) Representative plots and (G) quantitation of human PBMCs stimulated with anti-CD3/anti-CD28 gated on CD4+ or CD8+ T cells in the presence of EPO at the indicated doses (or vehicle control; means+SEMs; four experiments). *P<0.05; **P<0.01 versus 0 hours, isotype, or vehicle.
To test effects of EPO on human T-cell responses, we stimulated carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled PBMCs with anti-CD3/anti-CD28 mAbs in the presence of EPO or vehicle control and quantified proliferation in the CD4+ and CD8+ subsets by CFSE dilution using flow cytometry (Figure 1, F and G). These assays showed a statistically significant, dose-dependent, inhibitory effect in both T-cell subsets.
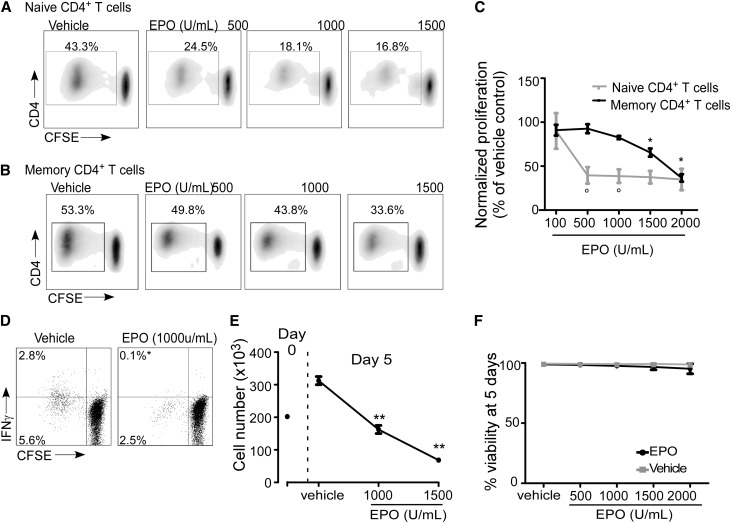
We next tested whether EPO impacts alloimmunity by focusing on naïve and memory CD4+ T cells (Figure 2). We isolated naïve CD45RA+CD45ROnegCD4+ and memory CD45RAnegCD45RO+CD4+ T cells by negative selection, labeled them with CFSE, and mixed them with allogeneic moDCs±varying doses of EPO in mixed lymphocyte reactions (MLRs). We used CFSE dilution (Figure 2, A–C), intracellular IFN-γ production (Figure 2D), and total cell number (Figure 2E) as readouts. These experiments showed dose-dependent inhibition of human alloreactive CD4+ T-cell proliferation, IFN-γ production, and expansion. Higher concentrations of EPO were required to inhibit proliferation of memory versus naïve T cells (Figure 2C). Viability staining (with FVS450 dye) and analysis by flow cytometry showed that EPO did not induce cell death (Figure 2F, Supplemental Figure 1).
Figure 2.
EPO inhibits CD4+ T-cell proliferation in a mixed lymphocyte reaction. Purified human naïve and memory CD4+ T cells were CFSE-labeled and cultured with allogeneic (mature) moDCs. (A and B) Representative flow cytometry histograms and (C) quantified results of CFSE dilution as a measure of cell proliferation in the presence of EPO at the indicated doses (or vehicle control; means+SEMs; seven experiments). (D) Representative flow cytometry histograms of IFN-γ production in CD4+ T cells cultured in MLRs with or without EPO and quantified results (percentages in the upper left are means of three separate experiments). *P<0.05. (E) Per-well cell count at day 0 and the end of the 5-day MLR culture. (F) Percent viability of CD4+ T cells at the end of the MLR in the presence of vehicle or EPO at indicated doses (means+SEMs; four experiments). *P<0.05; **P<0.01 versus vehicle. °P<0.05 versus naïve CD4+ T cells at the same EPO dose.
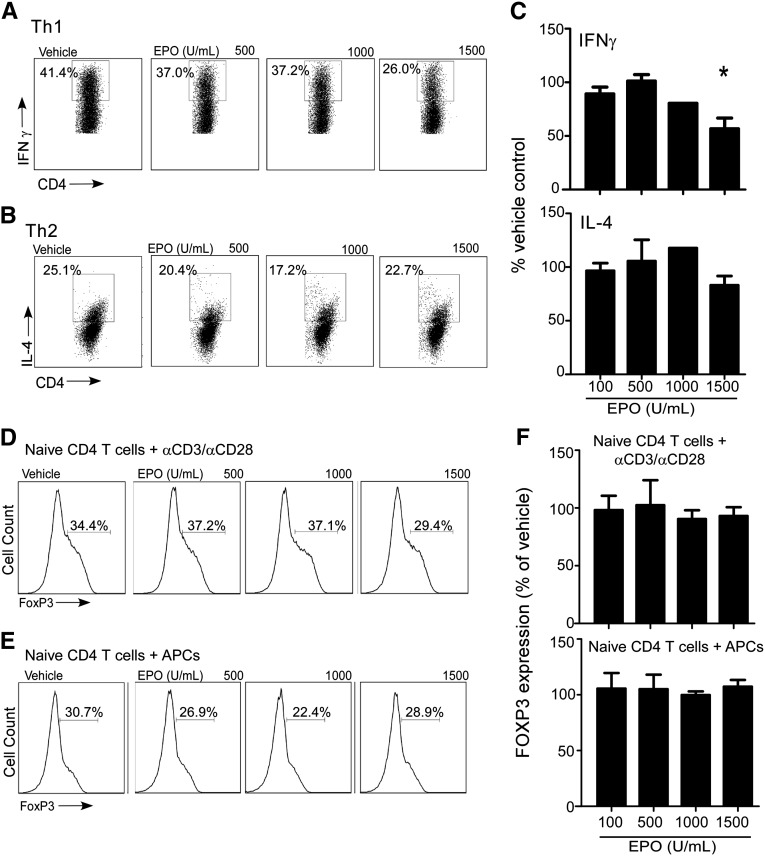
In separate experiments, we tested whether EPO alters T helper cell 1 (Th1)/Th2 differentiation in vitro (Figure 3, A–C). At the highest concentrations tested, EPO partially inhibited IFN-γ production under Th1 polarizing conditions (anti-CD3/anti-CD28+, IL-12, and blocking anti–IL-4 mAb) but did not inhibit IL-4 production under Th2 polarizing conditions (IL-4+blocking anti–IL-12/anti–IFN-γ mAb).
Figure 3.
EPO reduces Th1 but not Th2 polarization or Treg induction. Representative flow cytometry histograms and quantified results (n=5 experiments) of the expression of (A and C, upper panel) IFN-γ and (B and C, lower panel) IL-4 in naïve CD4+ T cells cultured under Th1- (means+SEMs; four experiments) or Th2-polarizing conditions (means+SEMs; six experiments), respectively, ±EPO at the indicated doses. (D–F) Representative flow cytometry histograms of FoxP3+ expression in naïve CD4+ T cells cultured with IL-2 and TGF-β±EPO and stimulated with (D) anti-CD3/anti-CD28 coated beads or (E) allogeneic moDCs. (F) Quantitation of the results (means+SEMs) for five individual experiments. *P<0.05 versus vehicle.
To test whether EPO affects induction of Tregs, we performed in vitro Treg induction assays by stimulating naïve CD4+ T cells with anti-CD3/anti-CD28 mAbs or allogeneic DCs (Figure 3, D–F), IL-2, and TGF-β±EPO or vehicle control. Although we observed induction of Foxp3+ T cells under all conditions, EPO had no discernible effect. CD4+CD25+FOXP3+ Tregs induced in the presence of EPO were capable of suppressing allogeneic CD8+ T-cell proliferation (percent suppression at 1:4 induced Treg to Teff was 95.1%; data not shown).
EPO Directly Prevents T-Cell Proliferation
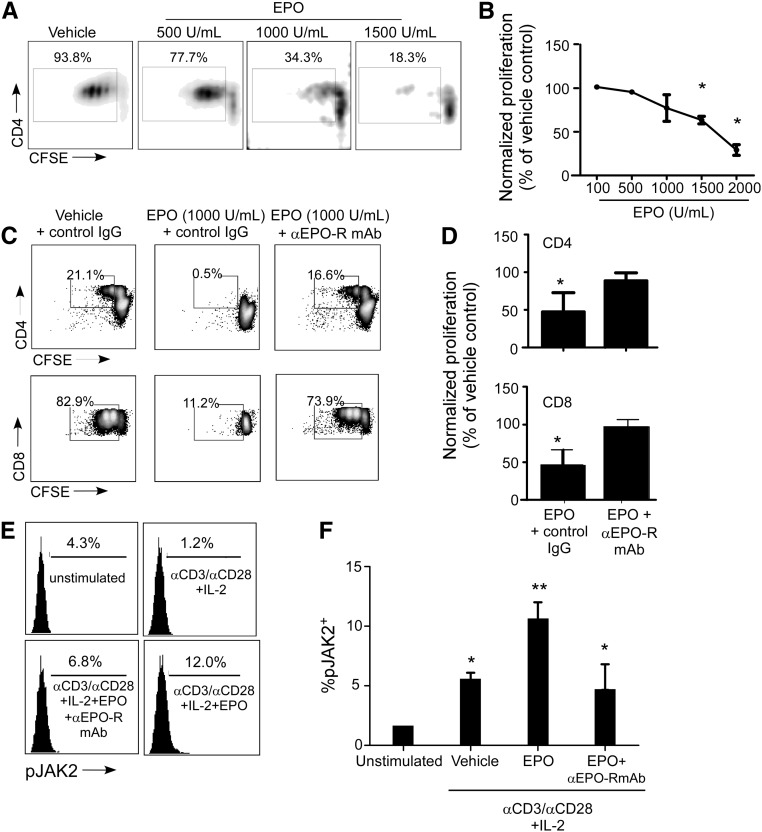
We reasoned that the EPO-induced inhibition of T-cell proliferation (and IFN-γ production) could be mediated through direct effects of EPO ligating EPO-R on T cells and/or indirectly through altering antigen presenting cell (APC) maturation or function. To test for a direct effect on T cells, we purified naïve CD4+ T cells and stimulated them with anti-CD3/anti-CD28 mAb±EPO or vehicle control (Figure 4, A and B). These assays unequivocally showed that EPO directly induced a dose-dependent reduction in naïve CD4+ T-cell proliferation in the absence of APCs. To test whether the inhibitory effects of EPO are mediated through the EPO-R, we repeated the experiment in the presence of a specific EPO-R blocking antibody (or isotype control) (Figure 4, C and D). Addition of the anti–EPO-R antibody but not the control IgG rescued T-cell proliferation, despite the presence of EPO.
Figure 4.
Inhibitory effects of EPO on T-cell proliferation are mediated through the EPO-R. (A) Representative flow cytometry histograms of enriched naïve CD4+ T cells stimulated with anti-CD3/anti-CD28 (no APCs) ±EPO at the indicated doses. (B) Quantified results of three separate experiments as performed in A. Proliferation rates in control wells from three donors were different, and therefore, the data are presented as means±SEMs of percent proliferation relative to the vehicle control. *P<0.05 versus vehicle. (C) Representative flow cytometry histograms and (D) quantified results of (upper panel) CD4+ and (lower panel) CD8+ T cells activated with anti-CD3/anti-CD28±EPO (1000 U/ml)±anti–EPO-R blocking antibody (5 μg/ml). Data in D are means+SEMs (four experiments; represented as percent proliferation relative to vehicle control). *P<0.05 versus vehicle. (E) Representative flow cytometry histograms and (F) quantified results of pJAK2 levels in unstimulated and anti-CD3/anti-CD28+IL-2–activated T cells±EPO (200 U/ml)±anti–EPO-R blocking antibody. Data in F are means+SEMs (four individual experiments). *P<0.05 versus unstimulated; **P<0.05 versus vehicle.
In erythroid cells, EPO-R signaling induces Janus kinase 2 (JAK2) phosphorylation.8 To test whether EPO/EPO-R signaling induces JAK2 phosphorylation in human T cells, we stimulated T cells with anti-CD3/anti-CD28 mAb±EPO and measured JAK2 phosphorylation by intracellular flow cytometry (Figure 4, E and F). EPO caused JAK2 phosphorylation, and the effect was abrogated in the presence of blocking anti–EPO-R mAb but not control IgG.
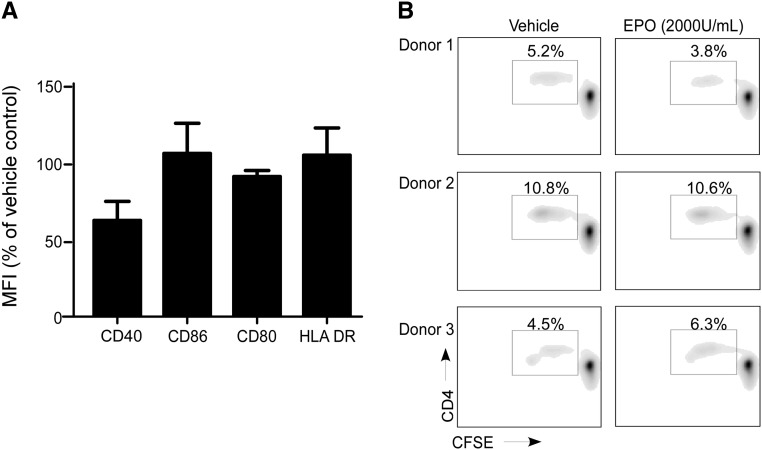
To formally test whether EPO also impacts DC function, we differentiated PBMCs into DCs by culturing them with GM-CSF and IL-4 for 5 days and then maturing them with LPS. We added EPO or vehicle control throughout the differentiation and maturation process, washed the resultant DCs, phenotyped them by flow cytometry, and then, tested their ability to stimulate allogeneic naïve CD4+ T cells in MLRs (Figure 5). Surface expression levels of class II HLA, CD40, CD80, and CD86 were similar on the DCs, regardless of EPO treatment (Figure 5A). Adding EPO during in vitro DC generation did not affect DC allostimulatory capacity (Figure 5B).
Figure 5.
EPO does not affect DC phenotype or function. (A) moDCs were induced in the presence of EPO (2000 U/ml) or vehicle control, analyzed for the cell surface markers indicated by flow cytometry, and reported as percentage of the mean fluorescence intensity (MFI) of each marker compared with vehicle-treated control DCs. Expression of CD40 was modestly but significantly lower (P<0.05) in the EPO-treated moDCs. The other three markers were not different between EPO-treated and vehicle-treated controls. (B) Proliferation of naïve CD4+ T cells in response to allogeneic moDCs pretreated with EPO or vehicle for 7 days (but not added in the MLR). Data are representative of three independent experiments using different donors. Similar results were obtained using memory CD4+ or total CD8+ T cells as responders (not shown).
Together, the functional and biochemical data support the conclusion that EPO-induced inhibition of T-cell proliferation is mediated through signals transmitted directly through the EPO-R expressed on the T cell.
EPO Inhibits T-Cell Proliferation by Uncoupling IL-2R Signaling
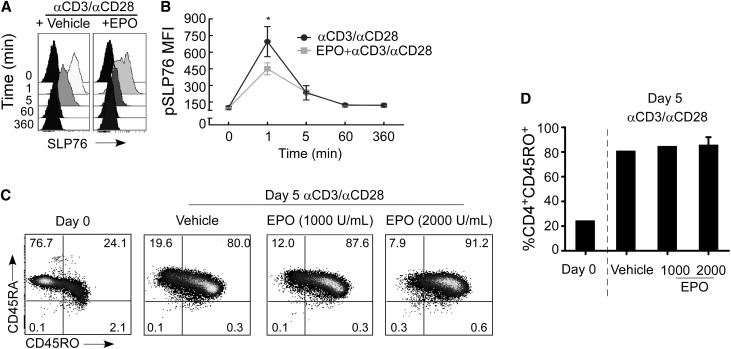
To address biochemical mechanisms that could account for the antiproliferative effects of EPO, we used flow cytometry to measure phosphorylation of intracellular signaling molecules downstream of the T-cell receptor (TCR) and CD28. As established by others,9,10 anti-CD3/anti-CD28 mAb stimulation of CD4+ T cells rapidly resulted in phosphorylation of TCR-associated Linker of Activated T cells (LAT; not shown) and SH2 domain–containing leukocyte protein of 76 kDa (SLP-76) (Figure 6, A and B). Addition of EPO to the cultures modestly but only transiently reduced early TCR activation-associated LAT (15%±5.65% reduction at 5 minutes; data not shown) and SLP76 phosphorylation (Figure 6, A and B). Similarly, whereas anti-CD3/anti-CD28 mAb stimulation induced phosphorylation of signal transducer and activator of transcription (STAT) 1, STAT6, ζ-chain–associated protein kinase 70 (ZAP70), phospholipase C (PLC)γ1, and PLCγ2, EPO did not impact phosphorylation of any of these proximal TCR/costimulation-associated signals (at 1, 5, 60, and 360 minutes; not shown). Also, EPO did not alter anti-CD3/anti-CD28–induced upregulation of the activation marker CD45RO (Figure 6, C and D), thereby confirming that EPO does not significantly alter T-cell activation.
Figure 6.
EPO does not affect early TCR activation or CD45RO expression. (A and B) pSLP76. Representative (A) flow cytometry histograms and (B) quantified kinetics of pSLP76 in anti-CD3/anti-CD28–stimulated CD4+ cells±EPO (means+SEMs; three experiments). *P<0.05 versus EPO treated at 1 minute. (C) Representative CD45RA and CD45RO expression on enriched naïve CD4+ T cells (day 0) and after 5 days of stimulation with anti-CD3/anti-CD28±EPO at the doses indicated. (D) Quantified percentages (means±SEMs; three separate experiments) of CD45RO+ CD4+ T cells on day 0 or after 5 days of stimulation in the presence or absence of EPO. MFI, mean fluorescence intensity.
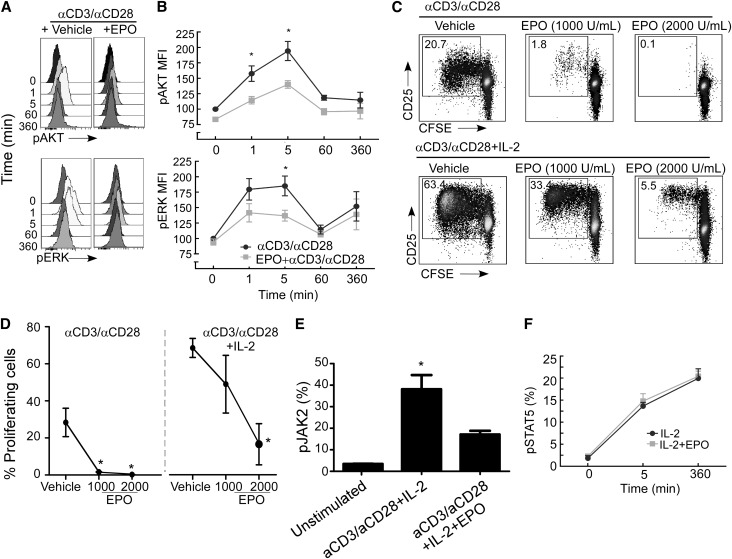
More distal TCR-initiated signals involved in T-cell proliferation include phosphoinositol-3-kinase-γ–dependent phosphorylation of AKT and mitogen-activated protein kinase–dependent phosphorylation of extracellular signal-regulated kinase (ERK).11 EPO significantly blunted anti-CD3/anti-CD28–induced phosphorylation of ERK and AKT at 1–5 minutes (Figure 7, A and B), the latter of which is a crucial intracellular signaling intermediary involved in T-cell proliferation and expansion.11
Figure 7.
EPO uncouples IL-2R signaling in anti-CD3/anti-CD28–stimulated CD4+ T cells. Representative (A) kinetic flow cytometry histograms and (B) quantified results of pERK and pAKT expression in anti-CD3/anti-CD28–stimulated CD4+ cells±EPO. Data in B are means±SEMs (three individual experiments). *P<0.05 versus time 0 (anti-CD3/anti-CD28 alone) and versus anti-CD3/anti-CD28+EPO tested at 1 and 5 minutes. (C) Representative flow cytometry histograms of CD4+ T cells activated with (upper panel) anti-CD3/anti-CD28 or (lower panel) anti-CD3/anti-CD28+recombinant IL-2 (0.5 μg/ml)±EPO. (D) Quantified results (means and SEMs) of one of four representative experiments, all of which showed similar results. *P<0.05 versus vehicle. (E) Representative flow cytometry histograms of pAKT in CD4+ T cells stimulated with anti-CD3/anti-CD28±IL-2±EPO at 6 hours after stimulation. Results shown in each panel are means of three experiments. *P<0.05 versus unstimulated. (F) Quantitation of kinetics of pSTAT5 in anti-CD3/anti-CD28+IL-2–stimulated CD4+±EPO (means+SEMs; three experiments). *P<0.05 versus vehicle.
Because T-cell proliferation and expansion as well as AKT phosphorylation are, in part, IL-2–dependent,12 we tested whether EPO alters IL-2 production and IL-2 receptor (IL-2R; CD25) expression and/or function in human T cells. EPO did not alter intracellular IL-2 production by CD4+ T cells 48 hours after stimulation with anti-CD3/anti-CD28 (vehicle=20% IL-2+; EPO 1000 U/ml=17.8% IL-2+ by intracellular flow cytometry, P=NS; data not shown). We next stimulated CD4+ T cells with anti-CD3/anti-CD28±IL-2±EPO and measured T-cell proliferation by CFSE dilution (Figure 7, C and D). As anticipated, IL-2 significantly augmented anti-CD3/anti-CD28–induced T-cell proliferation. Strikingly, EPO blocked the IL-2–induced proliferative effect without altering T-cell surface expression levels of the IL-2R.
IL-2R signaling is transduced through multiple parallel pathways, including phospho-STAT5 and AKT. EPO inhibited phosphorylation of AKT when we added recombinant IL-2 to the culture wells (Figure 7E). In contrast, EPO did not impact IL-2–induced phosphorylation of STAT5 (Figure 7F). Together, the data indicate that one mechanism through which EPO inhibits T-cell immune responses is disruption of IL-2R–dependent activation of the AKT but not STAT5 signaling pathways.
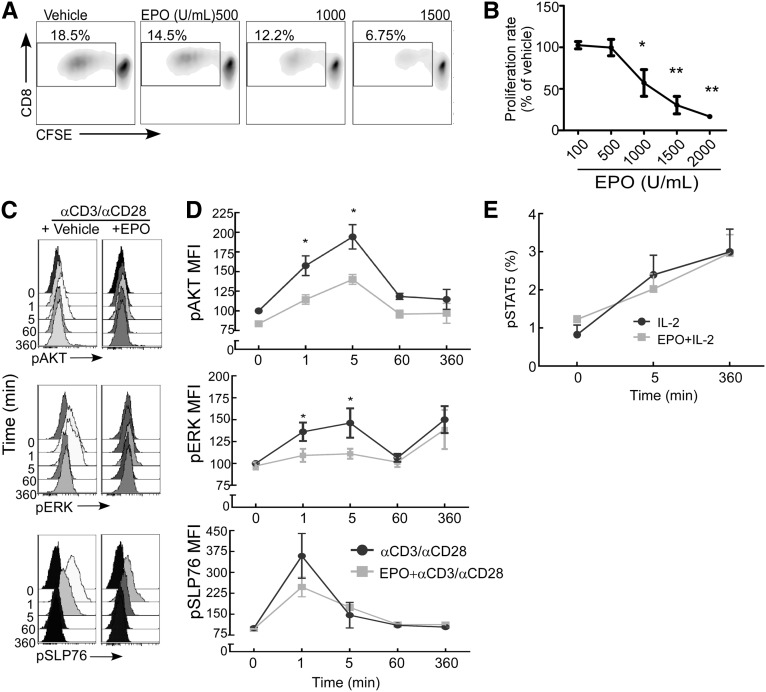
Analogous to the above-noted effects on CD4+ T cells, EPO inhibited CD8+ T-cell proliferation in MLRs (Figure 8, A and B) and blocked anti-CD3/anti-CD28 mAb-induced phosphorylation of ERK and AKT (Figure 8, C and D) but did not alter IL-2–induced STAT5 phosphorylation (Figure 8E).
Figure 8.
EPO inhibits proliferation of CD8+ T cells in response to anti-CD3/anti-CD28 stimulation. (A) Representative flow cytometry histograms of CD8+ T cells and (B) quantified results of CFSE dilution as a measure of cell proliferation in response to anti-CD3/anti-CD28 stimulation±EPO at the indicated doses (means+SEMs; three experiments). *P<0.05; **P<0.01 versus vehicle. (C) Representative flow cytometry histograms and (D) quantified kinetic results of pSLP76, pERK, and pAKT in anti-CD3/anti-CD28–stimulated CD8+ cells±EPO. Data in D represent means and SEMs of three individual experiments. *P<0.05 versus time 0 (anti-CD3/anti-CD28 alone) and versus anti-CD3/anti-CD28+EPO tested at 1 and 5 minutes. (E) Quantitation and kinetics of STAT5 phosphorylation in anti-CD3/anti-CD28+IL-2–stimulated CD8+±EPO (means+SEMs; three experiments). MFI, mean fluorescence intensity.
EPO Prevents Expansion of Alloreactive Naïve CD4+ T Cells In Vivo
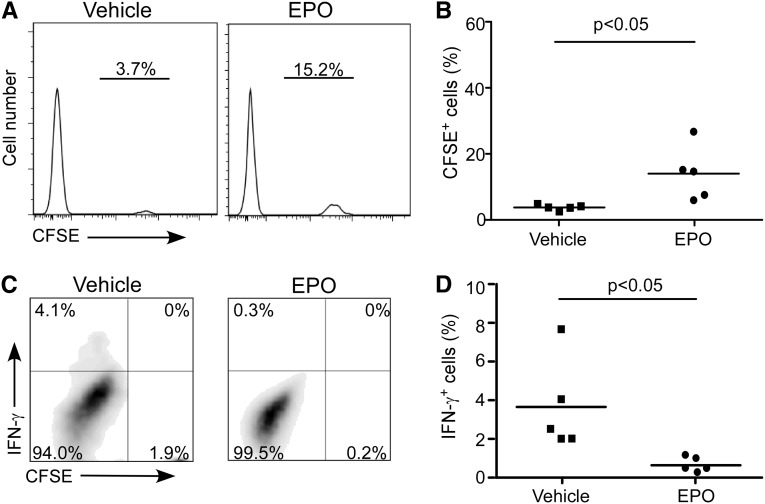
To test whether and how these in vitro findings apply in vivo, we transferred CFSE-labeled naïve human CD4+ T cells into NOD scid γcnull recipients, which are deficient in T, B, and natural killer cells.13 In this model, the human T cells proliferate in vivo in response to xenogeneic murine antigens. Groups of animals were treated with EPO or vehicle control. Three days later, we examined splenic T-cell responses by flow cytometry. These assays remarkably revealed less human T-cell proliferation (higher frequency of nondivided CFSEhi human T cells) (Figure 9, A and B) and diminished IFN-γ production (Figure 9, C and D) in the EPO-treated animals.
Figure 9.
EPO inhibits naïve CD4+ T-cell expansion in NOD scid γcnull mice. Five million CD45RA+CD4+ naïve human T cells were labeled with CFSE and injected into NOD scid γcnull mice. (A) Representative histograms and (B) data quantitation of remaining CFSE+ naïve CD4+ T cells 3 days after injection in NOD scid γcnull mice treated with EPO (n=5) or vehicle control (n=5). (C) Representative plots and (D) data quantitation of IFN-γ production by the same CD4+ T cells.
Activation of EPO Heterodimeric Receptor (CD131) Does Not Affect T-Cell Alloreactivity
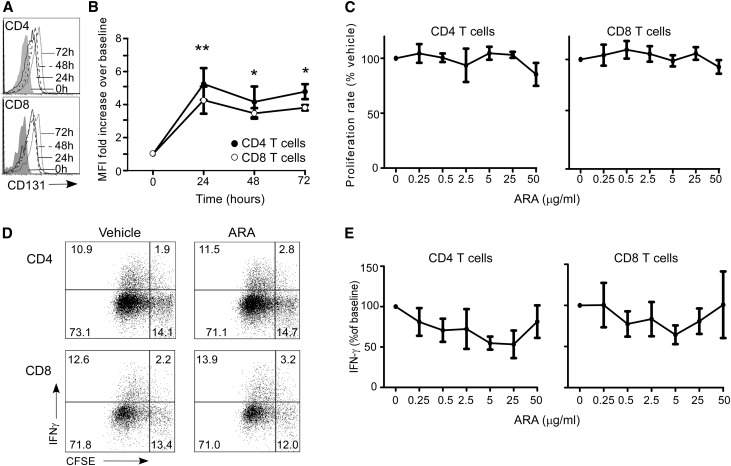
EPO-induced intracellular signaling is principally mediated through a homodimer of the EPO-R, but emerging evidence indicates that EPO can also signal through a heterodimeric receptor composed of an EPO-R monomer chain and CD131.14,15 This heterodimeric complex, the activation of which requires much higher concentrations of EPO compared with the activation of the homodimeric EPO-R,16 has been described on nonerythroid cells. When we stained human T cells for CD131 and evaluated expression levels by flow cytometry, we observed that, although CD131 was absent on resting T cells, CD131 was detectable on both CD4+ and CD8+ T-cell subsets 24 hours after anti-CD3/anti-CD28 mAb stimulation (Figure 10, A and B). We did not detect CD131 on monocytes or moDCs (not shown).
Figure 10.
Targeting CD131 does not affect T-cell expansion or IFN-γ production. (A and B) Representative (A) flow cytometry histograms and (B) MFI of CD131 expression on the cell surface of freshly isolated peripheral blood CD4+ and CD8+ T cells and after 24, 48, or 72 hours (means+SEMs; three experiments). *P<0.05 versus time 0; **P<0.01 versus time 0 for both CD4+ and CD8+ T cells. (C) Proliferation of CFSE-labeled naïve CD4+ and CD8+ T cells activated with anti-CD3/anti-CD28 cultured with vehicle or ARA290 at indicated doses (means+SEMS; five experiments). Representative (D) flow cytometry plots and (E) quantification of IFN-γ production in CD4+ and CD8+ T cells activated with anti-CD3/anti-CD28 cultured with vehicle or ARA290 at indicated doses (means of five separate experiments).
Previous work by others has shown that an 11-amino acid peptide derived from EPO, ARA290, specifically binds to and signals through the EPO-R/CD131 complex without effects on the EPO-R homodimer.16 The peptide has been shown to attenuate renal ischemia/reperfusion injury in pigs17 and improve neural function in separate model of neuropathic pain.18,19 When we added ARA290 to anti-CD3/anti-CD28 mAb-stimulated T cells, we did not observe significant alterations in T-cell proliferation (Figure 10, C and D) or IFN-γ production (Figure 10, D and E).
Discussion
Our results extend previously published work by others documenting EPO-R on human T cells20,21 by uniquely revealing that EPO exhibits immunosuppressive properties. EPO inhibits T-cell proliferation, expansion, and IFN-γ production without inducing T-cell death or altering Treg generation (Figures 1–3). The effects are mediated directly through ligation of the EPO-R expressed on the T cell; anti–EPO-R mAb overcame EPO’s antiproliferative effects and abrogated EPO-induced JAK2 phosphorylation (Figure 4). We did not detect a discernable effect of EPO on DC phenotype or function (Figure 5). We observed that anti-CD3/anti-CD28 induced EPO-R upregulation on the T-cell surface, raising the speculative possibility that signaling by the EPO-R could physiologically regulate T-cell expansion. The molecular basis for EPO-R surface upregulation, in part, is a consequence of increased EPO-R gene expression (Figure 1). Previous work by others showed that anti-CD3/anti-CD28 upregulates the Sp1 transcription factor known to positively regulate EPO-R transcription,22 further supporting this concept. Whether TCR signaling alters trafficking of EPO-R to the T-cell surface, which has been reported in erythroid cells,23 remains untested.
Mechanistic analyses indicate that early activation events induced by crosslinking the TCR with CD28-induced costimulation are minimally altered (Figure 6). In contrast, our data support the conclusion that EPO inhibits TCR-initiated downstream signals, including AKT and ERK, as well as blocks IL-2/IL-2R–dependent T-cell proliferation (Figure 7). EPO does not alter T-cell IL-2 production or T-cell surface expression of the IL-2R, but it does uncouple distal IL-2R signal transduction. IL-2Rαβ–initiated signals are known to activate phosphoinositol-3 kinase-γ/AKT and mitogen-activated protein kinase/ERK pathways,12 whereas inducible IL-2Rγ/JAK3-initiated signals activate the STAT5 pathway. Our data indicate that EPO inhibits phosphorylation of AKT and ERK without altering pSTAT5 (Figures 6–8), although additional work will be required to discern exact biochemical mechanisms. The novelty of our finding that EPO inhibits phosphorylation of AKT in T cells is underscored by contrasting our results with the reported effects of EPO-R signal transduction in erythropoietic cells, in which EPO-R signaling induces AKT phosphorylation.24 Together with the absence of a detectable effect after the addition of a peptide known to ligate the CD131/EPO-R heterodimer (Figure 10), our combined data support the conclusion that ligating the homodimeric EPO-R inhibits proximal TCR and IL-2Rαβ signaling.
STAT5 activation and IL-2R–dependent proximal signal transduction are required for Treg induction, function, and proliferation, whereas phosphorylation of AKT is constitutively blocked in Treg through endogenous phosphatase activities.25 Because our data show that EPO does not affect STAT5 phosphorylation and preferentially inhibits AKT phosphorylation in Teff cells, we speculate that EPO will facilitate, rather than inhibit, Treg stability, function, and proliferation in vivo. Although additional studies will be required to test this concept in humans, previous work published by others in the experimental autoimmune encephalomyelitis model revealed increased Treg frequencies in EPO-treated (and protected) animals,6 consistent with this hypothesis.
Importantly, our data indicate that EPO treatment can inhibit the expansion of alloreactive human naïve CD4+ T cells and their IFN-γ production in NOD scid γcnull mice (Figure 9), providing evidence that the EPO immunomodulatory effects can occur in vivo.
The immune-suppressive effects of EPO on T cells provide a potential explanation to account for why normalizing hemoglobin levels in transplant patients improves outcomes,2 whereas normalizing hemoglobin in the general CKD population is detrimental.26,27 The immunomodulatory actions of EPO may mitigate other negative cardiovascular effects related to the drug in this unique transplant patient population. The immunosuppressive properties of EPO also provide a possible mechanism to account for formerly published findings by others, including (1) the beneficial effects of EPO in accelerating recovery of early allograft ischemia,28 a phenomenon partially mediated by T cells,29 and (2) prolongation of T cell–mediated transplant survival in rats treated with EPO.5 Analogously, finding that EPO inhibits T-cell expansion in response to polyclonal stimuli and prevents Th1 differentiation of naïve CD4+ T cells may partially explain previous data showing that EPO downregulates proinflammatory immune effector pathways in response to chemical tissue damage, LPS stimulation, and Salmonella infection.6,30–32 In patients with rheumatoid arthritis, treatment with recombinant EPO not only resulted in amelioration of anemia but also improved disease activity.33 Intriguingly, EPO has been associated with adverse outcomes of tumor progression and impaired survival in a series of clinical trials,34,35 a finding that might be explained, at least in part, by EPO-induced inhibition of antitumor T-cell immunity. Our new identification of the inhibitory effects of EPO on T cells provides a rationale for the transplant research community to test these concepts in future studies.
We acknowledge that the EPO doses that optimally inhibited T-cell immunity are higher than serum levels in EPO-treated patients,36 but levels in immune organs have not been measured; also, it is conceivable that T-cell immunity is impaired at the doses generally provided to humans. Interestingly, because EPO is physiologically produced by renal tubular cells, it is tempting to speculate that high intragraft levels of EPO provide immune-modulating activities that could, at least in part, account for the better tolerogenicity of the kidney compared with other organs.37 We also recognize that EPO has other described in vivo effects that could influence graft survival, including reduction of oxidative stress and strengthening of antiapoptotic processes.3
In conclusion, we show, for the first time (to our knowledge), that EPO directly inhibits alloreactive human T-cell immunity. Our findings support the future testing of EPO and/or EPO-R agonists to target alloreactive T cells as adjuvant immune suppressants in kidney transplant recipients.
Concise Methods
Human Samples
PBMCs were isolated by Ficoll gradient centrifugation of deidentified buffy coats from healthy donors purchased from the New York Blood Bank.
EPO and ARA290
Epoetin-α (Procrit) was obtained from Janssen Cilag. ARA290 (pyroglutamic acid–Glu-Gln-Leu-Glu-Arg-Ala-Leu-Asn-Ser-Ser-OH) was synthesized by standard F-moc solid-phase peptide synthesis and purified by HPLC, and ion-exchange chromatography (GenScript).
Cell Isolation, Culture, and Expansion of T-Cell Subsets
Naïve CD4+ T cells were isolated from human PBMCs using the MACS cell separation kit (Miltenyi Biotec). Memory (CD45RO+) CD4+ T cells and total CD8+ T cells were isolated using EasySep negative selection from STEMCELL Technologies. After isolation, cells were stained for CD4, CD8, CD45RA, and CD45RO to confirm purity, which was consistently higher than 90% among all T-cell subsets.
Preparation of moDCs
CD14+ monocytes were isolated from PBMCs by positive bead selection (EasySep), and purity was consistently higher than 85%–90%. Monocytes were cultured in medium consisting of RPMI (Life Technologies) with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin and streptomycin, GM-CSF (1000 U/ml; Peprotech), and IL-4 (1000 U/ml; R&D Systems) at 37°C in 5% CO2 atmosphere for 5 days. On day 5, cells were stimulated with LPS (5 ng/ml; Sigma-Aldrich) for 48 hours. Before using these moDCs in MLRs, they were washed five times to eliminate residual LPS. EPO was added at the indicated doses to selected cultures as outlined in the text.
In Vitro Cultures/MLRs
Enriched T-cell subsets were stimulated with either soluble anti-CD3/anti-CD28 mAbs (1 μg/ml each; BD Biosciences) or anti-CD3/CD28 mAb-coated beads (25 μl/106 cells, Dynabeads; Invitrogen) in RPMI plus 10% heat-inactivated human AB serum (Gemini Bio-Products)±recombinant human IL-2 (BD Biosciences) for 5 days at 37°C and 5% CO2. EPO and equivalent amounts of vehicle were added to the cultures where indicated. Proliferation was assessed by CFSE (Invitrogen) dilution.
In MLRs, CFSE-labeled naïve or memory CD4+ and total CD8+ T cells were cultured in the presence of allogeneic moDCs (moDC to T-cell ratio was 1:5 to 1:20) at 37°C in 5% CO2 atmosphere in RPMI plus 10% heat-inactivated human AB serum for 5 days and then analyzed for CFSE expression by flow cytometry. EPO-R blocking antibody was purchased from R&D Systems.
In Vitro T-Cell Polarization
Naïve CD4+ T cells were stimulated with RPMI/10% human AB serum, anti-CD3/anti-CD28 mAb-coated beads, and recombinant human IL-2 (10 ng/ml; BD Bioscience) plus either IL-12 (1 ng/ml; R&D Systems) and neutralizing anti–IL-4 mAb (10 µg/ml; R&D Systems) to polarize to Th1 or IL-4 (20 ng/ml; R&D Systems), neutralizing anti–IL-12 mAb (10 µg/ml; R&D Systems), and neutralizing anti–IFN-γ mAb (10 µg/ml; R&D Systems) to polarize to Th2. Polarizing cytokines were replenished on day 3, and cells were harvested on day 6.
Human Tregs were generated by culturing 2×105 naive CD4+ T cells with 5×104 moDCs and anti-CD3 mAbs (or anti-CD3/anti-CD28 mAb-coated beads and no moDCs) along with IL-2 and TGF-β for 5 days.
Flow Cytometry
PE-Cy5–anti-CD4, APC-H7–anti-CD8, PE–anti-CD45RA, APC–anti-CD45RO, FITC–anti-CD4, PE-Cy7–anti–IFN-γ, PE-Cy5–anti–IL-2, PE, and PE-Cy7–anti-Foxp3 were obtained from BD Biosciences. PE–anti-human IL-4 and PE–anti-human CD131 were obtained from BioLegend. FITC–anti-human EPO-R was obtained from R&D Systems Inc. According to the manufacturer’s instructions for EPO-R and CD131 staining, pelleted cells were Fc-blocked with 2 µg human IgG (Endobulin; Baxter AG) for 15 minutes at room temperature.
For intracellular cytokine detection, brefeldin A (1:1000; eBioscience), 20 ng/ml PMA (Sigma-Aldrich), and 1 μM ionomycin (Sigma-Aldrich) were added 4–6 hours before recovery. For IL-2 detection, only brefeldin A was added. Dead cells were excluded by gating out those cells positive for FVS450 (Invitrogen). This reactive dye permeates the compromised membranes of necrotic cells and reacts with free amines both in the interior and on the cell surface, resulting in intense fluorescent staining. In contrast, only the cell surface amines of viable cells are available to react with the dye, resulting in relatively dim staining.
moDCs were identified and gated based on their positive HLA class II DR (BD Biosciences) and CD11c (R&D Systems). FITC anti-CD40, FITC anti-CD80, PE anti-CD86, and APC anti–HLA-DR were used to further characterize monocytes and moDC activation status (BD Pharmingen).
Data were acquired on a three-laser Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.). At least 50,000 events were acquired per sample.
Analysis of Signaling through Phosphoflow Cytometry
Phosphoflow cytometry was performed as described38 with slight modifications as follows: PBMCs were isolated, distributed into round-bottomed 12×75-mm tubes at 1×106 cells per tube, and allowed to rest in a 37°C water bath for 2 hours. Cells were then either stimulated or preincubated for 5 minutes with 2000 U/ml EPO before stimulation. Stimulation consisted of incubation with biotin-conjugated anti-CD3 and anti-CD28 mAbs for 2 minutes (5 μg/ml each; eBioscience) followed by cross-linking with avidin (50 μg/ml; Life Technologies) and incubating for the indicated time periods (1, 5, 60, and 360 minutes) at 37°C. Reactions were stopped by fixation with 4% paraformaldehyde for 10 minutes at room temperature. Cells were then washed with 1× PBS and permeabilized by resuspension in 90% methanol for 20 minutes at −20°C. Samples were rehydrated to prepare for flow staining by washing two times with 1 ml PBS and resuspended in 1% BSA in PBS.
Intracellular and surface stains were performed simultaneously. Surface stains consisted of Pacific Blue–conjugated anti-CD4 and APC-Cy7–conjugated anti-CD8.
Intracellular stains consisted of one of four panels: panel 1, PE anti-pAKT, AlexaFluor488 anti-pSTAT5, and AlexaFluor647 anti-pERK1/2; panel 2, PE-conjugated anti-SLP76, AlexaFluor488 anti-pNFkB, and AlexaFluor647 anti-pSTAT1; panel 3, PE anti-pZAP70, AlexaFluor488 anti-pLAT, and AlexaFluor647 anti-pPLCγ2; panel 4, PE anti-pSTAT6 and AlexaFluor647 anti-pPLCγ1. All antibodies were purchased from BD Bioscience. Experiments testing JAK2 phosphorylation were done by stimulating T cells with anti-CD3, anti-CD28 mAb, and IL-2±EPO and ±anti–EPO-R blocking antibody for 6 hours. Cells were processed as described above and stained with unconjugated rabbit anti-pJAK2 antibody (Cell Signaling Technology) and PE donkey anti-rabbit IgG secondary antibody (BD Bioscience).
Samples were read on an LSR Fortessa (BD Bioscience), and data analysis was performed using Stanford Cytobank software (http://cytobank.stanford.edu/public/openid/).
In Vivo Experiments
Six- to eight-week-old female NOD scid γcnull mice were housed in the Mount Sinai School of Medicine Center for Comparative Medicine and Surgery in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. Mice received retro-orbital injection of 5×106 CFSE-labeled human naïve CD4+ T cells together with syngeneic 5×106 CD4-depleted PBMCs. Animals were treated with EPO (5000 UI/kg per day intraperitoneally from day 0 to day 3)39 or vehicle control and euthanized at day 3 after injection for flow cytometric analysis of CSFE dilution and IFN-γ production. The study protocol described herein was reviewed and approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism (version 5 for Windows; GraphPad Software, Inc.). Group comparisons of paired data were analyzed by paired t tests. One-way repeated measures ANOVA was used for multiple comparisons among treatment groups, with pairwise multiple comparison procedures performed using Bonferroni post hoc tests. P values <0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Saghi Ghaffari (Icahn School of Medicine at Mount Sinai, NY) for her critical comments and suggestions. Erythropoietin (Epoetin alfa; Procrit) was provided by Janssen Cilag.
P.C. is a recipient of Fellowship Award 12POST12050294 from the American Heart Association. J.M. is the recipient of fellowship awards from the Institute Carlos III, Beca de Ampliacion de Estudios (BAE) and supported by a Spanish Society of Nephrology Foreign Studies Grant. A.M. is supported by Clinical Research Program Award 12CRP8850007 from the American Heart Association and a KL2 Award through National Center for Advancing Translational Sciences, National Institutes of Health Grant UL1TR000067. The work was supported by National Institutes of Health Grant R01 AI43578 (to P.S.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “When Erythropoietin Meddles in Immune Affairs,” on pages 1887–1889.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090945/-/DCSupplemental.
References
- 1.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O, Glowacki F, Moulin B, Lebranchu Y, Touchard G, Jaureguy M, Pallet N, Le Meur Y, Rostaing L, Martinez F, CAPRIT Study Investigators : Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 23: 360–368, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallet N, Bouvier N, Legendre C, Beaune P, Thervet E, Choukroun G, Martinez F: Antiapoptotic properties of recombinant human erythropoietin protects against tubular cyclosporine toxicity. Pharmacol Res 61: 71–75, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Rocchetta F, Solini S, Mister M, Mele C, Cassis P, Noris M, Remuzzi G, Aiello S: Erythropoietin enhances immunostimulatory properties of immature dendritic cells. Clin Exp Immunol 165: 202–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassis P, Gallon L, Benigni A, Mister M, Pezzotta A, Solini S, Gagliardini E, Cugini D, Abbate M, Aiello S, Rocchetta F, Scudeletti P, Perico N, Noris M, Remuzzi G: Erythropoietin, but not the correction of anemia alone, protects from chronic kidney allograft injury. Kidney Int 81: 903–918, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P: Erythropoietin: A potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS ONE 3: e1924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose S, Takahashi M, Ogawa R, Morimoto H, Izawa A, Sato H, Ise H, Hongo M, Ikeda U: Erythropoietin attenuates the development of experimental autoimmune myocarditis. Cardiovasc Drugs Ther 21: 17–27, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Broxmeyer HE: Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med 210: 205–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalland ME, Oberprieler NG, Vang T, Taskén K, Torgersen KM: T cell-signaling network analysis reveals distinct differences between CD28 and CD2 costimulation responses in various subsets and in the MAPK pathway between resting and activated regulatory T cells. J Immunol 187: 5233–5245, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Acuto O, Michel F: CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat Rev Immunol 3: 939–951, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Song J, Lei FT, Xiong X, Haque R: Intracellular signals of T cell costimulation. Cell Mol Immunol 5: 239–247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benczik M, Gaffen SL: The interleukin (IL)-2 family cytokines: Survival and proliferation signaling pathways in T lymphocytes. Immunol Invest 33: 109–142, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, Eto T, Ito M: Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation 87: 1654–1658, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Brines M, Cerami A: Discovering erythropoietin’s extra-hematopoietic functions: Biology and clinical promise. Kidney Int 70: 246–250, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Zhang YL, Radhakrishnan ML, Lu X, Gross AW, Tidor B, Lodish HF: Symmetric signaling by an asymmetric 1 erythropoietin: 2 erythropoietin receptor complex. Mol Cell 33: 266–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hand CC, Brines M: Promises and pitfalls in erythopoietin-mediated tissue protection: Are nonerythropoietic derivatives a way forward? J Investig Med 59: 1073–1082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rijt WG, Nieuwenhuijs-Moeke GJ, van Goor H, Jespersen B, Ottens PJ, Ploeg RJ, Leuvenink HG: ARA290, a non-erythropoietic EPO derivative, attenuates renal ischemia/reperfusion injury. J Transl Med 11: 9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulman KG, Smith M, Mengozzi M, Ghezzi P, Dilley A: The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model. Neuroscience 233: 174–183, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Swartjes M, Morariu A, Niesters M, Brines M, Cerami A, Aarts L, Dahan A: ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: An experimental study in rats and β-common receptor knockout mice. Anesthesiology 115: 1084–1092, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Lisowska KA, Bryl E, Witkowski JM: Erythropoietin receptor is detectable on peripheral blood lymphocytes and its expression increases in activated T lymphocytes. Haematologica 96: e12–e13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisowska KA, Debska-Slizień A, Bryl E, Rutkowski B, Witkowski JM: Erythropoietin receptor is expressed on human peripheral blood T and B lymphocytes and monocytes and is modulated by recombinant human erythropoietin treatment. Artif Organs 34: 654–662, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Lisowska KA, Frackowiak JE, Mikosik A, Witkowski JM: Changes in the expression of transcription factors involved in modulating the expression of EPO-R in activated human CD4-positive lymphocytes. PLoS ONE 8: e60326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Verma R, Pradeep A, Leu K, Mortensen RB, Young PR, Oyasu M, Schatz PJ, Green JM, Wojchowski DM: Dynamic ligand modulation of EPO receptor pools, and dysregulation by polycythemia-associated EPOR alleles. PLoS ONE 7: e29064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao H, Jacobs-Helber SM, Lawson AE, Penta K, Wickrema A, Sawyer ST: Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells). Blood 93: 3757–3773, 1999 [PubMed] [Google Scholar]
- 25.Cheng G, Yu A, Malek TR: T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 241: 63–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Cassis P, Azzollini N, Solini S, Mister M, Aiello S, Cugini D, Scudeletti P, Gagliardini E, Abbate M, Gallon L, Remuzzi G, Noris M: Both darbepoetin alfa and carbamylated erythropoietin prevent kidney graft dysfunction due to ischemia/reperfusion in rats. Transplantation 92: 271–279, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G: Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 34: 61–74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H, Retnakaran R, Woo M: Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 207: 2831–2842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzzocrea S, Mazzon E, di Paola R, Genovese T, Patel NS, Britti D, de Majo M, Caputi AP, Thiemermann C: Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum 52: 940–950, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kaltwasser JP, Kessler U, Gottschalk R, Stucki G, Möller B: Effect of recombinant human erythropoietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. J Rheumatol 28: 2430–2436, 2001 [PubMed] [Google Scholar]
- 34.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A: Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 373: 1532–1542, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Arcasoy MO: Erythropoiesis-stimulating agent use in cancer: Preclinical and clinical perspectives. Clin Cancer Res 14: 4685–4690, 2008 [DOI] [PubMed] [Google Scholar]
- 36.McMahon FG, Vargas R, Ryan M, Jain AK, Abels RI, Perry B, Smith IL: Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood 76: 1718–1722, 1990 [PubMed] [Google Scholar]
- 37.Wang C, Cordoba S, Hu M, Bertolino P, Bowen DG, Sharland AF, Allen RD, Alexander SI, McCaughan GW, Bishop GA: Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol 24: 149–156, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Krutzik P, Trejo A, Schulz K, Nolan G: Phospho flow cytometry methods for the analysis of kinase signaling in cell lines and primary human blood samples. In: Flow Cytometry Protocols, edited by Hawley TS, Hawley RG, Humana Press, New York, 2011, pp 179–202 [DOI] [PubMed] [Google Scholar]
- 39.Hagemeyer N, Boretius S, Ott C, Von Streitberg A, Welpinghus H, Sperling S, Frahm J, Simons M, Ghezzi P, Ehrenreich H: Erythropoietin attenuates neurological and histological consequences of toxic demyelination in mice. Mol Med 18: 628–635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.