Abstract
AKI predicts the future development of CKD, and one proposed mechanism for this epidemiologic link is loss of peritubular capillaries triggering chronic hypoxia. A precise definition of changes in peritubular perfusion would help test this hypothesis by more accurately correlating these changes with future loss of kidney function. Here, we have adapted and validated a fluorescence microangiography approach for use with mice to visualize, analyze, and quantitate peritubular capillary dynamics after AKI. A novel software-based approach enabled rapid and automated quantitation of capillary number, individual area, and perimeter. After validating perfusion in mice with genetically labeled endothelia, we compared peritubular capillary number and size after moderate AKI, characterized by complete renal recovery, and after severe AKI, characterized by development of interstitial fibrosis and CKD. Eight weeks after severe AKI, we measured a 40%±7.4% reduction in peritubular capillary number (P<0.05) and a 36%±4% decrease in individual capillary cross-sectional area (P<0.001) for a 62%±2.2% reduction in total peritubular perfusion (P<0.01). Whereas total peritubular perfusion and number of capillaries did not change, we detected a significant change of single capillary size following moderate AKI. The loss of peritubular capillary density and caliber at week 8 closely correlated with severity of kidney injury at day 1, suggesting irreparable microvascular damage. These findings emphasize a direct link between severity of acute injury and future loss of peritubular perfusion, demonstrate that reduced capillary caliber is an unappreciated long-term consequence of AKI, and offer a new quantitative imaging tool for understanding how AKI leads to future CKD in mouse models.
Keywords: acute renal failure, chronic kidney disease, vascular disease
AKI is a strong risk factor for the future development of CKD and ESRD. In a recent meta-analysis, Coca and colleagues compared the risk for CKD, ESRD, and death in patients with or without AKI in 13 cohort studies that included >1.4 million patients.1 They demonstrated that patients with AKI are at high risk to develop CKD, with a pooled adjusted hazard ratio (HR) of 8.8 (95% confidence interval [95% CI], 3.1 to 25.5); ESRD (pooled HR, 3.1; 95% CI, 1.9 to 5); and mortality (pooled HR, 2.0; 95% CI, 1.3 to 3.1).1
Possible mechanisms underlying the link between AKI to future CKD include nephron loss, glomerular hypertrophy, inflammation, interstitial fibrosis, epithelial cell-cycle abnormalities, and endothelial injury with capillary rarefaction.2 Acute ischemic injury causes epithelial injury and death, but the tubule can repair completely after mild-to-moderate injury (restitutio ad integrum).3,4 After multiple ischemic events or severe ischemia-reperfusion injury (IRI), the repair process is often incomplete, resulting in fibrogenesis and development of CKD.2,5,6
In contrast to renal tubules, the microvasculature lacks substantial regenerative capacity, resulting in a persistent vascular rarefaction after severe or recurrent injury.7,8 Indeed, the loss of the renal microvasculature is hypothesized to be a key component in fibrogenesis and CKD progression after AKI. As the molecular pathways regulating capillary rarefaction begin to be unraveled, preventing this process has been proposed as a therapeutic target.9,10 Capillary rarefaction induces focal hypoxia, activating an injury cascade with inflammation and ultimately interstitial fibrosis.2,11 It has been described in a variety of rodent AKI models,7,12–15 and in human patients with CKD the loss of capillary density correlates with the severity of fibrosis.16–18
Despite the established association between AKI and peritubular capillary loss, the precise nature of microvascular changes occurring after AKI are poorly defined because high-resolution techniques have not been used to examine this question. To date, microvascular density has been measured by immunostaining and genetic labeling of the endothelium, techniques that assess the surface area of endothelial cells or the number of visually identified capillaries but not the actual capillary lumen.12–15,19 Endothelial cell antigens used for immunostaining, such as CD31 or genetic labeling (such as Tie 2), are reported to be expressed on nonendothelial cell types, which may introduce error.20–22 Other approaches have included Microfil infusion7,23 and radiation microangiography.24 Whereas radiation microangiography does not give information about the capillaries,24 the other techniques allow an estimation of microvascular density; however, they do not provide high-resolution information about perfusion characteristics, such as single capillary cross-sectional area and perimeter. A further limitation to date is the absence of a nonbiased and high-throughput means of quantitating capillary changes. Finally, when the endothelium is used as a surrogate for capillary perfusion, overestimation is possible because endothelial cells of clogged or collapsed vessels with microembolism will still be stained, even though the capillary is actually nonfunctional.
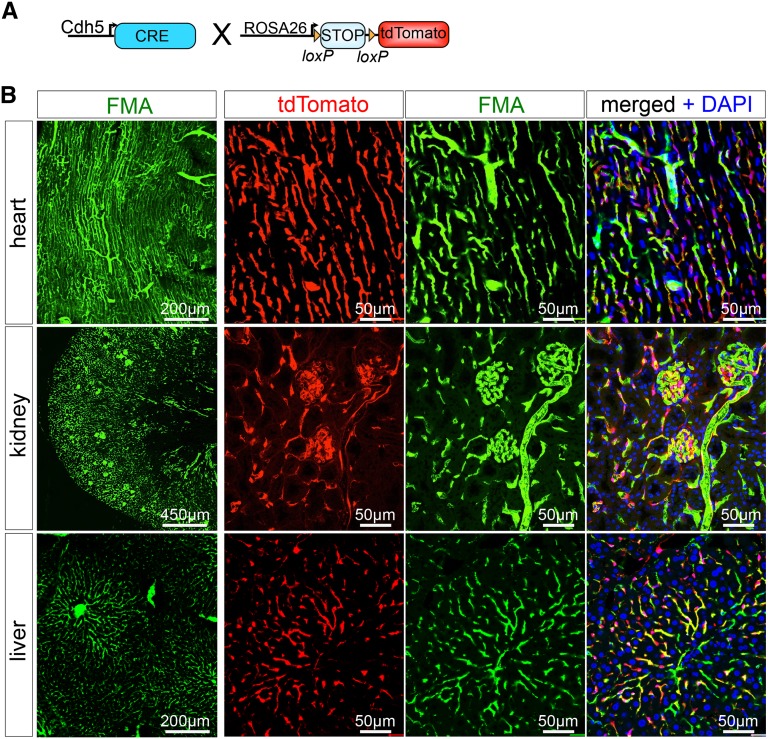
Recently, Advani and colleagues reported use of fluorescence microangiography (FMA) by renal artery injection in the rat 5/6 nephrectomy model.20 They describe changes in glomerular perfusion and a global reduction in peritubular perfusion in this CKD model. Here we modify this technique for use in mice by intracardiac injection and show that it allows visualization of microvasculature in all solid organs tested. We additionally created a MATLAB-based script that allows for precise and rapid analysis of microvascular characteristics, including single capillary cross-sectional area and perimeter (Supplemental Figure 1). (We provide a detailed step-by-step description, including notes and a reagent list for the mouse FMA procedure, and describe how to analyze FMA pictures automatically using our MATLAB script in the Supplemental Material. We also provide all script files needed for this analysis.) To validate our modified approach, we genetically labeled endothelial cells using the VE-Cadherin-Cre driver line (Tg[Cdh5-cre]7Mlia) bred against the R26Tomato reporter mouse (Gt[ROSA]26Sortm9[CAG-tdTomato]Hze/J) (Figure 1A). Using our modified FMA protocol, which includes solutions prewarmed to 41°C and intracardiac injection rather than renal artery injection (see Concise Methods), the fluorescence microbead (0.02 µm)–low-melting-point agarose mixture successfully perfuses the microvasculature of all solid organs tested (Figure 1B).
Figure 1.
FMA provides highly efficient high-resolution perfusion imaging of the kidney, heart, and liver. (A) To genetically label endothelial cells in solid organ vascular beds, we bred the VE-Cadherin-Cre driver mouse against the Rosa26-tdtomato reporter line, resulting in endothelial cell–specific cre-recombination with removing of the LoxP flanked STOP codon and expression of the bright red fluorochrome tdtomato. (B) FMA was validated in Ve-CadherinCre+, R26Tomato+ mice, showing highly efficient delineation of the microvasculature in heart, kidney, and liver. DAPI, 4′,6-diamidino-2-phenylindole.
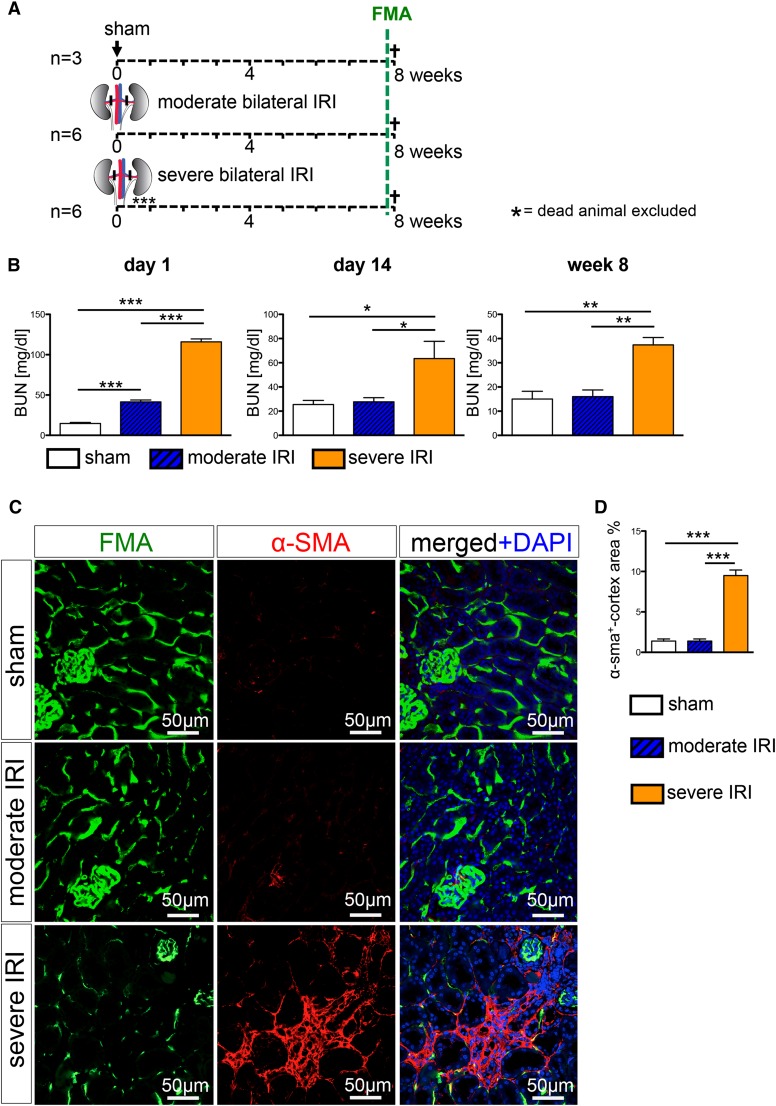
To more precisely define renal peritubular capillary dynamics during progression of AKI to CKD, wild-type mice were subjected to severe IRI (28 minutes of clamping), moderate IRI (23 minutes of clamping), or sham surgery followed by FMA 8 weeks after the surgery (Figure 2A). Whereas the BUN levels in the moderate group showed a complete recovery at 14 days after surgery, BUN levels remained significantly elevated at day 14 and week 8 after severe IRI, indicating a progression to CKD (Figure 2B). Immunostaining and quantification of α-smooth muscle actin–positive surface area demonstrated development of interstitial renal fibrosis only in the severe IRI group, whereas severity of interstitial fibrosis in the moderate IRI group did not differ significantly compared with the sham group (Figure 2, C and D). All mice were subjected to the FMA procedure immediately before euthanasia at 8 weeks after surgery. Confocal microscopy pictures of the kidneys demonstrate that FMA allows precise delineation of the peritubular capillary network, including by three-dimensional reconstruction of Z-stack images (Figure 3A, Supplemental Videos 1 and 2).
Figure 2.
Severe ischemia reperfusion injury leads to CKD with interstitial fibrosis. (A) Wild-type mice were subjected to severe bilateral IRI (clamping for 28 minutes), moderate bilateral IRI (clamping for 23 minutes), or sham surgery and were euthanized following FMA at 8 weeks. (B) BUN measurement revealed significantly increased BUN values in both IRI groups at day 1 after surgery. After moderate IRI, mice showed a complete recovery, with BUN levels returned to normal values at day 14 after surgery, whereas severe IRI lead to persistently increased BUN levels at 8 weeks after surgery. (C and D) α-Smooth muscle actin staining and quantification revealed induction of interstitial fibrosis only after severe IRI. (Graphs show mean±SEM; *P<0.05; **P<0.01; ***P<0.001, one-way ANOVA with post hoc Bonferroni correction). DAPI, 4′,6-diamidino-2-phenylindole.
Figure 3.
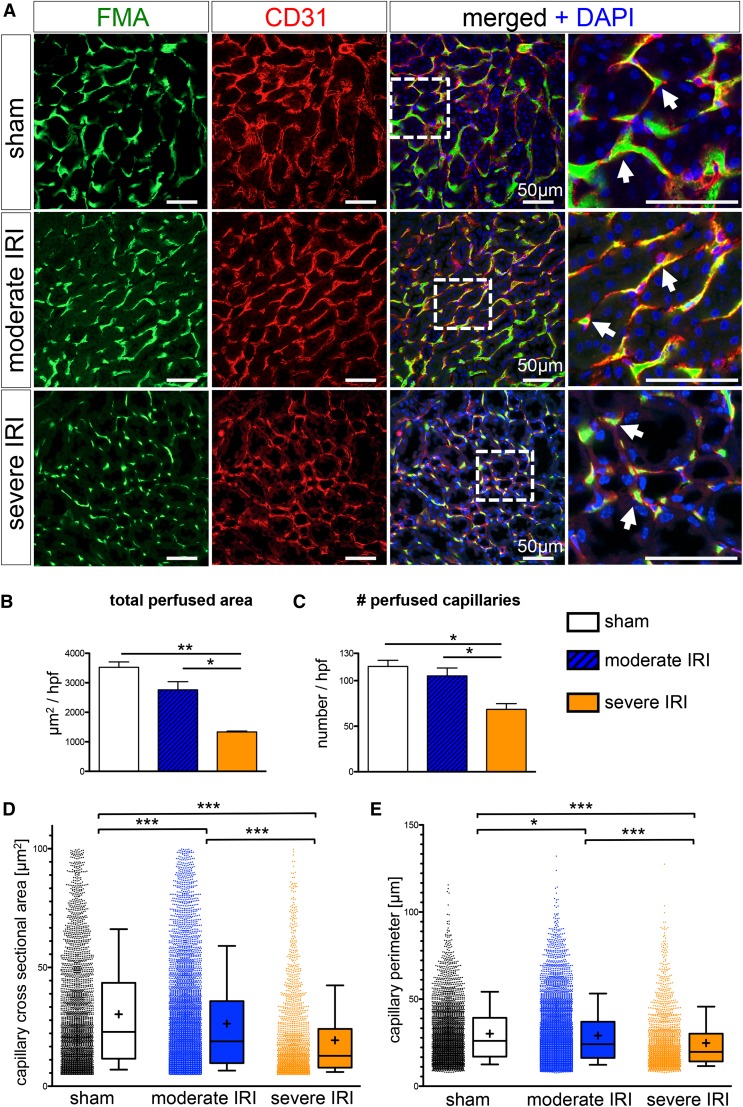
High-throughput software–based analysis of fluorescence microangiography reveals reduced capillary number and caliber after severe IRI. (A) FMA after sham surgery and moderate and severe IRI, together with CD31 immunostaining, demonstrates capillary rarefaction in response to severity of injury (arrows indicate capillaries with red CD31+endothelial cells surrounding the green FMA solution; all scale bars are 50 µm). (B and C) Severe IRI results in a significant reduction of the total cortical cross-sectional capillary area per high-power field [×400, inner cortex] (B) and a significant reduction in capillary number (C). (D and E) The cortical individual capillary cross-sectional area (D) (mean±SEM, sham: 30.31±0.42 µm2; moderate IRI: 26.29±0.28 µm2; severe IRI: 19.34±0.38 µm2) and perimeter (E) (sham: 30.19±0.31 µm; moderate IRI: 29.13±0.22 µm; severe IRI: 24.77±0.35 µm) was significantly reduced after both moderate and severe IRI. (Of note, data represent n=3 mice in the sham group and severe IRI group and n=6 mice in the moderate IRI group; mean±SEM in B and C; box and whiskers with 10th–90th percentiles in D and E; + indicates mean in D and E. *P<0.05; **P<0.01; ***P<0.001, one-way ANOVA with post hoc Bonferroni correction). DAPI, 4′,6-diamidino-2-phenylindole.
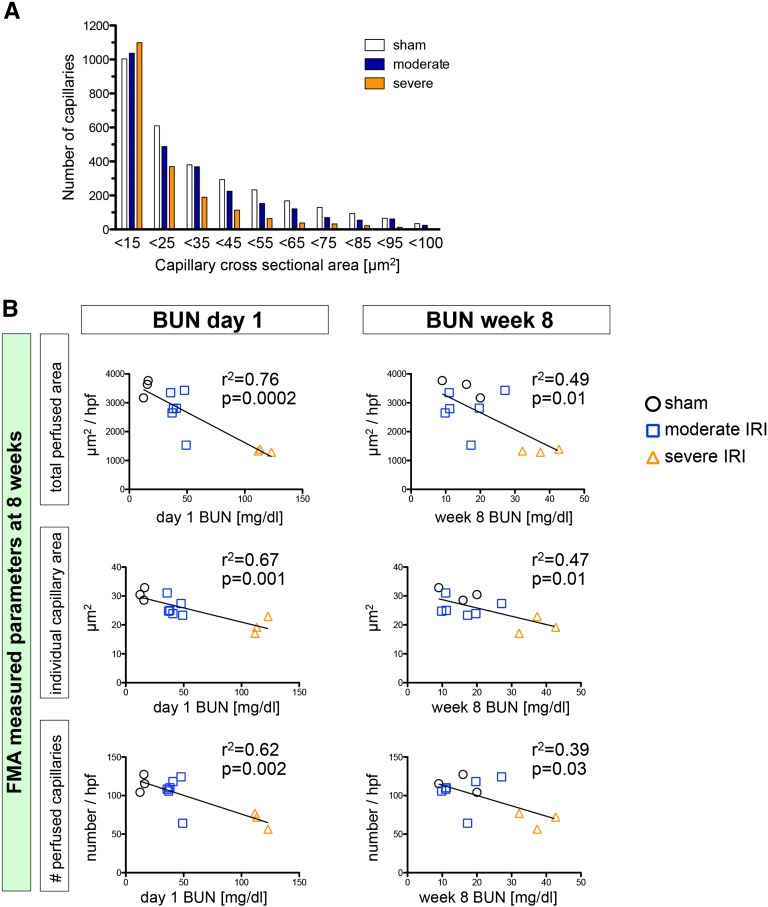
Our software-based analysis (script available in Supplemental Material) demonstrates a significant 62%±2% reduction of total cortical perfused area (µm2/high-power field of the inner cortex) in the severe IRI group compared with the sham group and a significant 52%±1% reduction compared with the moderate IRI group (Figure 3B). This reduction of total perfused cortical area was due to both a reduction of capillary number (Figure 3C) and individual capillary cross-sectional area and perimeter (Figure 3, B and D), indicating a loss of both capillary number and a reduction of the caliber in remaining capillaries after severe IRI. Interestingly, whereas total perfused cortical area and number of capillaries did not significantly change after moderate IRI compared with the sham group, the single cortical capillary surface area and size did change significantly (Figure 3, B–E). The distribution of cross-sectional area in counted capillaries revealed a biphasic trend: The number of smallest capillaries (cross-sectional area <15 µm2) increased slightly after IRI, whereas the number of larger capillaries (>15 µm2) was reduced after IRI (Figure 4A). The most likely explanation for this observation is a shift in categorization of larger capillaries to the smallest group after IRI. Interestingly, quantification of medullary perfusion and the number of medullary capillaries did show a more dramatic reduction following severe IRI (total medullary perfused area µm2/high-power field, −78%±3% versus sham, capillary number −64%±3% versus sham) and a similar reduction of individual capillary cross-sectional area and perimeter (Supplemental Figure 2).
Figure 4.
Severity of initial injury determines shift in capillary size and extent of capillary rarefaction. (A) Distribution of capillary size demonstrates a loss of larger capillaries (>15 µm2) after moderate and severe IRI, with a slight increase in counted small capillaries (<15 µm2) (data represent three mice per group). (B) FMA assessed total capillary cross-sectional area (total perfused area, µm2/high-power field), individual capillary cross-sectional area (µm2), and capillary number (number/high-power field) shows highly significant correlation with day 1 BUN and lower correlation with week 8 BUN.
The use of genetic lineage tracing is limited by the specificity of the Cre drivers used. During this study, we noted that 15%±1% of F4/80+ macrophages were labeled in VE-Cadherin+; tdTomato+ kidneys (Supplemental Figure 3). Because macrophage infiltration intensifies in the postischemic kidney,25 this macrophage labeling would induce an error during quantitative measurements after injury. Therefore, we decided to compare the performance of FMA with standard CD31 immunostaining instead. Comparison between FMA and CD31 staining following IRI demonstrated a larger reduction of the perfused FMA+ capillary area compared with the CD31+ endothelial cell surface area (Supplemental Figure 4), suggesting that some capillaries might lack perfusion following IRI. Indeed, we observed areas where CD31+ capillaries did not contain FMA+ luminal signal following IRI (Supplemental Figure 4).
For the automated software-based analysis of the FMA sections, we used a lower cutoff value of 4.9 µm2 because it has been previously reported that the critical diameter at which erythrocytes cannot pass through capillaries is 2.5 µm (π×r2=4.9 µm2)26; to exclude venules, glomerular capillary convolutes, and other larger vessels (arterioles, arteries, veins), we used an upper cutoff value of 100 µm2 (Supplemental Figure 5). Importantly, the raw data obtained without applying these cutoffs still showed the same significant differences after IRI (Supplemental Figure 5).
The loss of total perfused peritubular cross-sectional area (µm2/high-power field), peritubular capillary number, and individual capillary cross-sectional area (µm2/capillary) and perimeter (µm/capillary) at week 8 correlated very closely with BUN at day 1, and to a lesser degree at day 14 and week 8 after IRI (Figure 4B, Supplemental Figure 6), suggesting that severity of initial injury is a critical factor for future peritubular capillary rarefaction.
In summary, this method reveals novel insight into capillary dynamics in response to AKI, including a previously unappreciated reduction not only in the number but also the caliber of surviving peritubular capillaries. Basile et al. previously used Microfil injection to demonstrate that rats develop a significant reduction of peritubular capillary density in the kidney (cortex, −25% to 30%; inner stripe of outer medulla, −35% to 40%) at 4–40 weeks after IRI.7 Horbelt et al. subsequently identified endothelium by immunostaining of postischemic mice and also reported a 45% decrease of microvascular density at 30 days after IRI.14 Although the experimental protocols differ, our results confirm a substantial reduction in perfusion after acute injury but also report that surviving capillaries are smaller. Thus, an advantage of FMA in assessing peritubular microvasculature is its ability to define perfused capillaries and their precise architecture.
Several future applications can be envisioned. This mouse FMA approach should be useful for the study of the microvasculature, not just in the kidney but in a variety of solid organs, where capillary rarefaction after injury is also considered to be an important component of chronic disease progression.27–29 It should also enable a more precise definition of changes within the interstitial space that accompany injury and repair, such as visualizing pericyte migration away from endothelium during CKD, which has been proposed to underlie fibrogenesis.19,30,31 Finally, mouse FMA may serve as a useful functional readout for therapeutics targeting vascular survival, such as angiogenic growth factors or drugs.19
Concise Methods
All mouse experiments were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Harvard University. Wild-type mice were 8- to 10-week-old C57Bl/6J males from Charles River Laboratories (Wilmington, MA). The Ve-Cadherin-Red Rosa reporter mice were created by crossing homozygous VE-Cadherin-Cre (Tg(Cdh5-cre)7Mlia) with homozygous R26-tdTomato (Gt[ROSA]26Sortm9[CAG-tdTomato]) reporter mice (The Jackson Laboratories; stock # 006137 and 007909). IRI was performed as previously described.4 Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg body wt intraperitoneally); buprenorphine (0.1 mg/kg body wt intraperitoneally) was used to achieve analgesia; kidneys were exposed through flank incisions; and mice were subjected to ischemia by clamping the renal pedicle with nontraumatic microaneurysm clamps (Roboz, Rockville, MD) for 28 minutes (severe IRI) or 23 minutes (moderate IRI) or no clamping (sham surgery). Reperfusion was visually verified. During the surgery, 1 ml of 0.9% NaCl was subcutaneously administered. Body temperatures were controlled at 36.5°C–37.5°C throughout the procedure. Mice were bled at 24 hours, 14 days, and 8 weeks after the surgery via the tail vein. BUN was measured using the Infinity Urea assay (Thermo Fisher Scientific) according to the manufacturer instructions.
FMA
The agarose-fluorescent microbead mixture was made immediately before the procedure by microwaving low-melting-point agarose (Lonza; #50080) 1% by mass in distilled water (4.5 ml dH20+0.05 g agarose/mouse), according to the protocol published by Advani et al.20 Following complete dilution of the agarose, 0.02 μm FluoSpheres sulfate (Invitrogen; #F8845, yellow-green) were added to the mixture such that they were 10% by volume (i.e., 500 µl FluoSpheres plus 4.5 ml 1% agarose/mouse). Mice were anesthetized with pentobarbital (60 mg/kg of body wt intraperitoneally); buprenorphine (0.1 mg/kg body wt intraperitoneally) was given to achieve analgesia; and mice were placed on a surgical heating pad (37°C). The abdomen and thorax were cut via a midline incision extending from the symphysis pubis to the jugulum. We did not achieve satisfactory results when following the Advani protocol in mice until all solutions were prewarmed to 41°C rather than room to body temperature. One milliliter of heparinized saline (100 IU/ml heparin [Sagent Pharmaceuticals] in 0.9% NaCl) followed by 1 mm of 3 M KCl (41°C) was injected in the beating left ventricle using a 27-gauge butterfly catheter (Exel, Corp., #26709). The inferior vena cava was then cut and the mouse was perfused with 41°C prewarmed PBS (10 ml), immediately followed by 5 ml of the agarose-microbead mixture (41°C). (Note: A rapid switch between the different syringes is critical [i.e., PBS → agarose].) Immediately after the perfusion, kidneys, heart, and liver were excised and carefully placed in an ice bucket (surrounded by ice) for 10 minutes. Thereafter, the tissues were fixed in 4% paraformaldehyde on ice for 2 hours, then incubated in 30% sucrose in PBS at 4°C overnight and optimum cutting temperature (OCT) embedded (Sakura Finetek). OCT-embedded organs were cryosectioned into 7- to 40-µm sections and mounted on Superfrost slides (Thermo Fisher Scientific) using ProLong Gold Antifade reagent (Invitrogen). Sections were washed in 1× PBS (3×5 minutes), stained with 4′,6-diamidino-2-phenylindole and mounted in ProLong Gold (Life Technologies). For immunofluorescence staining, sections were blocked in 10% normal goat serum (Vector Labs) and incubated with an primary antibody specific for CD31 (1:100; eBioscience; #14–0311), F4/80 (1:200; Abcam, Inc.; #ab6640) or α-smooth muscle actin (1:200; Sigma-Aldrich; #A2547) followed by a Cy5-conjugated secondary antibody (1:200; Jackson ImmunoResearch Laboratories). All images were obtained by confocal microscopy (Nikon C1 Eclipse; Nikon, Melville, NY).
Ten images of each kidney section (×400 magnification) were taken at random (inner cortex), or seven images of each kidney medulla were obtained using the same laser power and gain intensity for all pictures with the Nikon C1 Eclipse confocal microscope. All images were split in RGB channels using ImageJ (National Institutes of Health, Bethesda, MD), and the green channels were saved in grayscale as a PNG file. Pictures were then automatically analyzed using a MATLAB-based script (Supplemental Material, Supplemental Figure 1). The script removes the background noise and creates a binary image of the capillaries. Through an array loop, it sorts out measurements that do not meet the basic requirements for a capillary. For the analysis, the script was written to exclude measured areas smaller than the size of an erythrocyte (i.e., 4.9 µm2) as background noise (i.e., probably no functional capillary) and areas>100 μm2 as too large for a capillary (i.e., arterioles, arteries, venules, and glomerular convolutes).26 The same analysis was run without the array loop to include all data without any cutoff value (Supplemental Figure 5). Quantification of α-smooth muscle actin–positive surface area was performed by taking random cortical pictures (×200; n=5/kidney) of each mouse using the number of stained pixels per total pixels in Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA).
Statistical Analyses
Data are presented as mean±SEM. For multiple group comparisons, ANOVA with post hoc Bonferroni correction was applied. All statistical analyses, including linear regression analyses, were performed using GraphPad Prism software, version 5.0c (GraphPad Software Inc., San Diego, CA). A P value<0.05 was considered to indicate a statistically significant difference.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant DK088923 (to B.D.H.), by an Established Investigator Award of the American Heart Association (to B.D.H.), and by a fellowship from the RWTH Aachen University and the Deutsche Forschungsgemeinschaft (to R.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101121/-/DCSupplemental.
References
- 1.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 28: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV: Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Basile DP: Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS: EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24: 559–572, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kida Y, Tchao BN, Yamaguchi I: Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol 29: 3330–3342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T, Nangaku M: Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol 9: 211–222, 2013 [DOI] [PubMed] [Google Scholar]
- 12.O’Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS: Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol 292: H285–H294, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS: Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 163: 2289–2301, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA: Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, Shim SI, Suki WN, Truong LD: Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: Altered expression of vascular endothelial growth factor. Hum Pathol 31: 1491–1497, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Serón D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS: Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol Dial Transplant 5: 889–893, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Bohle A, Mackensen-Haen S, Wehrmann M: Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19: 191–195, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Advani A, Connelly KA, Yuen DA, Zhang Y, Advani SL, Trogadis J, Kabir MG, Shachar E, Kuliszewski MA, Leong-Poi H, Stewart DJ, Gilbert RE: Fluorescent microangiography is a novel and widely applicable technique for delineating the renal microvasculature. PLoS ONE 6: e24695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L: Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8: 211–226, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH: PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 104: 1010–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Lennon GM, Ryan PC, Gaffney EF, Fitzpatrick JM: Changes in regional renal perfusion following ischemia/reperfusion injury to the rat kidney. Urol Res 19: 259–264, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Eppel GA, Jacono DL, Shirai M, Umetani K, Evans RG, Pearson JT: Contrast angiography of the rat renal microcirculation in vivo using synchrotron radiation. Am J Physiol Renal Physiol 296: F1023–F1031, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Dirocco D, Bisi J, Roberts P, Strum JC, Wong KK, Sharpless N, Humphreys BD: CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol 306: F379–F388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henquell L, LaCelle PL, Honig CR: Capillary diameter in rat heart in situ: Relation to erythrocyte deformability, O2 transport, and transmural O2 gradients. Microvasc Res 12: 259–274, 1976 [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde AV, Frangogiannis NG: Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res 113: 1331–1344, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitas F, Estato V, Carvalho VF, Torres RC, Lessa MA, Tibiriçá E: Cardiac microvascular rarefaction in hyperthyroidism-induced left ventricle dysfunction. Microcirculation 20: 590–598, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Vollmar B, Menger MD: The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89: 1269–1339, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Schrimpf C, Duffield JS: Mechanisms of fibrosis: The role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS: Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.