Abstract
Vascular calcification (VC) is a life-threatening complication of CKD. Severe protein restriction causes a shortage of essential amino acids, and exacerbates VC in rats. Therefore, we investigated the effects of dietary l-lysine, the first-limiting amino acid of cereal grains, on VC. Male Sprague-Dawley rats at age 13 weeks were divided randomly into four groups: low-protein (LP) diet (group LP), LP diet+adenine (group Ade), LP diet+adenine+glycine (group Gly) as a control amino acid group, and LP diet+adenine+l-lysine·HCl (group Lys). At age 18 weeks, group LP had no VC, whereas groups Ade and Gly had comparable levels of severe VC. l-Lysine supplementation almost completely ameliorated VC. Physical parameters and serum creatinine, urea nitrogen, and phosphate did not differ among groups Ade, Gly, and Lys. Notably, serum calcium in group Lys was slightly but significantly higher than in groups Ade and Gly. Dietary l-lysine strongly suppressed plasma intact parathyroid hormone in adenine rats and supported a proper bone-vascular axis. The conserved orientation of the femoral apatite in group Lys also evidenced the bone-protective effects of l-lysine. Dietary l-lysine elevated plasma alanine, proline, arginine, and homoarginine but not lysine. Analyses in vitro demonstrated that alanine and proline inhibit apoptosis of cultured vascular smooth muscle cells, and that arginine and homoarginine attenuate mineral precipitations in a supersaturated calcium/phosphate solution. In conclusion, dietary supplementation of l-lysine ameliorated VC by modifying key pathways that exacerbate VC.
Medial vascular calcification is common in aging, diabetes, and CKD.1–4 Because the presence of vascular calcification is strongly associated with increased cardiovascular morbidity and mortality, several studies in both animals and humans have sought ways to reduce the extent of vascular calcification.5–10 However, satisfactory therapies have not yet been established.11
Adenine-induced renal failure is one of the commonly used animal models for studying the development of vascular calcification, but the prevalence of vascular calcification in this model is not very high. Indeed, Price et al. reported that vascular calcification was detected in only 30% of rats with adenine-induced chronic renal failure (adenine rats) fed a normal-protein diet.5 These authors speculated that consistent vascular calcification might require a longer period of adenine feeding. On the basis of this idea, they designed a low-protein (LP) diet in an attempt to reduce the nitrogen load and thus enable the rats to thrive on the adenine diet for longer periods. As a result of this attempt, Price et al. unexpectedly found that adenine rats fed a LP diet had extensive vascular calcification without a longer feeding period.5 All 13 adenine rats fed the LP diet had uniform alizarin red staining of the aorta, whereas only 3 of the 11 adenine rats fed a normal-protein diet had partial calcification.5 These findings indicated that dietary protein deficiency correlates with the extent of vascular calcification.
Proteins are usually made from 20 kinds of amino acids. On the basis of nutritional requirements, these amino acids can be divided into two groups: essential amino acids (EAAs) and non-EAAs. Because restriction of dietary protein results in a shortage of EAAs, the level of dietary EAAs may be relevant to the extent of vascular calcification. Among nine EAAs, this study focused on l-lysine (l-Lys) based on the following three reasons. First, l-Lys is the first-limiting amino acid in most cereal grains.12 Second, the safety of l-Lys supplementation has been verified in the area of animal husbandry. l-Lys has long been added to feed grains in order to improve the utility of feed proteins.13 Third, several studies have demonstrated that dietary supplementation with l-Lys protects bones from osteoporosis, a pathologic condition that often coexists with vascular calcification.14,15 These points prompted us to hypothesize that supplementation with l-Lys would ameliorate vascular calcification. Therefore, in this study, we tested this hypothesis using adenine rats.
Results
l-Lys Ameliorated Arterial Calcification in Adenine Rats
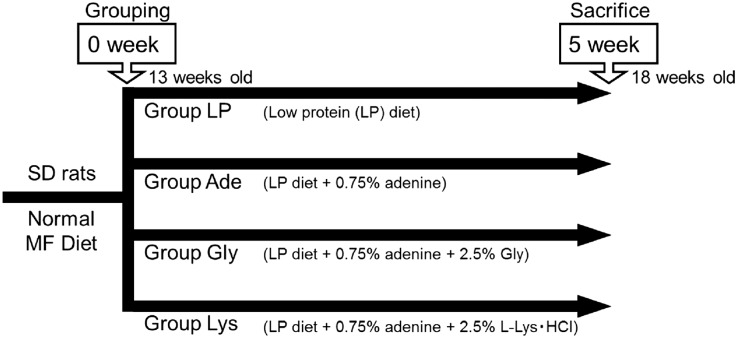
To assess the effects of l-Lys on vascular calcification, we used LP diet–based adenine rats. The rats at age 13 weeks were divided randomly into four groups: LP diet (group LP), LP diet+0.75% adenine (group Ade), LP diet+0.75% adenine+2.5% glycine (group Gly), and LP diet+0.75% adenine+2.5% l-Lys·HCl (group Lys) (Figure 1). Glycine served as an amino acid control in this study because glycine is the amino acid with the simplest structure.
Figure 1.
Experimental design of adenine-induced uremic rats. Twelve-week-old male Sprague-Dawley (SD) rats are maintained under a normal MF diet for one week. At age 13 weeks, the animals are divided randomly into four groups: LP diet (group LP), LP diet+0.75% adenine (group Ade), LP diet+0.75% adenine+2.5% glycine (group Gly), and LP diet+0.75% adenine+2.5% l-Lys·HCl (group Lys). At age 18 weeks, the rats are anesthetized and processed.
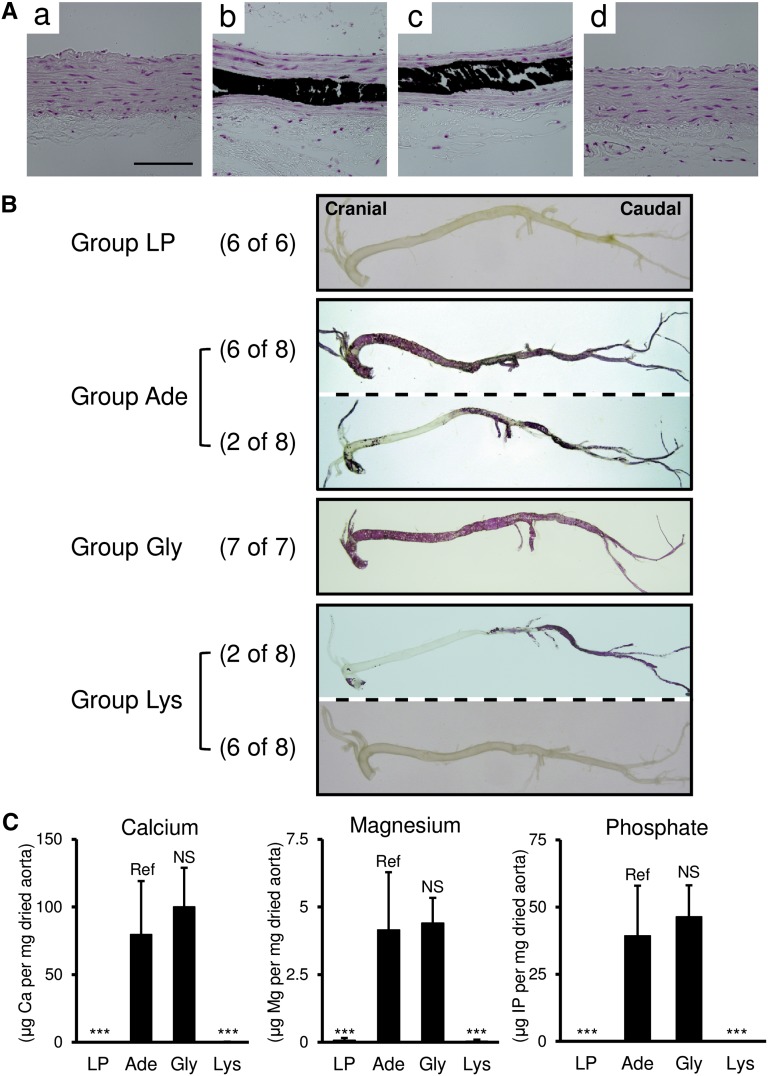
Von Kossa staining of the thoracic aorta revealed that the rats in group LP had no calcification (Figure 2Aa), whereas the rats in groups Ade and Gly had medial calcification (Figure 2, Ab and Ac). Calcification was not observed in adenine rats supplemented with l-Lys (Figure 2Ad). Alizarin red staining of the aorta showed similar results (Figure 2B). We extracted minerals from the aortic tissues and quantified the levels of calcium, magnesium, and phosphate. In accordance with the results of von Kossa and alizarin red staining, the levels of these minerals were elevated in groups Ade and Gly, but not in group Lys (Figure 2C). Analyses of aortic root tissues also confirmed that l-Lys inhibited vascular calcification in adenine rats (Figure 3).The inhibitory effect of l-Lys on ectopic calcification was not limited to vascular calcification. We also found that l-Lys ameliorated nephrocalcinosis in parathyroid hormone (PTH)–treated rats (Supplemental Figure 1).
Figure 2.
l-Lys ameliorates vascular calcification in adenine-induced uremic rats. (A) Calcification of the thoracic aorta is visualized by von Kossa staining. Representative micrographs from group LP (a), group Ade (b), group Gly (c), and group Lys (d) are shown. Group LP has no calcification, whereas groups Ade and Gly show extensive medial calcifications (black area). Group Lys has no von Kossa–positive area. (B) Macroscopically, alizarin red staining shows no vascular calcification in group LP. The majority of group Ade (six of eight animals) and all rats in group Gly have extensive calcification (red area) throughout the vasculature. In group Ade, the calcification of the thoracic aorta is relatively weak in two of eight animals. The majority (six of eight animals) of group Lys has no calcification. Only two of eight animals in group Lys develop partial vascular calcification. (C) In contrast with arteries from group LP, those from groups Ade and Gly contain high levels of calcium, magnesium, and phosphate. Compared with group Ade, arteries from group Lys have significantly low amounts of these minerals. All results are presented as means±SD (n=6–8 in each group). ***P<0.001 (Dunnett’s test). Scale bar, 100 μm.
Figure 3.
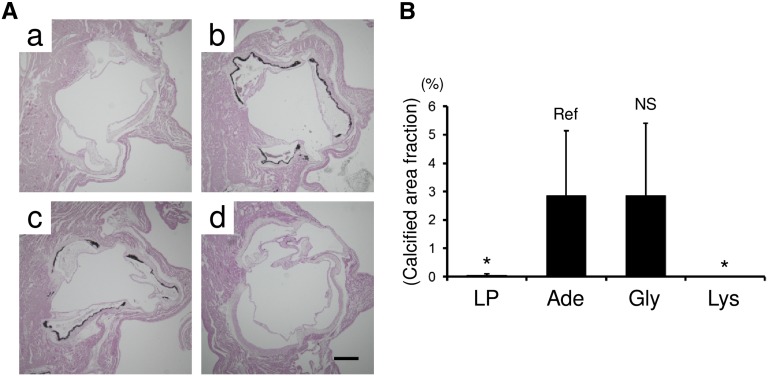
l-Lys ameliorates valvular calcification in adenine-induced uremic rats. (A) Calcified areas of aortic root tissues from group LP (a), group Ade (b), group Gly (c), and group Lys (d) are visualized by von Kossa staining. (B) Calcified area fractions (von Kossa–positive area/cross-sectional area of aortic root) are quantitatively analyzed. All results are presented as means±SD (n=6–7 in each group). *P<0.05 (Dunnett’s test). Scale bar, 500 μm.
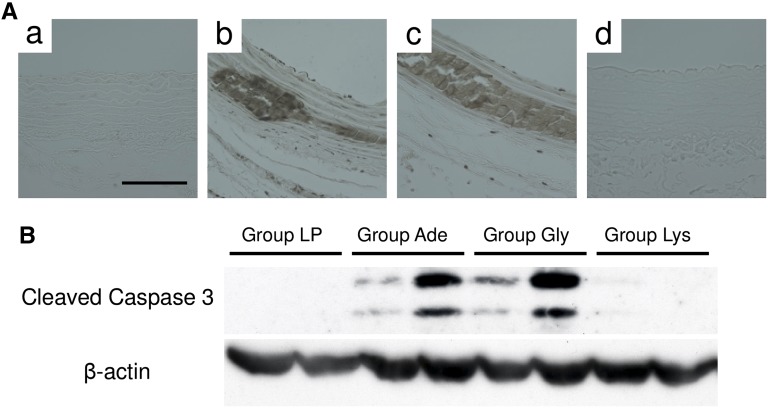
Because apoptosis of vascular smooth muscle cells (VSMCs) is one of the major promoters of vascular calcification, we examined the effects of dietary l-Lys on apoptosis (Figure 4). Both terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining and Western blot analysis demonstrated that uremia induced apoptosis of the thoracic aorta in groups Ade and Gly, but not in group Lys (Figure 4).
Figure 4.
l-Lys suppresses apoptosis of VSMCs in adenine rats. Apoptosis of the aorta is assessed by TUNEL staining (A) and Western blotting (B). (A) Thoracic aortae are TUNEL stained in brown. Representative micrographs from group LP (a), group Ade (b), group Gly (c), and group Lys (d) are shown (n=6–7 in each group). (B) Using anti-cleaved caspase-3 antibody, thoracic aortic tissues are subjected to Western blot analysis. Three independent experiments show similar results. Scale bar, 100 μm.
l-Lys Ameliorated Arterial Calcification without Affecting Renal Function
We measured several parameters to investigate the underlying mechanism of how dietary l-Lys ameliorated arterial calcification in adenine rats (Tables 1–4). Compared with group LP, three adenine-loaded groups (groups Ade, Gly, and Lys) had low levels of food intake, low body weight, and high levels of water intake (Table 1). These parameters were not different among the three adenine-loaded groups (Table 1). Serum creatinine, urea nitrogen, magnesium, and phosphate equally increased, whereas serum pH, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D equally decreased in the three adenine-loaded groups (Table 2). All four groups had similar levels of serum albumin (Table 2). Serum calcium levels in groups Ade and Gly were lower than in group LP. The rats in group Lys had slightly but significantly higher serum calcium levels than in group Ade (Table 2). Reflecting the high serum calcium level, a high urinary calcium/creatinine ratio was observed in group Lys (Table 3). The urinary volume and urinary phosphate/creatinine ratio were equally elevated in the three adenine-loaded groups (Table 3). All four groups had comparable levels of the urinary magnesium/creatinine ratio (Table 3). These results indicate that we need to explore other mechanisms to understand the effects of dietary l-Lys.
Table 1.
Physical parameters of the adenine rats
| Parameter | Adenine Administration Period | |||
|---|---|---|---|---|
| 0 wk | 2 wk | 4 wk | 5 wk | |
| Food intake (g/d) | ||||
| Group LPa | 18.7±2.4 | 27.0±3.0 | 29.0±7.9 | 33.0±7.6 |
| Group Adeb | 19.7±1.4 | 18.3±3.9 | 11.7±3.5 | 11.5±1.0 |
| Group Gly | 21.1±2.5 | 15.4±7.3 | 15.1±8.9 | 10.0±5.5 |
| Group Lys | 22.3±3.4 | 15.3±7.2 | 17.0±9.5 | 14.8±8.1 |
| Water intake (ml/d) | ||||
| Group LPc | 33.0±3.3 | 21.7±1.5 | 18.3±3.9 | 18.3±4.5 |
| Group Adeb | 36.6±6.3 | 52.9±4.9 | 41.4±6.4 | 38.5±14.4 |
| Group Gly | 35.7±5.6 | 46.6±6.7 | 44.6±6.7 | 36.3±4.8 |
| Group Lys | 35.3±3.7 | 57.0±5.6 | 48.7±4.8 | 44.4±15.2 |
| Body weight (g) | ||||
| Group LPa | 396.0±8.4 | 400.0±9.7 | 389.0±18.9 | 388.3±16.7 |
| Group Adeb | 400.3±6.9 | 332.9±10.3 | 289.7±18.5 | 255.5±19.8 |
| Group Gly | 400.0±14.8 | 324.3±16.1 | 283.4±11.9 | 240.3±10.5 |
| Group Lys | 400.7±5.8 | 315.0±11.8 | 279.7±14.2 | 242.8±18.0 |
Physical parameters are summarized. All results are presented as means±SD. Statistical significance was evaluated by multivariate ANOVA (n=4–7 in each group).
P<0.001.
Reference group.
P<0.05.
Table 4.
Plasma amino acid levels of the adenine rats at 5 weeks
| Amino Acid (nmol/ml) | Group LP | Group Adea | Group Gly | Group Lys |
|---|---|---|---|---|
| Glycine (Gly) | 249.0±74.8 | 265.6±83.1 | 400.4±216.5 | 416.0±193.9 |
| Alanine (Ala) | 561.9±68.4b | 346.5±100.1 | 304.6±81.9 | 473.7±126.7c |
| Serine (Ser) | 339.1±49.7b | 118.6±30.0 | 167.0±76.9 | 163.7±52.1 |
| Threonine (Thr) | 31.5±6.0b | 417.6±120.1 | 203.9±80.5b | 268.1±96.9d |
| Valine (Val) | 78.4±10.4 | 86.3±26.5 | 110.3±53.0 | 142.1±155.7 |
| Isoleucine (Ile) | 37.9±5.3 | 40.6±11.3 | 52.0±22.7 | 57.6±56.1 |
| Leucine (Leu) | 77.1±6.8 | 72.4±22.1 | 92.0±42.6 | 111.6±114.5 |
| Lysine (Lys) | 210.0±35.9d | 432.3±110.0 | 405.6±164.1 | 498.6±242.9 |
| Arginine (Arg) | 89.2±18.0 | 91.3±18.9 | 94.2±24.2 | 125.6±28.0d |
| Histidine (His) | 53.2±10.2d | 86.0±12.7 | 92.5±29.7 | 121.8±28.5d |
| Tyrosine (Tyr) | 29.9±8.5 | 29.5±4.3 | 36.8±14.3 | 36.2±17.2 |
| Phenylalanine (Phe) | 28.6±3.4c | 42.3±6.5 | 53.1±19.2 | 45.7±11.0 |
| Tryptophan (Trp) | 47.0±7.9b | 12.5±1.8 | 13.6±4.6 | 12.5±2.9 |
| Methionine (Met) | 23.2±4.0 | 25.2±3.7 | 28.5±9.9 | 28.9±6.4 |
| Cystin (Cys2) | 17.6±4.3 | 25.3±10.2 | 24.9±8.8 | 26.8±5.7 |
| Proline (Pro) | 192.3±29.1b | 120.6±29.8 | 107.8±21.9 | 173.4±43.1d |
| Glutamine (Gln) | 574.0±81.6 | 520.7±107.8 | 522.9±106.3 | 759.1±169.2d |
| Glutamic acid (Glu) | 78.7±19.3d | 45.4±12.8 | 45.4±14.8 | 49.7±16.3 |
| Asparagine (Asn) | 34.2±5.6 | 38.6±6.3 | 36.6±8.2 | 53.7±8.7b |
| Asparatic acid (Asp) | 6.9±1.9 | 7.3±1.8 | 7.6±2.7 | 6.1±2.1 |
| α-Aminoadipic acid | 1.2±0.5 | 2.3±0.4 | 2.2±0.5 | 4.1±2.5c |
| Homoarginine (Homo-Arg) | 3.2±0.5 | 4.4±0.7 | 3.8±1.0 | 6.5±3.0c |
| α-aminobutyric acid | 3.0±0.5 | 3.5±1.4 | 2.5±0.7 | 4.7±2.3 |
| β-aminoisobutyric acid | Not detectable | Not detectable | Not detectable | 0.1±0.3 |
| γ-aminobutyric acid | Not detectable | Not detectable | Not detectable | Not detectable |
Plasma amino acid levels are summarized. All results are presented as means±SD. Statistical significance was evaluated by Dunnett’s test (n=10 in each group).
Reference group.
P<0.001.
P<0.05.
P<0.01.
Table 2.
Serum parameters of the adenine rats at 5 weeks
| Parameter | Group LP | Group Adea | Group Gly | Group Lys |
|---|---|---|---|---|
| Creatinine (mg/100 ml) | 0.51±0.13b | 6.32±0.95 | 5.76±0.92 | 5.37±1.53 |
| Urea nitrogen (mg/100 ml) | 6.64±3.66b | 180.9±49.1 | 220.8±64.4 | 129.7±71.6 |
| pH | 7.65±0.04b | 7.23±0.12 | 7.22±0.14 | 7.19±0.12 |
| Albumin (g/100 ml) | 3.14±0.31 | 3.03±0.21 | 3.00±0.28 | 3.35±0.44 |
| Calcium (mg/100 ml) | 9.65±0.35b | 8.60±0.40 | 8.26±0.56 | 10.08±0.34b |
| Magnesium (mg/100 ml) | 2.42±0.40b | 4.31±0.81 | 3.99±0.41 | 4.12±0.34 |
| Phosphate (mg/100 ml) | 6.89±3.04b | 32.19±4.35 | 36.63±8.02 | 26.33±6.20 |
| 25-hydroxyvitamin D (ng/ml) | 37.81±8.73b | 23.19±4.87 | 22.13±3.04 | 20.26±3.01 |
| 1,25-dihydroxyvitamin D (pg/ml) | 103.97±33.79b | 5.03±0.90 | 5.02±0.73 | 5.13±0.98 |
Serum parameters are summarized. All results are presented as means±SD. Statistical significance was evaluated by Dunnett’s test. n=10 in each group for creatinine, urea nitrogen, pH, albumin, calcium, magnesium, and phosphate. n=9 in each group for 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D.
Reference group.
P<0.001.
Table 3.
Urinary parameters of the adenine rats
| Parameter | Adenine Administration Period | |||
|---|---|---|---|---|
| 0 wk | 2 wk | 4 wk | 5 wk | |
| Urinary volume (ml/6 h) | ||||
| Group LPa | 5.83±1.79 | 2.37±1.44 | 1.95±1.20 | 2.31±2.12 |
| Group Adeb | 5.59±2.49 | 7.65±3.16 | 6.71±2.28 | 4.50±4.80 |
| Group Gly | 3.27±1.53 | 5.99±1.90 | 5.04±2.21 | 3.37±2.82 |
| Group Lys | 4.09±1.99 | 5.85±2.68 | 6.42±2.59 | 3.01±2.95 |
| Calcium/creatinine | ||||
| Group LP | 0.141±0.090 | 0.041±0.021 | 0.098±0.067 | 0.085±0.033 |
| Group Adeb | 0.146±0.070 | 0.018±0.012 | 0.123±0.073 | 0.224±0.037 |
| Group Gly | 0.164±0.060 | 0.012±0.011 | 0.138±0.055 | 0.306±0.111 |
| Group Lysc | 0.176±0.078 | 0.064±0.043 | 0.274±0.084 | 0.490±0.155 |
| Magnesium/creatinine | ||||
| Group LP | 0.534±0.251 | 0.123±0.042 | 0.130±0.064 | 0.212±0.125 |
| Group Adeb | 0.533±0.225 | 0.396±0.099 | 0.351±0.108 | 0.453±0.135 |
| Group Gly | 0.448±0.115 | 0.260±0.065 | 0.399±0.093 | 0.203±0.170 |
| Group Lys | 0.369±0.172 | 0.282±0.082 | 0.363±0.060 | 0.152±0.137 |
| Phosphate/creatinine | ||||
| Group LPa | 0.098±0.072 | 2.044±1.112 | 2.503±0.686 | 2.338±1.076 |
| Group Adeb | 0.399±0.486 | 2.117±0.340 | 2.930±0.526 | 4.474±0.868 |
| Group Gly | 0.405±0.403 | 1.889±0.459 | 2.760±0.306 | 4.450±0.958 |
| Group Lys | 0.334±0.383 | 2.185±0.415 | 2.775±0.531 | 4.142±0.548 |
Urinary parameters are summarized. All results are presented as means±SD. Statistical significance was evaluated by multivariate ANOVA (n=4–7 in each group).
P<0.05.
Reference group.
P<0.01.
l-Ly Protected the Femora from Osteoporotic Changes in Adenine Rats
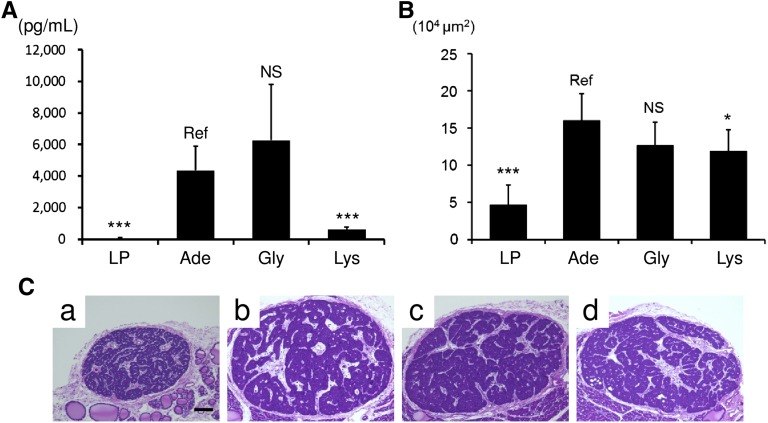
We found that the rats in group Lys had strongly suppressed plasma intact PTH (iPTH) (Figure 5A). The strong suppression seemed to depend on the elevated serum calcium in group Lys (Table 2) because the reduction of maximum cross-sectional area of the parathyroid glands in group Lys was minor (Figure 5, B and C).
Figure 5.
Dietary l-Lys suppresses plasma iPTH level in adenine rats. (A) The plasma iPTH level is determined by ELISA. Compared with group LP, groups Ade and Gly have elevated plasma iPTH. l-Lys supplementation strongly suppresses plasma iPTH. Results are presented as means±SD (n=10 in each group). (B) Maximum cross-sectional area of the parathyroid glands is assessed using hematoxylin and eosin–stained sections. Results are presented as means±SD (n=5–13 in each group). (C) Representative hematoxylin and eosin–stained micrographs of parathyroid glands from group LP (a), group Ade (b), group Gly (c), and group Lys (d) are shown. *P<0.05; ***P<0.001 (Dunnett’s test). Scale bar, 100 μm.
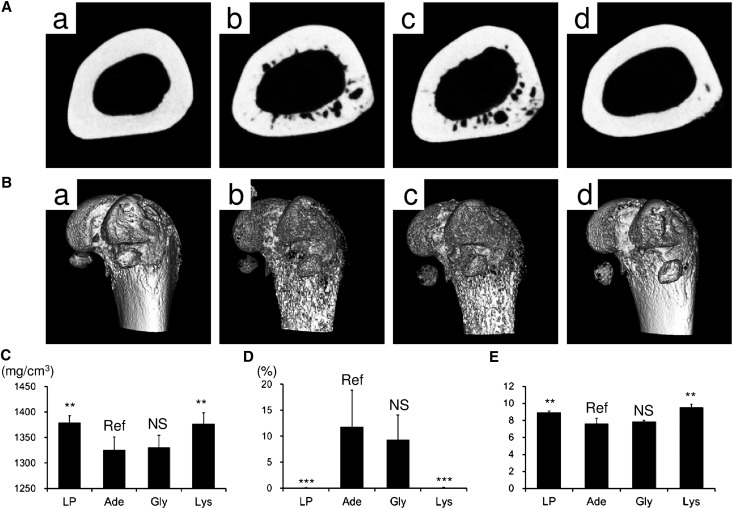
Although hyperparathyroidism often correlates with the presence of vascular calcification, PTH itself cannot directly induce calcification in cultured VSMCs or in cultured aortic rings.16 The causal relationship between hyperparathyroidism and vascular calcification depends, at least in part, on the bone-vascular axis.17 Therefore, we examined the effects of l-Lys on the femora. Both transverse sections of the femoral diaphyses (Figure 6A) and three-dimensional reconstructed images of the distal femora (Figure 6B) demonstrated that the rats in group LP had intact femora, whereas the rats in groups Ade and Gly had osteoporotic femora. Dietary supplementation with l-Lys suppressed these osteoporotic changes (Figure 6). Three vital predictors of bone strength—bone mineral density (Figure 6C), porosity (Figure 6D), and the orientation of biologic apatite (Figure 6E)—in group Lys were identical to those in group LP, representing the bone-protective effects of dietary l-Lys.18–21
Figure 6.
l-Lys protects the femora from osteoporotic changes. Bone morphology is assessed by micro x-ray computed tomography. Transverse sections of the femoral diaphyses (A) and three-dimensional reconstructed images of the distal femora (B) from group LP (a), group Ade (b), group Gly (c), and group Lys (d) are shown. In addition to these morphologic changes, bone mineral density (C), porosity (D), and orientation of the biologic apatite (E) demonstrate the bone-protective effects of l-Lys (n=5 in each group in A and B and n=6–7 in each group in C–E). **P<0.01; *** P<0.001 (Dunnett’s test).
Dietary Supplementation with l-Lys in Adenine Rats Did Not Elevate Plasma Lys Level
Using liquid chromatography–electrospray ionization–tandem mass spectrometry, we assessed plasma amino acid levels (Table 4).22 All four groups had comparable levels of plasma Gly, Val, Ile, Leu, Tyr, Met, Cys2, Asp, and α-aminobutyric acid. Both β-aminoisobutyric acid and γ-aminobutyric acid were almost undetectable in all groups. The three adenine-loaded groups had equally elevated plasma Lys, Phe, and equally suppressed Ser, Trp, and Glu. Plasma Thr and the His levels did not correlate with the presence of arterial calcification. Elevated plasma α-aminoadipic acid and homoarginine, two metabolites of l-Lys, in group Lys indicated that the rats in group Lys had increased intestinal absorption of l-Lys.23–25 However, the elevation of plasma Lys in group Lys was not statistically significant. As far as we examined, plasma Ala, Pro, Arg, Gln, Asn, homoarginine, and α-aminoadipic acid in group Lys seemed to play some roles in the suppression of vascular calcification. Dietary l-Lys–dependent elevation of plasma Ala, Pro, Arg, Gln, Asn, homoarginine, and α-aminoadipic acid was not limited to the condition of CKD. Compared with the rats in group LP (Table 4), rats fed a LP diet containing 2.5% l-Lys·HCl without 0.75% adenine had elevated plasma Ala, Pro, Arg, Gln, Asn, homoarginine, and α-aminoadipic acid (Supplemental Table 1).
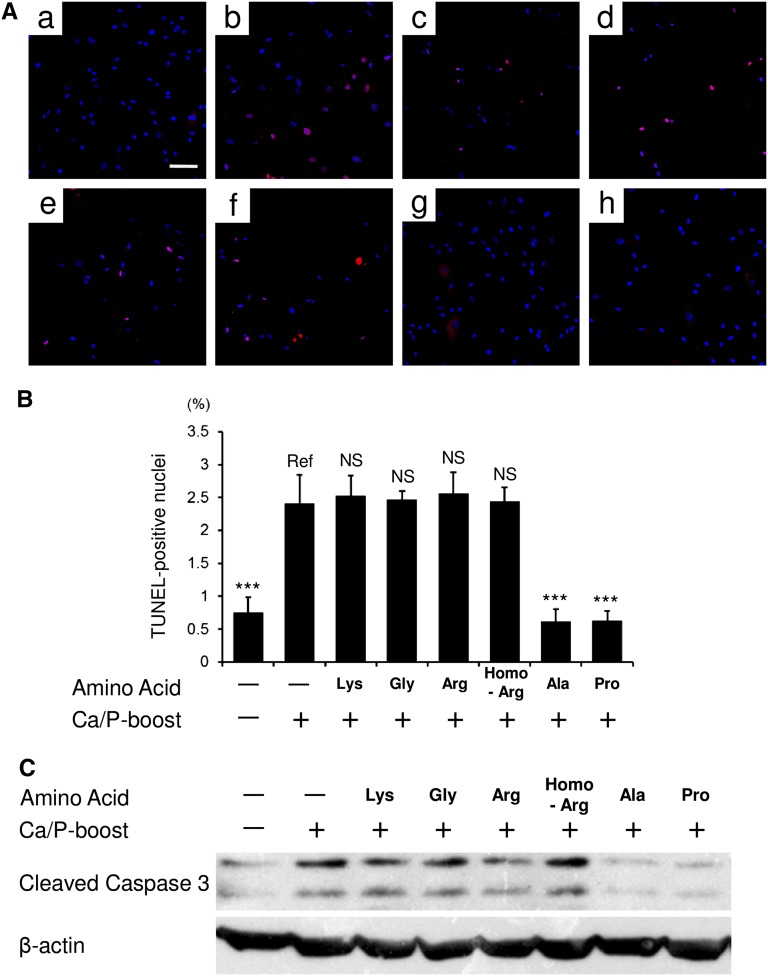
l-Ala and l-Pro Inhibited Apoptosis of Cultured Human VSMCs
Using the hydrophilic amino acids among Ala, Pro, Arg, Gln, Asn, homoarginine, and α-aminoadipic acid (i.e., Ala, Pro, Arg, and homoarginine), we further analyzed the underlying mechanism. Both passive and active pathways play roles in the pathogenesis of vascular calcification.26,27 Among several active pathways, apoptosis of VSMCs is one of the key phenomena that promote vascular calcification.28–30 Therefore, using cultured human VSMCs (hVSMCs), we examined the effects of l-Ala, l-Pro, l-Arg, and l-homoarginine on apoptosis. As shown in Figure 7, calcium/phosphate-boosted DMEM induced cell apoptosis. Both TUNEL staining (Figure 7, A and B) and Western blotting (Figure 7C) revealed that l-Ala and l-Pro, but not l-Lys, Gly, l-Arg, or l-homoarginine, inhibited the apoptosis. These results indicated that elevated plasma Ala and Pro in group Lys (Table 4) contributed, at least in part, to the inhibition of aortic apoptosis (Figure 4).
Figure 7.
l-Ala and l-Pro suppress apoptosis of cultured hVSMCs. Apoptosis of hVSMCs is analyzed. Apoptosis is induced by calcium/phosphate (Ca/P)-boosted medium in the absence or presence of amino acid supplementation. (A) TUNEL-positive nuclei are stained in red. All nuclei are counterstained in blue. Representative micrographs are shown as follows: (a) negative control [Ca/P-boost (−), amino acid supplementation (−)], (b) positive control [Ca/P-boost (+), amino acid supplementation (−)], and (c–h) Ca/P-stimulated hVSMCs treated with l-Lys (c), Gly (d), l-Arg (e), l-homoarginine (l-Homo-Arg) (f), l-Ala (g), or l-Pro (h). (B) TUNEL-positive nuclei are quantified using the In Cell Analyzer 6000. (C) Western blot analysis for cleaved caspase-3 is shown. l-Ala and l-Pro, but not l-Lys, Gly, l-Arg, or l-Homo-Arg inhibit cell apoptosis. All results are presented as means±SD (n=8 in each group). ***P<0.001 (Dunnett’s test). Scale bar, 100 μm.
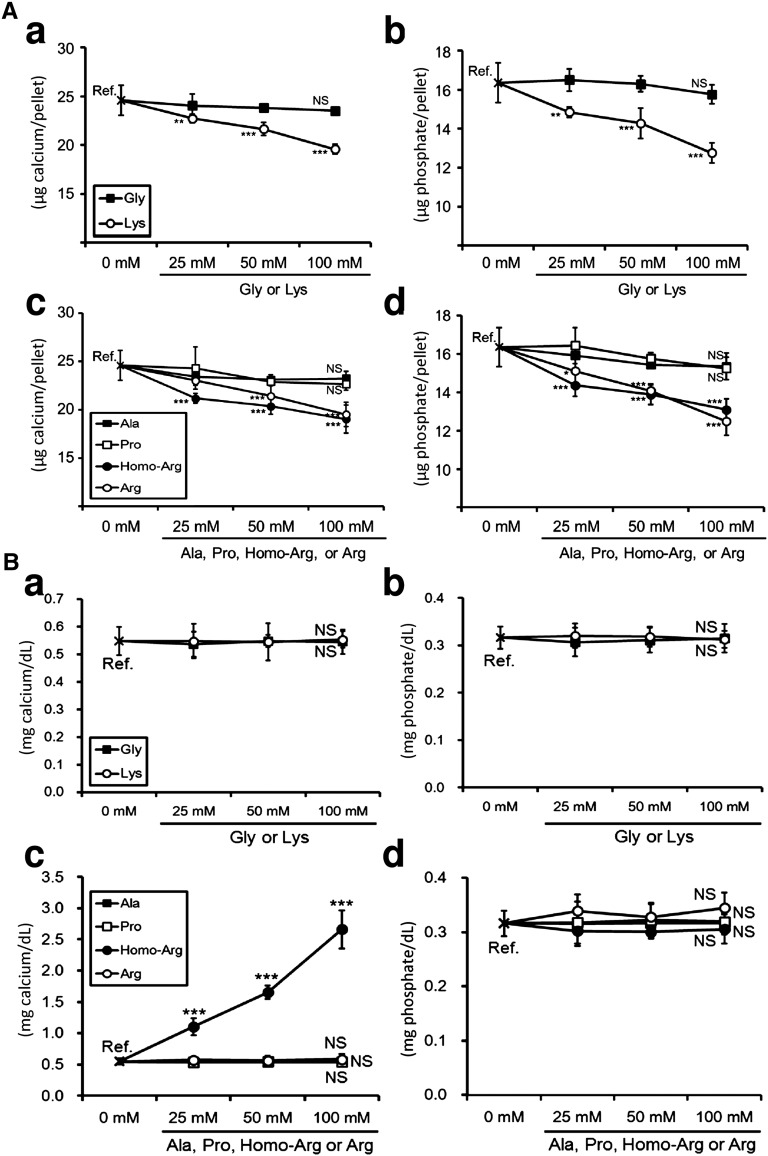
l-Lys, l-Arg, and l-Homoarginine Inhibited the Precipitation of Minerals in a Solution of Supersaturated Calcium/Phosphate
Finally, we examined the effects of amino acids on the passive pathway of vascular calcification. We used HEPES-buffered amino acid solutions (pH 7.4) supersaturated with calcium/phosphate for the analyses of mineral precipitations. Because the solubility product of hydroxyapatite is extremely low, the mixed calcium/phosphate solutions immediately developed mineral precipitates (data not shown). The quantification of calcium and phosphate in the resultant precipitates showed that l-Lys, l-Arg, and l-homoarginine, but not Gly, l-Ala, or l-Pro, attenuated the mineral precipitations (Figure 8A). These findings indicated that l-Arg and l-homoarginine, two amino acids elevated in the plasma of group Lys (Table 4), inhibited the passive pathway of vascular calcification. The importance of l-Arg in the suppression of arterial calcification was confirmed in adenine rats supplemented with dietary 2.5% l-Arg·HCl (Supplemental Figure 2).
Figure 8.
l-Lys, l-Arg, and l-homoarginine (l-Homo-Arg) inhibit the precipitation of minerals in a solution of supersaturated calcium/phosphate. (A) HEPES-buffered calcium/phosphate solution (pH 7.4) is incubated at room temperature for 10 minutes in the absence or presence of amino acids. All mixtures contain 10 mM calcium and 10 mM phosphate. After incubation, the precipitates are collected by centrifugation at 1890×g for 10 seconds. Calcium (a and c) and phosphate (b and d) levels in the resultant pellet are examined. l-Lys, l-Arg, and l-Homo-Arg inhibit mineral precipitation in a dose-dependent manner (n=5 in each point). (B) HEPES-buffered hydroxyapatite mixture (2.0 mM) is incubated at 37°C for 12 hours in the absence or presence of amino acids. After incubation, the mixtures are centrifuged at 1890×g for 10 seconds. The resultant supernatants are subjected to calcium (a and c) or phosphate (b and d) quantification. (c) Contamination with calcium in purchased l-Homo-Arg causes calcium elevation in the supernatant. (a–d) All amino acids do not dissolve hydroxyapatite (n=5 in each point). *P<0.05; **P<0.01; ***P<0.001 (Dunnett’s test).
We also assessed the effects of these six amino acids on the dissolution of hydroxyapatite (Figure 8B). HEPES-buffered hydroxyapatite mixtures were incubated in the presence or absence of amino acids for 12 hours. The resultant supernatants were subjected to calcium/phosphate quantification. Figure 8B shows that Gly, l-Lys, l-Ala, l-Pro, and l-Arg did not dissolve hydroxyapatite. Although l-homoarginine increased the supernatant calcium levels in a dose-dependent manner, it seemed that calcium contamination in purchased l-homoarginine caused the elevation (Figure 8Bc). Indeed, calcium quantification of purchased l-homoarginine by both the methylxylenol blue method and atomic absorption spectroscopy indicated that the reagent had calcium contamination (data not shown). The constant phosphate levels in the supernatant confirmed that l-homoarginine did not dissolve hydroxyapatite (Figure 8Bd). l-Arg and l-homoarginine inhibited the precipitation of minerals, but they did not dissolve hydroxyapatite.
Discussion
In this study, we investigated the effects of dietary l-Lys on vascular calcification. Our results clearly demonstrated that dietary l-Lys ameliorates vascular calcification by modifying several key pathways.
Three observations in our study suggested that dietary l-Lys increased intestinal absorption of calcium. First, three adenine-loaded groups had similar levels of food intake (Table 1). Second, group Lys had an increased urinary calcium/creatinine ratio (Table 3), in addition to elevated serum calcium (Table 2). Third, l-Lys protected the bones from adenine-induced osteoporotic changes (Figure 6). Indeed, using orally administered radioactive calcium as a tracer, Comar et al. reported that dietary supplementation of l-Lys facilitates intestinal absorption of calcium.31,32 Because intraperitoneally injected l-Lys does not facilitate calcium absorption,32 it is likely that dietary l-Lys works within the gastrointestinal tract. Our results in Figure 8A showed that l-Lys elevated the solubility of calcium. This phenomenon seems to explain the reason, at least in part, why dietary l-Lys facilitates intestinal absorption of calcium.
Osteoporosis is one of the serious pathologic conditions associated with vascular calcification. In addition to morphologic changes, this study revealed that the orientation of biologic apatite in the femora was degraded in groups Ade and Gly (Figure 6E). Dietary l-Lys almost completely prevented these changes (Figure 6E). Because the orientation of biologic apatite is the key determinant of the bone intrinsic material property, a bone possessing a more elevated degree of apatite orientation shows a higher Young’s modulus in the oriented direction.19 Therefore, all of our data in Figure 6 confirm the bone-protective effects of l-Lys. Dietary l-Lys plays a vital role in bone microstructure and mechanical function.
In accordance with some previous reports, we demonstrated that both l-Ala and l-Pro inhibit Ca/P-induced apoptosis in cultured hVSMCs (Figure 7).33,34 Therefore, elevated plasma Ala and Pro in group Lys seemed to be responsible, at least in part, for the protection of VSMCs from apoptotic death (Figure 4, Table 4). Several preceding studies revealed the underlying mechanisms of how l-Ala and l-Pro inhibit apoptosis. Grosser et al. demonstrated that l-Ala protects cultured human endothelial cells from hydrogen peroxide–mediated cytotoxicity by inducing heme oxygenase and ferritin proteins.35 Furthermore, Krishnan et al. demonstrated that l-Pro modulates the intracellular redox status and protects cells from H2O2 stress.36 These molecular mechanisms might also be responsible for the protection of VSMCs under Ca/P stimuli (Figure 7) and adenine-induced uremia (Figure 4).
Hydroxyapatite is one of the main components of calcified aortic tissues.37 Because of its hexagonal crystallographic structure, hydroxyapatite has two crystal planes: a-planes and c-planes.18,38,39 The a-planes are rich in calcium ions and are positively charged, whereas the c-planes are rich in phosphate ions and are negatively charged.40,41 This property of hydroxyapatite affects the growth of hydroxyapatite itself. Using self-assembled monolayers that have CH3, PO4H2, COOH, CONH2, OH, or NH2 terminal functional groups, Tanahashi et al. revealed that the negatively charged groups strongly promote hydroxyapatite formation.42 The results of Tanahashi et al. suggest that the electrical adsorption of calcium ions on the negatively charged surface, but not the adsorption of phosphate ions on the positively charged surface, promotes hydroxyapatite formation.42 Indeed, Tanase et al. demonstrated that the growth of hydroxyapatite on the c-plane of crystallographically aligned hydroxyapatite is faster than that on the a-plane.43 In this study, we revealed that l-Lys, l-Arg, and l-homoarginine, but not Gly, l-Ala, or l-Pro, attenuated mineral precipitations in supersaturated calcium/phosphate solutions (Figure 8A). As shown in Supplemental Figure 3, the precipitate-inhibitory amino acids have two amino groups (−NH2) and are positively charged under physiologic pH. Positively charged amino acids might interfere with the electrical interaction between calcium and a negatively charged surface, although this is not experimentally confirmed in this study.
Homoarginine has caught researchers’ attention in recent years because results of two clinical studies revealed that low serum homoarginine, a metabolite of l-Lys, is independently associated with cardiovascular mortality and fatal strokes.23,44 The causal relationship between low homoarginine and an increased incidence of cardiovascular events has been mainly attributed to suppressed nitric oxide (NO), because homoarginine induces NO by two pathways: (1) homoarginine itself serves as a substrate for NO synthase; and (2) homoarginine inhibits arginase and thereby increases arginine, a major substrate for NO synthase.23 In addition to the property of homoarginine as an inducer of NO, our current study revealed that homoarginine works as an inhibitor of mineral precipitations (Figure 8A). The property of homoarginine as an inhibitor of arginase seems to explain the reason, at least in part, why plasma Arg, another precipitate-inhibitory amino acid, was elevated in group Lys (Figure 8A, Table 4).23 Therefore, homoarginine is likely to have both direct and indirect precipitate-inhibitory effects in vivo. Because vascular calcification has been revealed to be associated with an increased incidence of cardiovascular events,1–4 the property of homoarginine as a precipitate-inhibitor seems to play important roles.
Dietary supplementation of l-Lys in adenine rats elevated plasma Ala, Pro, Arg, and homoarginine, but not Lys itself (Table 4). The putative underlying metabolic pathways that caused these changes in group Lys are summarized in Supplemental Figure 4. First, the statistically unchanged plasma Lys level in group Lys might be caused by an induction of catabolic enzymes for l-Lys due to prolonged dietary supplementation of l-Lys. Indeed, the elevation of plasma α-aminoadipic acid and homoarginine, two metabolites of l-Lys, in group Lys indicated that the rats in group Lys more rapidly catabolized l-Lys than the rats in group Ade. In contrast with the rats in group Lys (Table 4), however, the rats fed the LP diet containing 2.5% l-Lys·HCl without 0.75% adenine (Supplemental Table 1) had elevated plasma Lys. Because the adenine rats ate a lower amount of the diet (Table 1), a balance between intestinal absorption and the catabolism of l-Lys seems to determine plasma Lys levels. Second, an induction of amino acid transporters, such as b0,+-AT and y+LAT-1 systems, in the intestine might increase intestinal absorption of Arg and His in group Lys. He et al. recently reported that dietary supplementation of l-Lys upregulates jejunal b0,+-AT and y+LAT-1 in piglets.45 Absorbed l-Arg and l-His can be converted to l-Pro, l-Gln, and/or l-Ala through the pathway shown in Supplemental Figure 4. The presence of elevated plasma ornithine, a metabolite of l-Arg, in group Lys supports the idea that the metabolic flow from l-Arg to l-Pro, l-Gln, and/or l-Ala was very high in group Lys (Supplemental Table 2). Third, skeletal muscle of group Lys might serve as a source of plasma Gln and Ala. l-Gln and l-Ala are two major amino acids supplied from skeletal muscles.46 Although we did not directly evaluate the effect of dietary l-Lys on skeletal muscle in adenine rats, the observed suppression of plasma 3-methylhistidine, an index of the muscular breakdown rate, in group Lys suggested that the rats in group Lys were protected from muscular degradation (Supplemental Table 2).47 Our findings agreed with those of a study of Ishida et al., in which they demonstrated that dietary l-Lys downregulates E3 ubiquitin ligase atrogin-1 (also known as muscle atrophy F-box), a key enzyme for muscle degradation.48 Fourth, the similar plasma Glu levels among the three adenine-loaded groups might be attributed to proximal tubular damage by adenine. The proximal tubule is the major expression site of glutaminase, a key enzyme that generates Glu+NH4+ from Gln.49 Because the proximal tubules were severely damaged, the rats in group Lys might have been unable to convert Gln to Glu+NH4+, even under the high Gln condition. The rats fed the LP diet containing l-Lys without adenine showed an elevation in Glu, which is in sharp contrast with the rats with adenine. This observation supports the idea, at least in part, that proximal tubular glutaminase contributed to the elevation of plasma Glu (Supplemental Table 1).
Although we have demonstrated in this study that dietary l-Lys prevents arteries from calcification in adenine-induced uremic rats, the therapeutic potential of dietary l-Lys for treating preexisting vascular calcification remains obscure. Further studies are required to address this issue.
In conclusion, dietary supplementation with l-Lys prevented arteries from calcification by modifying several key pathways via the following mechanisms: (1) dietary l-Lys strongly suppresses plasma iPTH; (2) dietary l-Lys supports a proper bone-vascular axis; (3) dietary l-Lys elevates plasma alanine and proline, thereby inhibiting apoptosis of VSMCs; and (4) dietary l-Lys elevates plasma arginine and homoarginine, thereby inhibiting mineral precipitation. Our findings provide a novel preventive approach for managing vascular calcification.
Concise Methods
Animals
Twelve-week-old male Sprague-Dawley rats were purchased from Japan SLC (Hamamatsu, Japan) and were fed a normal MF diet (Oriental Yeast, Tokyo, Japan) for 1 week. At age 13 weeks, the animals were divided randomly into four groups (Figure 1). Glycine served as an amino acid control in this study because it is the amino acid that has the simplest structure. Adenine rats fed a LP diet containing 2.5% l-Arg·HCl were prepared in a similar way (Supplemental Figure 2). Another group of rats were fed a LP diet with 2.5% l-Lys·HCl but without 0.75% adenine; this group was prepared for the purpose of testing whether the changes in plasma amino acid levels (Table 4) were unique to CKD (Supplemental Table 1). Based on the composition of the TD05030 diet (Harlan Teklad, Madison, WI), a LP diet containing 2.5% protein, 1.062% calcium, and 0.923% phosphorus was prepared by CLEA Japan (Tokyo, Japan). Adenine, glycine, l-Lys·HCl, and l-Arg·HCl were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Nephrocalcinosis in 6-week-old male Wistar rats was induced by continuous injection of rat PTH 1-34 (Bachem, Bubendorf, Switzerland) at a dosage of 40 μg/kg per day via an osmotic mini-pump (Alzet, Cupertino, CA) for 50 hours.50 l-Lys·HCl or glycine at a dose of 20 mmol/kg was administered via a gastric tube at 2 hours, 14 hours, 26 hours, and 38 hours after the implantation of the osmotic pump.
At the indicated periods, the rats were processed in similar ways, as previously described.5,50,51 All rats were handled in a humane manner in accordance with the guidelines of the Animal Committee of Osaka University.
Biochemical Parameters
Serum and urinary biochemical parameters, urea nitrogen, albumin, calcium, inorganic phosphate, and magnesium were determined by clinical diagnostic reagents (Wako Pure Chemical Industries; and Kainos Laboratories, Inc., Tokyo, Japan). Serum creatinine and plasma iPTH were measured by Kainos CRE reagent and the Rat BioActive Intact PTH ELISA Kit (Immutopics, Inc., San Clemente, CA), respectively. Serum 1,25(OH)2D levels were determined by RIA (SRL, Inc., Tokyo, Japan). Serum samples were sent to Kyowa Medex Inc. (Shizuoka, Japan) for the serum 25(OH)D assay using the DiaSorin LIAISON 25-hydroxy OH vitamin D TOTAL Assay (DiaSorin, Inc., Stillwater, MN).52 Plasma amino acid levels were evaluated by liquid chromatography–electrospray ionization–tandem mass spectrometry.22 To investigate mineral accumulation levels in the aorta, the appropriate alizarin red–stained tissues were minced and freeze-dried. After weighing the dried aorta, the minerals were extracted with 150 mM HCl overnight at room temperature.
Histologic Analyses
The thoracic aorta, abdominal aorta, and femoral arteries from each rat were dissected as a unit, stained with Alizarin Red S (Sigma-Aldrich, St. Louis, MO), and photographed. Histologic analyses by von Kossa staining and TUNEL staining (In Situ Apoptosis Detection Kit; Takara Bio, Shiga, Japan) were performed using 4% paraformaldehyde-fixed aortic valve and thoracic aorta sections.
Bone Analyses
Morphometric analyses of isolated femoral midshafts were performed using micro x-ray computed tomography (R_mCT2; Rigaku, Tokyo, Japan). The settings of the R_mCT2 were as follows: The unit operated with x-ray power at 90 kV and 160 μA with an exposure time of 3 minutes. Three-dimensional microstructural images were visualized using TRI/3D-BON software (RATOC, Tokyo, Japan).
Bone mineral density of the femoral midshafts was measured using peripheral quantitative computed tomography (XCT Research SA+; Stratec Medizintechnik, Birkenfeld, Germany) with a resolution of 80×80×460 μm. Bone tissue was judged over a threshold value of 690 mg/cm3. The bone mineral density in the femoral midshaft cross-sections was averaged.
The crystallographic orientation of the biologic apatite c axis was analyzed by conducting microbeam x-ray diffraction (R-Axis BQ; Rigaku) with a transmission optical system. The apatite orientation is a valuable index for the analysis of bone microstructure (which largely depends on the bone type, such as long bones, skull, and mandible), prediction of bone mechanical function, evaluation of regenerative and pathologic conditions, and validation of administration of antiosteoporotic agents.18–20,53,54 Molybdenum-Kα radiation was generated at 50 kV and 90 mA. The whole femur was vertically mounted on the specimen holder, and the incident beam was collimated into an 800-μm circular spot by a double-pinhole metal collimator. The incident x-ray radiated vertically onto the specimen in the anteroposterior axis, and diffracted x-rays were collected by an imaging plate (FujiFilm, Tokyo, Japan) placed behind the specimen for 300 seconds. From the Debye ring obtained, the degree of biologic apatite orientation was quantitatively analyzed as the intensity ratio of (002) diffraction peak to (310) peak along the femur long axis.21 The (002) crystal plane is the representative plane of the biologic apatite c axis, and the (310) plane is orthogonal to the (002) plane.
Calcium-Phosphate Precipitation Assays
The formation of calcium-phosphate precipitates in vitro was assessed in HEPES-buffered-glycine, -l-Lys, -l-Arg, -l-homoarginine, -l-Ala, and -l-Pro solution. All amino acids were purchased from Wako Pure Chemical Industries. Amino acid solutions at desired concentrations were obtained by adding amino acid stock solutions (pH 7.4) to 500 mM HEPES buffer (pH 7.4). To increase the concentrations of calcium and phosphate, 0.4 M CaCl2 stock solution was added to an aliquot of HEPES-buffered amino acid solution to achieve twice the desired final levels, and 0.4 M sodium phosphate (pH 7.4) stock solution was added to a second aliquot of HEPES-buffered amino acid solution to achieve twice the desired final levels. Fifty microliters of calcium-boosted amino acid solution was then expelled rapidly into 50 μl of phosphate-boosted amino acid solution, and vortexed. After 10 minutes of incubation at room temperature, the mixed solutions were centrifuged at 1890×g for 10 seconds. The resultant pellets were dissolved in 150 mM HCl for the analyses of calcium and phosphate.
Hydroxyapatite Dissolution Assays
The solubility of hydroxyapatite (Sigma-Aldrich) was assessed in HEPES-buffered amino acid solutions. Amino acid solutions at desired concentrations were obtained by adding amino acid stock solutions (pH 7.4) to 500 mM HEPES buffer (pH 7.4). We added hydroxyapatite to an aliquot of HEPES-buffered amino acid solution to achieve the desired final levels. After 12 hours of rotation at 37°C, the incubated mixtures were centrifuged at 1890×g for 10 seconds. The resultant supernatants were subjected to calcium/phosphate quantification.
Cell Culture
hVSMCs were maintained in DMEM supplemented with 10% FCS. For the evaluation of cell apoptosis, hVSMCs at 90% confluence were stimulated with calcium/phosphate-boosted (final calcium 2.7 mM and phosphate 2.0 mM) DMEM supplemented with 0.5% BSA. Amino acid stock solution (pH 7.4) was added to the calcium-boosted medium to elevate each amino acid concentration by 2.0 mM. After incubation for 24 hours, samples were subjected to Western blot and TUNEL analyses. TUNEL-stained cells were analyzed using the In Cell Analyzer 6000 (GE Healthcare Bio-Sciences, Piscataway, NJ).
Western Blot Analyses
Antibodies against specific molecules were obtained as follows: cleaved caspase-3 (no. 9664; Cell Signaling Technology, Danvers, MA) and β-actin (A5441; Sigma-Aldrich). The dilution rates of the primary antibodies were 1:10,000 for β-actin and 1:1000 for cleaved caspase-3. Western blot analyses were carried out in similar ways, as previously described.55
Statistical Analyses
Statistical significance among multiple experimental values was evaluated by Dunnett’s test. To analyze the time course of serum and urine parameters with respect to treatment assignment, changes in these parameters over time were analyzed with linear mixed models for repeated measures with random intercept and slope. This method was applied to take into account the correlation between repeated measurements within the same rat. The multivariate models contained the treatment group as well as the number of measurements (time) as independent variables. The group difference was investigated by adding interaction terms between the exposures (groups) and time. All results are presented as means±SD. Statistical significance was defined as P<0.05. All data were statistically analyzed using JMP Pro 10.0.2 for Windows (SAS Institute, Cary, NC) or STATA/SE 11.0 for Windows (StataCorp LP, College Station, TX).
Disclosures
K.T. is an employee of Ajinomoto Pharmaceuticals Co. Ltd. Y.K. is an employee of Ajinomoto Co. Inc. T.H. and Y.Ts. are members of a department that has received donations from Chugai Pharmaceutical Co. Ltd.
Supplementary Material
Acknowledgments
The authors thank Naoko Horimoto for her technical assistance. The authors also thank the Center of Medical Research and Education, Osaka University Graduate School of Medicine, for technical support.
This research was supported by a grant-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (24790844 to I.M.), and grants from the Osaka Kidney Foundation (OKF12-0001 to I.M.) and the Kidney Foundation, Japan (JKFB11-8 to I.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090967/-/DCSupplemental.
References
- 1.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M: Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16: 978–983, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM: Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107: 363–369, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Moe SM, O’Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA: Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19: 2387–2393, 2004 [DOI] [PubMed] [Google Scholar]
- 4.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Price PA, Roublick AM, Williamson MK: Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 70: 1577–1583, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Persy V, De Broe M, Ketteler M: Bisphosphonates prevent experimental vascular calcification: Treat the bone to cure the vessels? Kidney Int 70: 1537–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M, CARE-2 Investigators : A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 51: 952–965, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Jamal SA, Fitchett D, Lok CE, Mendelssohn DC, Tsuyuki RT: The effects of calcium-based versus non-calcium-based phosphate binders on mortality among patients with chronic kidney disease: A meta-analysis. Nephrol Dial Transplant 24: 3168–3174, 2009 [DOI] [PubMed] [Google Scholar]
- 11.O’Neill WC, Lomashvili KA: Recent progress in the treatment of vascular calcification. Kidney Int 78: 1232–1239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kies C, Fox HM: Determination of the first-limiting amino acid of wheat and triticale grain for humans. Cereal Chem 47: 615–625, 1970 [Google Scholar]
- 13.Shimada A, Cline TR: Limiting amino acids of triticale for the growing rat and pig. J Anim Sci 38: 941–946, 1974 [DOI] [PubMed] [Google Scholar]
- 14.Civitelli R, Villareal DT, Agnusdei D, Nardi P, Avioli LV, Gennari C: Dietary L-lysine and calcium metabolism in humans. Nutrition 8: 400–405, 1992 [PubMed] [Google Scholar]
- 15.Fürst P: Dietary L-lysine supplementation: A promising nutritional tool in the prophylaxis and treatment of osteoporosis. Nutrition 9: 71–72, 1993 [PubMed] [Google Scholar]
- 16.Lomashvili K, Garg P, O’Neill WC: Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int 69: 1464–1470, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhães AO, Custódio MR, Batista DG, Jorgetti V, Moysés RM: Vascular calcification: Contribution of parathyroid hormone in renal failure. Kidney Int 71: 1262–1270, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Kaibara K, Tabata Y, Nagata N, Enomoto S, Marukawa E, Umakoshi Y: Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone 31: 479–487, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto T, Nakano T, Umakoshi Y, Yamamoto M, Tabata Y: Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J Bone Miner Res 28: 1170–1179, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, Kaibara K, Ishimoto T, Tabata Y, Umakoshi Y: Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 51: 741–747, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Noyama Y, Nakano T, Ishimoto T, Sakai T, Yoshikawa H: Design and optimization of the oriented groove on the hip implant surface to promote bone microstructure integrity. Bone 52: 659–667, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Shimbo K, Oonuki T, Yahashi A, Hirayama K, Miyano H: Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 23: 1483–1492, 2009 [DOI] [PubMed] [Google Scholar]
- 23.März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C: Homoarginine, cardiovascular risk, and mortality. Circulation 122: 967–975, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Struys EA, Jakobs C: Metabolism of lysine in alpha-aminoadipic semialdehyde dehydrogenase-deficient fibroblasts: Evidence for an alternative pathway of pipecolic acid formation. FEBS Lett 584: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Ryan WL, Wells IC: Homocitrulline and homoarginine synthesis from lysine. Science 144: 1122–1127, 1964 [DOI] [PubMed] [Google Scholar]
- 26.Giachelli CM: Vascular calcification mechanisms. J Am Soc Nephrol 15: 2959–2964, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL: Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 87: 1055–1062, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM: Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot D, Skepper JN, Hegyi L, Farzaneh-Far A, Shanahan CM, Weissberg PL: The role of apoptosis in the initiation of vascular calcification. Z Kardiol 90[Suppl 3]: 43–46, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Comar CL, Nold MM, Wasserman RH: The influence of amino acids and other organic compounds on the gastrointestinal absorption of calcium 45 and strontium 89 in the rat. J Nutr 59: 371–383, 1956 [DOI] [PubMed] [Google Scholar]
- 32.Raven AM, Lengemann FW, Wasserman RH: Studies of the effect of lysine on the absorption of radiocalcium and radiostrontium by the rat. J Nutr 72: 29–36, 1960 [DOI] [PubMed] [Google Scholar]
- 33.Franěk F, Chládková-Šrámková K: Apoptosis and nutrition: Involvement of amino acid transport system in repression of hybridoma cell death. Cytotechnology 18: 113–117, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Franĕk F, Srámková K: Protection of B lymphocyte hybridoma against starvation-induced apoptosis: Survival-signal role of some amino acids. Immunol Lett 52: 139–144, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schröder H: Antioxidant action of L-alanine: Heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun 314: 351–355, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Krishnan N, Dickman MB, Becker DF: Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 44: 671–681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Morrisett JD, Tung CH: Detection of hydroxyapatite in calcified cardiovascular tissues. Atherosclerosis 224: 340–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki T: Theory of chromatography of rigid molecules on hydroxyapatite columns with small loads: IV. Estimation of adsorption energy of nucleoside polyphosphates. J Chromatogr A 151: 95–112, 1978 [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki T, Niikura M, Kobayashi Y: Fundamental study of hydroxyapatite high-performance liquid-chromatography. II. Experimental analysis on the basis of the general theory of gradient chromatography. J Chromatogr A 515: 91–123, 1990 [Google Scholar]
- 40.Kawasaki T, Takahashi S, Ikeda K: Hydroxyapatite high-performance liquid chromatography: Column performance for proteins. Eur J Biochem 152: 361–371, 1985 [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki T, Ikeda K, Takahashi S, Kuboki Y: Further study of hydroxyapatite high-performance liquid chromatography using both proteins and nucleic acids, and a new technique to increase chromatographic efficiency. Eur J Biochem 155: 249–257, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Tanahashi M, Matsuda T: Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J Biomed Mater Res 34: 305–315, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Tanase T, Akiyama J, Iwai K, Asai S: Characterization of surface biocompatibility of crystallographically aligned hydroxyapatite fabricated using magnetic field. Mater Trans 48: 2855–2860, 2007 [Google Scholar]
- 44.Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, März W, Wanner C: Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail 13: 852–859, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Yang H, Hou Y, Li T, Fang J, Zhou X, Yin Y, Wu L, Nyachoti M, Wu G: Effects of dietary L-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45: 383–391, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Garber AJ: The regulation of skeletal muscle alanine and glutamine formation and release in experimental chronic uremia in the rat: Subsensitivity of adenylate cyclase and amino acid release to epinephrine and serotonin. J Clin Invest 62: 633–641, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagasawa T, Yoshizawa F, Nishizawa N: Plasma N tau-methylhistidine concentration is a sensitive index of myofibrillar protein degradation during starvation in rats. Biosci Biotechnol Biochem 60: 501–502, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Ishida A, Kyoya T, Nakashima K, Katsumata M: Muscle protein metabolism during compensatory growth with changing dietary lysine levels from deficient to sufficient in growing rats. J Nutr Sci Vitaminol (Tokyo) 57: 401–408, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Solbu TT, Boulland JL, Zahid W, Lyamouri Bredahl MK, Amiry-Moghaddam M, Storm-Mathisen J, Roberg BA, Chaudhry FA: Induction and targeting of the glutamine transporter SN1 to the basolateral membranes of cortical kidney tubule cells during chronic metabolic acidosis suggest a role in pH regulation. J Am Soc Nephrol 16: 869–877, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Matsui I, Hamano T, Mikami S, Inoue K, Shimomura A, Nagasawa Y, Michigami T, Ohnishi T, Fujii N, Nakano C, Kusunoki Y, Kitamura H, Iwatani H, Takabatake Y, Kaimori JY, Matsuba G, Okoshi K, Kimura-Suda H, Tsubakihara Y, Rakugi H, Isaka Y: Retention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in rats. Am J Physiol Renal Physiol 304: F751–F760, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Matsui I, Hamano T, Mikami S, Fujii N, Takabatake Y, Nagasawa Y, Kawada N, Ito T, Rakugi H, Imai E, Isaka Y: Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int 75: 915–928, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Wagner D, Hanwell HE, Vieth R: An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 42: 1549–1556, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Shiraishi A, Miyabe S, Nakano T, Umakoshi Y, Ito M, Mihara M: The combination therapy with alfacalcidol and risedronate improves the mechanical property in lumbar spine by affecting the material properties in an ovariectomized rat model of osteoporosis. BMC Musculoskelet Disord 10: 66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JW, Nakano T, Toyosawa S, Tabata Y, Umakoshi Y: Areal distribution of preferential alignment of biological apatite (BAp) crystallite on cross-section of center of femoral diaphysis in osteopetrotic (op/op) mouse. Mater Trans 48: 337–342, 2007 [Google Scholar]
- 55.Inoue K, Matsui I, Hamano T, Fujii N, Shimomura A, Nakano C, Kusunoki Y, Takabatake Y, Hirata M, Nishiyama A, Tsubakihara Y, Isaka Y, Rakugi H: Maxacalcitol ameliorates tubulointerstitial fibrosis in obstructed kidneys by recruiting PPM1A/VDR complex to pSmad3. Lab Invest 92: 1686–1697, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.