Abstract
The intracellular concentration of retinoic acid is determined by two sequential oxidation reactions that convert retinol to retinoic acid. We recently demonstrated that retinoic acid synthesis is significantly impaired in glomeruli of HIV-1 transgenic mice (Tg26), a murine model of HIV-associated nephropathy. This impaired retinoic acid synthesis correlates with reduced renal expression of retinol dehydrogenase 9, which catalyzes the rate-limiting step of retinoic acid synthesis by converting retinol to retinal. Because retinoic acid has renal protective effects and can induce podocyte differentiation, we hypothesized that restoration of retinoic acid synthesis could slow the progression of renal disease. Herein, we demonstrate that overexpression of retinol dehydrogenase 9 in cultured podocytes induces the expression of podocyte differentiation markers. Furthermore, we confirm that podocyte-specific overexpression of retinol dehydrogenase 9 in mice with established kidney disease due to either HIV-associated nephropathy or adriamycin-induced nephropathy decreases proteinuria, attenuates kidney injury, and restores podocyte differentiation markers. Our data suggest that restoration of retinoic acid synthesis could be a new approach to treat kidney disease.
Retinoic acids (RAs) are derivatives of vitamin A and have multiple cellular functions, including inhibition of proliferation, induction of cell differentiation, and inhibition of inflammation.1 In addition to their established benefits in the treatment of a variety of cancers, RA has also been shown to protect against renal injury in multiple experimental models of kidney disease,2 including HIV-associated nephropathy (HIVAN).3 Both in vitro and in vivo studies suggest that RA restores the expression of podocyte differentiation markers, including nephrin, podocin, and synaptopodin.3,4 These studies provide strong evidence supporting the therapeutic benefit of RA in kidney diseases with podocyte injury. In fact, a phase II clinical trial examining the efficacy of RA for treatment of podocyte diseases, including minimal change disease, FSGS, or collapsing glomerulopathy, is ongoing (ClinicalTrials.gov identifier NCT00098020). However, clinical use of RA is challenging because of its side effects.5
After cellular uptake, retinol is converted to RA by two sequential oxidation reactions. Retinol dehydrogenases (RHDs) oxidize retinol to retinal,6 which is further metabolized to retinoic acid by retinaldehyde dehydrogenases (ALDHs).7 The expression of these enzymes varies greatly among different cell types and at different stages of cell differentiation.7 Both the synthesis and metabolism of the bioactive metabolites of retinol are impaired in cancer cells.8 The kidney is a major organ for retinoid metabolism. However, not much is known regarding how retinoid metabolism is altered in renal disease.
Our previous work showed that although the expression of retinoic acid receptor-α–target genes is suppressed in kidneys of a murine model of HIVAN (Tg26) and of patients with HIVAN, the expression of retinoic acid receptor-α is not different between normal and diseased kidneys.9 The concentration of RA, however, is significantly reduced in the kidney cortex and isolated glomeruli of Tg26. We also found that the glomerular concentration of RA is >10-fold higher than the concentration in the kidney cortex.9 Examination of enzymes involved in RA metabolism reveal that two key enzymes in the RA synthetic pathway, retinol dehydrogenase type 1 and type 9 (RDH1 and RDH9), were significantly downregulated in Tg26 glomeruli.9 RDH9 is a rate-limiting enzyme in RA synthesis. Because it is known that RA has renal protective effects and is able to induce podocyte differentiation, we hypothesize that overexpression of RDH9 to restore endogenous RA synthesis could slow the progression of renal disease.
Consistent with our findings, recent studies also showed that endogenous RA synthesis is impaired in kidneys of diabetic db/db mice10 and TGF-β transgenic mice.11 These data together with ours suggest that endogenous RA synthesis is impaired in diseased kidneys. Thus, a better understanding of RA metabolism in kidney disease could help us to identify potential new therapy for kidney diseases.
Results
Overexpression of RDH9 Induced the Expression of Differentiation Markers in Cultured Podocytes
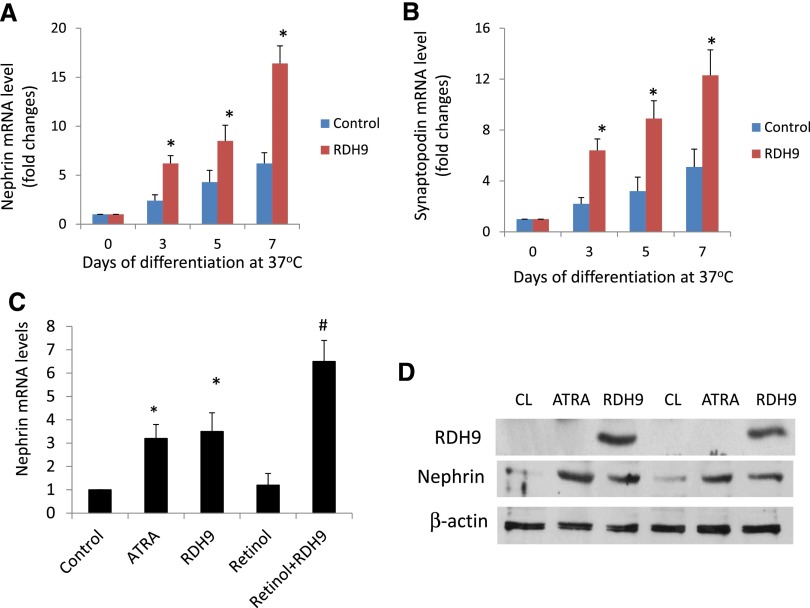
We observed that overexpression of RDH9 in cultured mouse podocytes significantly induced nephrin and synaptopodin expression compared with podocytes without RDH9 overexpression after 7 days of incubation at 37°C (Figure 1, A and B). The effect of RDH9 overexpression on nephrin expression was similar to that observed in cells treated with all-trans-retinoic acid (Figure 1C). Supplementation of retinol in the culture media further increased nephrin expression in podocytes with RDH9 overexpression (Figure 1C). These effects on nephrin expression were also confirmed by Western blot (Figure 1D). Taken together, these in vitro data suggest that improvement of RA synthesis induces podocyte differentiation.
Figure 1.
RDH9 overexpression increases expression of markers of differentiated podocytes. (A and B) Mouse conditionally immortalized podocytes infected with control vector or RDH9 expression vector are cultured at 37°C for 3, 5, and 7 days to induce differentiation. RNA is isolated from these cells for real-time PCR analysis of nephrin and synaptopodin. (C) Podocytes are treated with all-trans retinoic acid (atRA), or overexpressed with RDH9 with or without retinol (1.25 µM) at 37°C for 7 days. Real-time PCR is performed in these cells for nephrin. *P<0.01 compared with control cells; #P<0.01 compared with RDH9 without retinol treatment (n=4). (D) Mouse podocytes are cultured at 37°C for 7 days with atRA (1 µM) in serum-free medium or infected with a RDH9 expression vector, and Western blots are performed for RDH9, nephrin, and β-actin. The representative blots of three independent experiments are shown. CL, control cell.
An Inducible Podocyte-Specific RDH9 Overexpression Mouse Model
We obtained 14 mice positive for the pTRE2 FLAG-Rdh9 transgene, which encodes a fusion FLAG-tagged Rdh9 under the transcriptional control of a tetracycline-responsive promoter element (TRE), from two rounds of pronuclear injection of fertilized FVB-N embryos. Germ-line transmission of the transgene was confirmed in eight founders. Mice derived from the F1 generation were bred with FVB-N mice homozygous for podocin-rtTA (gift from Dr. J. Kopp, Bethesda, MD) expressing the reverse tetracycline transactivator (rtTA) specifically in podocytes to generate double transgenic mice, podocin-rtTA; TRE2-FLAG-Rdh9, which are on a pure FVB-N background (hereafter referred to as PODRDH9). First-generation offspring carrying both transgenes were studied for doxycycline-inducible transgene expression. Five-week-old adult PODRDH9 mice were administered with either doxycycline or vehicle for 6 weeks. Both sexes showed a similar phenotype and were analyzed together. By Western blotting, we confirmed that RDH9 was expressed highly in kidney cortex, highly in liver, and weakly in heart (Supplemental Figure 1A). PODRDH9 mice that were exposed to doxycycline showed significantly (P<0.05) increased RDH9 levels in isolated glomeruli compared with PODRDH9 mice without doxycycline feeding (Supplemental Figure 1B). We also confirmed that mRNA levels of RDH9 were increased in glomeruli of PODRDH9 mice fed with doxycycline compared with PODRDH9 mice without doxycycline feeding (Supplemental Figure 1C).
Doxycycline feeding of PODRDH9 mice had no effect on the body weight and serum levels of urea nitrogen and creatinine (Table 1). Induction of RDH9 expression in podocytes did not affect expression of podocyte differentiation markers at baseline (Supplemental Figure 2).
Table 1.
Body weight, serum urea nitrogen, and creatinine
| Treatment Group | Mice (n) | Body Weight (g) | Serum Urea Nitrogen (mg/dl) | Serum Creatinine (mg/dl) |
|---|---|---|---|---|
| PODRDH9+vehicle | 4 | 27.25±1.01 | 22.87±4.75 | 0.10±0.02 |
| PODRDH9+doxycycline | 4 | 28.18±1.09 | 22.22±4.01 | 0.09±0.03 |
| Tg26;PODRDH9+vehicle | 8 | 24.82±4.73 | 31.24±9.19a | 0.17±0.04a |
| Tg26;PODRDH9+doxycycline | 10 | 24.23±4.31 | 24.09±3.79b | 0.13±0.04b |
Body weight was recorded and serum samples were obtained at the end of experiments after feeding of doxycycline or vehicle for 6 weeks. Serum creatinine was measured by HPLC-based assay.
P<0.05 compared with all other groups.
P<0.05 compared with the Tg26;PODRDH9+vehicle group.
Induction of RDH9 in Podocytes Improved Renal Function and Reduced Proteinuria in Tg26 Mice
To determine whether podocyte-specific overexpression of RDH9 is sufficient to slow the progression of kidney disease, Tg26 mice were crossed with PODRDH9 mice and triple transgenic mice (Tg26;PODRdh9) harboring the Tg26, TRE2-FLAG-RDH9, and podocin-rtTA transgenes, and were fed with doxycycline at age 5 weeks for a total of 6 weeks. Our preliminary studies showed that doxycycline was able to induce RDH9 expression in podocytes after feeding of doxycycline for 1 week (data not shown) and Tg26 mice are known to develop proteinuria starting at 4 weeks of age. Urine was collected biweekly to assess for proteinuria and mice were euthanized at the end of experiments for assessment of renal function and histology.
After 6 weeks of doxycycline feeding (11 weeks of age), we found that the body weight was not significantly different between Tg26;PODRDH9 mice fed with doxycycline and vehicle. The levels of serum BUN and creatinine were significantly lower in Tg26;PODRDH9 mice fed with doxycycline compared with mice fed with vehicle (Table 1; P<0.05). No significant difference in BUN and creatinine were observed between the Tg26;PODRDH9 group fed with doxycycline and the PODRDH9 fed with either doxycycline or vehicle (data not shown).
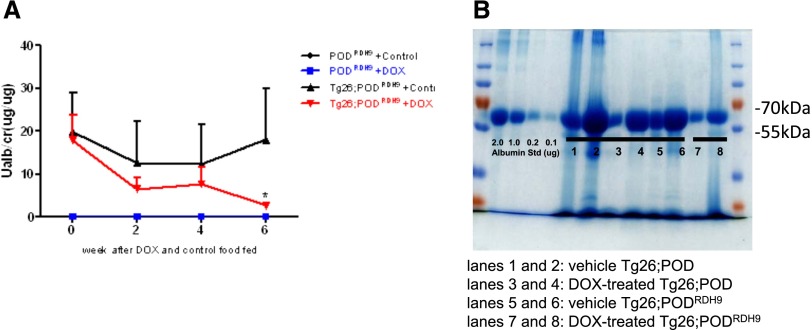
Urinary albumin excretion measured by the urinary albumin/creatinine ratio (UACR) in Tg26;PODRDH9 mice fed with either doxycycline or vehicle was significantly higher than that of PODRDH9 mice fed with either doxycycline or vehicle at 2, 4, and 6 weeks (Figure 2A; P<0.05). However, the UACR of Tg26;PODRDH9 mice fed with doxycycline was significantly lower than Tg26;PODRDH9 mice fed with vehicle only after 6 weeks of doxycycline feeding (P<0.05). Coomassie blue staining confirmed that urinary proteins were predominately albumin, which was reduced in two doxycycline-fed Tg26;PODRDH9 mice compared with vehicle-fed Tg26;PODRDH9 mice and Tg26;POD mice fed with either doxycycline or vehicle (Figure 2B).
Figure 2.
RDH9 overexpression suppresses albuminuria of Tg26 mice. (A) Urinary albumin excretion. The UACR is determined in Tg26;PODRDH9 and PODRDH9 mice before feeding and after feeding with doxycycline or vehicle for 2, 4, and 6 weeks (n=4 in the vehicle and doxycycline-treated PODRDH9 groups; n=8 in the vehicle Tg26;PODRDH9 group; n=10 in the doxycycline-treated Tg26;PODRDH9 group). The UACRs in both vehicle and doxycycline-treated Tg26;PODRDH9 are significantly higher than in vehicle and doxycycline-treated PODRDH9 groups. *P<0.05 compared with the control Tg26;PODRDH9 group. (B) Coomassie stain reveals that the change in proteinuria is mainly albuminuria. The representative gel shows two mice in each group (lanes 1 and 2, vehicle Tg26;POD; lanes 3 and 4, doxycycline-treated Tg26;POD; lanes 5 and 6, vehicle Tg26;PODRDH9; and lanes 7 and 8, doxycycline-treated Tg26;PODRDH9). DOX, doxycycline.
Induction of RDH9 Expression in Podocytes Improved Kidney Injury in Tg26 Mice
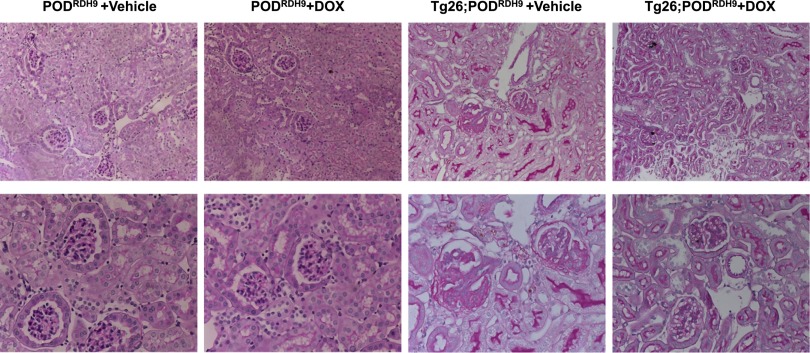
Tg26;PODRDH9 developed significant glomerulosclerosis, interstitial nephritis, and tubular microcystic dilation. However, induction of RDH9 expression in podocytes of mice fed with doxycycline from age 5 weeks to 11 weeks significantly reduced glomerulosclerosis and tubular injuries compared with mice fed with vehicle (Figure 3, Table 2).
Figure 3.
Kidney histology. Periodic acid–Schiff staining is performed in kidneys of PODRDH9 and Tg26;PODRDH9 fed with doxycycline or vehicle for 6 weeks. Mice are euthanized at age 11 weeks. Representative pictures of these mice are shown. Original magnification, ×200 in top row; ×400 in bottom row. DOX, doxycycline.
Table 2.
Summary of kidney histology
| Treatment Group | Glomerulosclerosis Index | Podocyte Hypertrophy | Tubular Casts/Cysts |
|---|---|---|---|
| Tg26;PODRDH9+vehicle | 0 | 0 | 0.59±0.13 |
| Tg26;PODRDH9+doxycycline | 32±19.64a | 2.47±0.84a | 7.81±6.43a |
Kidney histologic analysis was performed in Tg26;PODRDH9 mice treated with doxycycline or vehicle. The glomerulosclerosis index, podocyte hypertrophy, and tubular casts/cysts were quantified as described in the Concise Methods.
P<0.05 compared with the vehicle group.
Induction of RDH9 Expression in Podocytes Increased Expression of Podocyte Differentiation Markers and Inhibited Glomerular Cell Proliferation in Tg26 Mice
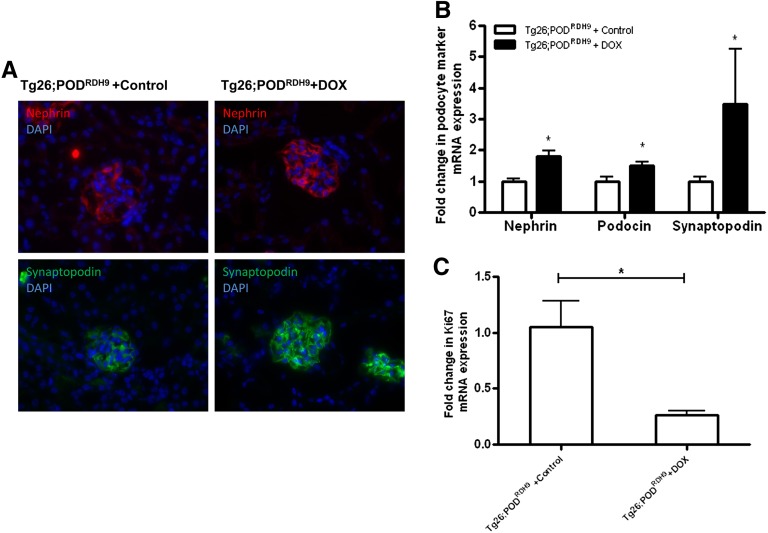
We assessed the expression of nephrin, podocin, and synaptopodin in the glomeruli of these mice by both real-time PCR and immunostaining. We found that induction of RDH9 expression in podocytes increased nephrin and synaptopodin expression at both mRNA and protein levels in Tg26;PODRDH9 mice fed with doxycycline, whereas expression of these markers remained suppressed in Tg26;PODRDH9 mice fed with vehicle (Figure 4, A and B; P<0.05). Similarly, the mRNA level of podocin was also increased in Tg26;PODRDH9 mice fed with doxycycline compared with those fed with vehicle (Figure 4B; P<0.05). Because glomerular cell proliferation is considered as a hallmark of collapsing FSGS in HIVAN,12 we examined the expression of Ki67 in kidneys of Tg26;PODRDH9 mice and found that Ki67 was markedly reduced in Tg26;PODRDH9 mice fed with doxycycline compared with Tg26;PODRDH9 mice fed with vehicle (Figure 4C; P<0.01).
Figure 4.
Podocyte differentiation and cell proliferation markers. (A) Immunofluorescence labeling for nephrin and synaptopodin. Nuclei are labeled by DAPI in blue. (B) Quantitative real-time PCR for nephrin, podocin, and synaptopodin mRNA expression is performed (n=4 in vehicle group and n=6 in doxycycline group). (C) Quantitative real-time PCR for Ki67 mRNA expression is performed (n=4 in vehicle group and n=6 in doxycycline group). *P<0.05 compared with vehicle group. DAPI, 4′6-diamidino-2-phenylindole; DOX, doxycycline.
Induction of RDH9 Expression in Podocytes Modulated RA Target Gene Expression in Tg26
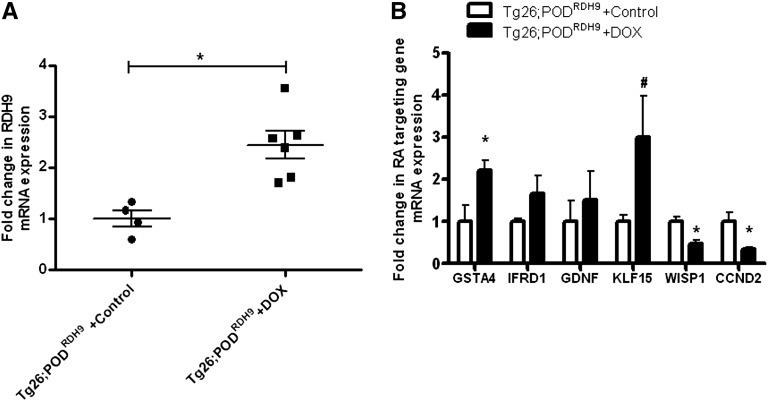
We also determined whether induction of RDH9 expression in podocytes affected the expression of RA target genes that are involved in cell differentiation, cell proliferation, anti-inflammatory processes, and reactive oxygen species detoxification as we previously described.13 We first confirmed that expression of RDH9 was significantly increased in glomeruli of Tg26;PODRDH9 mice fed with doxycycline compared with Tg26;PODRDH9 mice fed with vehicle (Figure 5A). Expression of Kruppel-like factor 15 (KLF15), a transcription factor involved in podocyte differentiation,13 was significantly increased in Tg26;PODRDH9 mice fed with doxycycline compared with Tg26;PODRDH9 mice fed with vehicle (Figure 5B). Glutathione S-transferase-α-4 (GSTA4), which is involved in the inhibition of reactive oxygen species generation, was also increased in Tg26;PODRDH9 mice fed with doxycycline (Figure 5B). Consistent with our previous findings, Cyclin D2 (Ccnd2) and WNT1-inducible signaling pathway protein 1 (Wisp1) were significantly suppressed in Tg26;PODRDH9 mice fed with doxycycline (Figure 5B). Glial cell line–derived neurotrophic factor (GDNF), a growth factor that is critical for neural and renal development, was recently found to function as a survival factor for injured podocytes in puromycin nephropathy nephrotic rats.14 IFN-related developmental regulator 1 (IFRD1) is the human homolog of the rat PC4 gene.15 The rat PC4 gene was initially isolated as a nerve growth factor–inducible sequence in PC12 cells and it was recently shown that PC4 is necessary to muscle differentiation16 (Figure 5B). These findings illustrate potential mechanisms to explain how restoration of RA synthesis by induction of RDH9 expression in podocytes restored cell differentiation and inhibited cell proliferation in our model.
Figure 5.
Quantitative real-time PCR for RA synthesis and RA targeting gene mRNA expression is performed (n=4 in vehicle group and n=6 in doxycycline group). *P<0.05 compared with the vehicle group; #P<0.01 compared with vehicle group. DOX, doxycycline.
Induction of RDH9 Expression in Podocytes Protected Mice from Adriamycin-Induced Nephropathy
To further confirm whether overexpression of RDH9 in podocyte could improve kidney disease and podocyte injury, we induced nephropathy by injection of adriamycin (18.8 mg/kg intravenously)17 in PODRDH9 mice. Mice were fed with doxycycline or vehicle foods at day 7 postinjection when they developed kidney injury. Urine was collected at days 14 and 28 postinjection to assess the UACR. Mice were euthanized at day 28 postinjection to assess renal function and kidney histology.
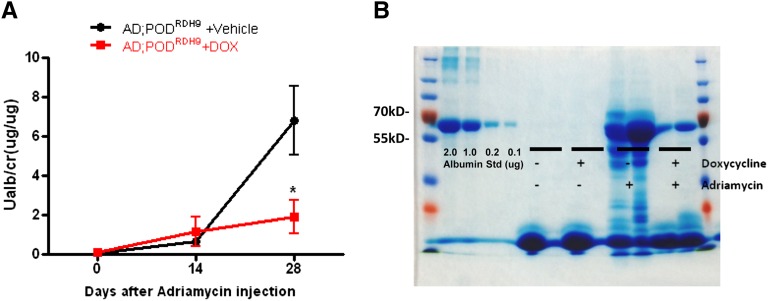
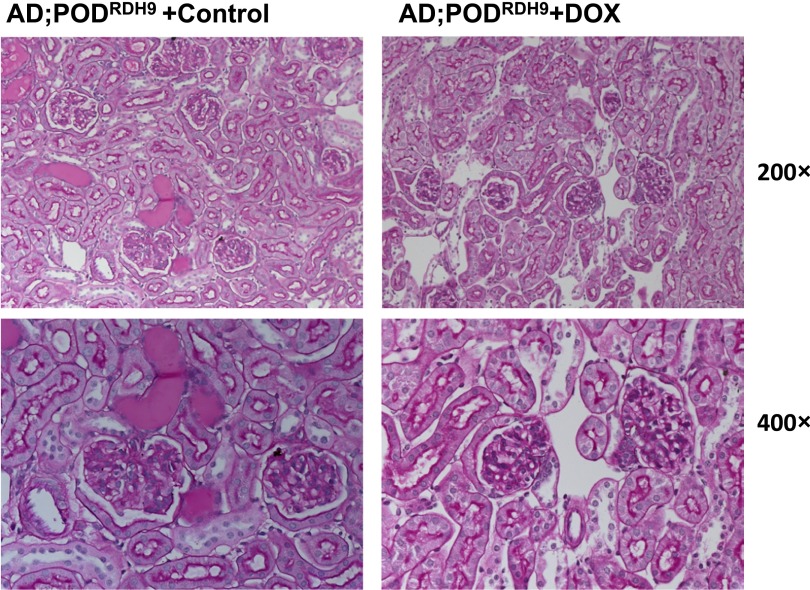
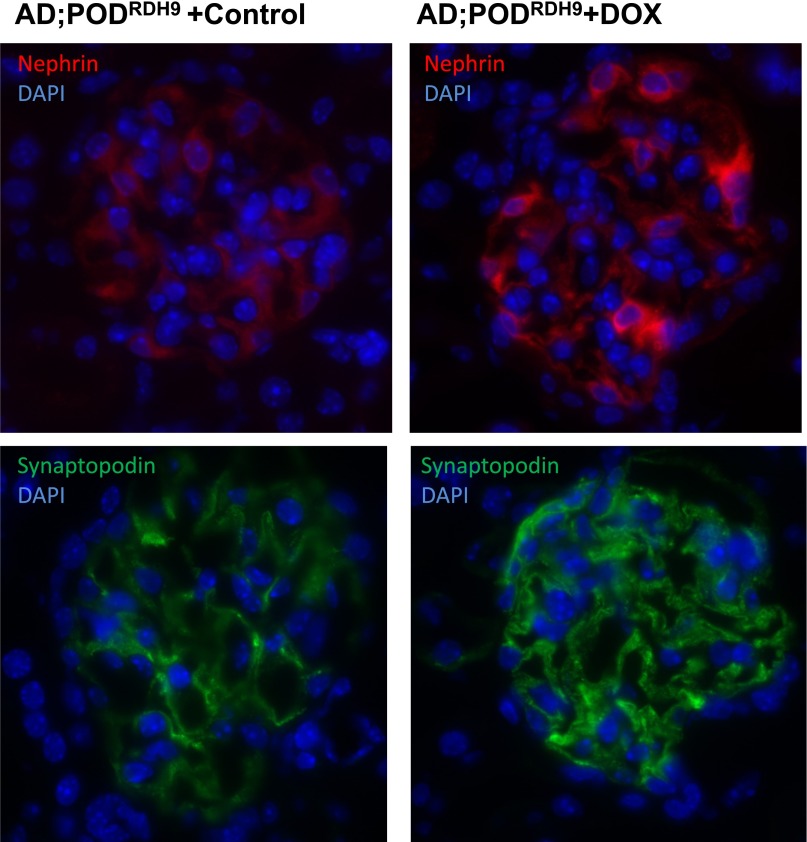
We found that PODRDH9 mice treated with doxycycline exhibited significantly reduced proteinuria compared with mice in the vehicle group at day 28 postinjection (Figure 6A; P<0.05). Coomassie staining revealed that the proteinuria was predominantly albumin and confirmed a reduction of albuminuria in mice treated with doxycycline (Figure 6B). The kidney sections stained with periodic acid–Schiff showed that PODRDH9 mice treated with doxycycline had attenuated glomerulosclerosis and tubular cast/cyst formation compared with mice in the vehicle group at day 28 postinjection (Figure 7). BUN and serum creatinine were not different between the doxycycline and vehicle groups before and after adriamycin injection and within each group before and after adriamycin injection (Table 3; P>0.05). The body weight of mice after adriamycin injection was higher than before injection in the doxycycline group (Table 3; P<0.05), but was not different between the other groups. In addition, the expression of synaptopodin and nephrin was significantly increased in PODRDH9 mice fed with doxycycline compared with the mice fed with vehicle, as determined by immunofluorescence staining of kidney sections from mice at day 28 postinjection (Figure 8).
Figure 6.
Urinary albumin excretion. (A) The UACR is assessed in mice before and after injection of adriamycin for 14 and 28 days. Mice are fed with either doxycycline or vehicle food from days 7 to 28 postinjection (n=6 in AD;PODRDH9+vehicle group; n=5 in AD;PODRDH9+doxycycline group). *P<0.05 compared with AD;PODRDH9+vehicle. (B) Coomassie stain reveals that the proteinuria is mainly albuminuria. The representative gel of two mice in each group is shown. AD, adriamycin; DOX, doxycycline.
Figure 7.
Kidney histology. Periodic acid–Schiff staining is performed in kidneys of AD;PODRDH9 mice, which are fed with doxycycline or vehicle food from days 7 to 28. The kidneys are obtained when mice are euthanized at day 28 postinjection. Representative pictures are shown here. DOX, doxycycline. Original magnification, ×200 in top row; ×400 in bottom row.
Table 3.
Body weight, serum urea, and creatinine in the adriamycin-induced nephropathy model
| Treatment Group | Mice (n) | Body Weight (g) | Serum Urea Nitrogen (mg/dl) | Serum Creatinine (mg/dl) | |||
|---|---|---|---|---|---|---|---|
| Pregroup | Postgroup | Pregroup | Postgroup | Pregroup | Postgroup | ||
| AD;PODRDH9+vehicle | 6 | 25.15±2.17 | 26.28±1.71 | 26.22±4.86 | 29.16±7.99 | 0.11±0.04 | 0.15±0.04 |
| AD;PODRDH9+doxycycline | 5 | 23.94±1.2 | 27.44±1.63a | 26.41±3.17 | 22.84±4.43 | 0.10±0.02 | 0.14±0.06 |
Body weight was recorded and serum samples were obtained in mice before and after injection of adriamycin for 28 days in both the doxycycline feeding and control groups. Serum creatinine was measured by HPLC-based assay. Pregroups are before the injection of adriamycin, whereas the postgroups are 28 days after injection of adriamycin. AD, adriamycin.
P<0.05 compared with mice in the pregroups.
Figure 8.
Immunofluorescence staining for nephrin (red) and synaptopodin (green). Nuclei are labeled by DAPI (blue). DAPI, 4′6-diamidino-2-phenylindole; DOX, doxycycline.
Discussion
It is known that the kidney is one of the major organs for RA metabolism. However, it remains unclear whether RA metabolism is altered in the diseased kidney and how RA metabolism contributes to the progression of kidney disease. Our studies suggest RA synthesis is impaired in the diseased kidney and contributes to the progression of kidney disease. We previously found that endogenous RA synthesis is impaired in kidneys of Tg26 mice due to downregulation of key enzymes—RDH9 and RDH1—involved in RA synthesis.9 A similar deficiency of RA synthesis was also reported in diabetic kidney and in kidneys of TGF-β transgenic mice.10,11 In addition, recent studies suggest that albumin in the urine could sequester endogenous RA synthesized locally by kidney cells, leading to the progression of kidney disease.18 Previous studies also suggest that local RA concentration is critical for kidney development.2 These studies together with ours support a critical role of endogenous RA in the progression of kidney disease.
Previous studies strongly support a role of RA in inducing podocyte differentiation.4,19 Recent studies suggest that RA also induces regeneration of podocytes from parietal epithelial cells.18,20 Here, we showed that overexpression of RDH9 in podocytes induced expression of differentiation markers. In addition, induction of RDH9 expression in podocytes in vivo improved proteinuria and podocyte differentiation markers in two animal models with podocyte injury. Our studies provided additional evidence to support the critical role of RA in podocyte differentiation.
In addition, our studies showed that induction of RDH9 in podocytes regulated several RA target genes in Tg26 mice, including KLF15, GSTA4, Ccnd2, Wisp1, GDNF, and IFRD1. Our recent studies confirmed that RA could induce KLF15 expression both in vivo and in vitro and that KLF15 is a critical transcriptional factor mediating RA-induced podocyte differentiation.13 Our unpublished data suggest that GSTA4 mediates the antioxidative effect of RA in podocytes. It is known that HIV infection activates Ccnd2 leading to podocyte proliferation21,22 and RA suppresses podocyte proliferation in Tg26 mice through inhibition of Ccnd2. Wisp1, GDNF, and IFRD1 are RA target genes that mediate cell proliferation and differentiation.14,16,23 These data suggest that induction of RDH9 expression in podocytes activates these RA target genes to improve podocyte differentiation and attenuate kidney injury.
Many studies have shown that exogenous RA is able to improve kidney diseases in various experimental models, including the HIV-1 transgenic mouse (Tg26),3 anti-Thy-1 nephritis,24 puromycin aminonucleoside-induced nephrosis,25 lupus nephritis,26 diabetic nephropathy,27 and anti-glomerular basement membrane nephritis.4 However, clinical application of RA is limited by its side effects. Our studies suggest that restoration of endogenous RA synthesis could be a new approach to treat patients with kidney disease. In addition, we showed that induction of RDH9 in mice with established kidney disease was able to attenuate kidney injury and reduce proteinuria, indicating that restoration of RA synthesis could be developed to slow the progression of kidney disease.
Vitamin A is absorbed from animal food and derived from beta-carotene of plants.28 In the blood, retinol binds to serum retinol-binding protein 4 and is then taken up by cells via a cell membrane receptor for retinol-binding protein 4.29 The intracellular concentrations of various retinoids are regulated by the activities of several key metabolic enzymes.30 RHDs oxidize retinol to retinal, which is further metabolized to retinoic acid by ALDHs.7 RA is further oxidized to more polar metabolites by cytochrome P450 family members such as CYP26A1.31 RDH9 converts 9-cis-retinol into 9-cis-retinal, has the highest efficiency (Vm/Km) among RHDs. Interestingly, when we induced RDH9 expression in kidney of Tg26 mice, RDH1 expression was also significantly increased. Consistent with this finding, RHD1 was downregulated 3- to 8-fold in RDH9-null mice.32 However, there were no changes in the expression of other enzymes in the RA synthetic pathway such as Aldh1a1,2. There are different RDH isoforms between human and mouse. We previously showed that RDH1 was also reduced in human diseased kidney.9 Further studies are required to determine how we could restore RA synthesis in humans to treat kidney disease.
In conclusion, our studies suggest that restoration of endogenous RA synthesis induces podocyte differentiation in vitro and improves kidney injury in vivo. Therefore, restoration of RA synthesis could be considered as another approach to treat patients with kidney disease.
Concise Methods
Generation of Transgenic Animals
Construct generation (TRE, construct) was as follows. Mouse RDH9 cDNA corresponding to NM_153133.2 was purchased from Thermo Fisher Scientific. A fusion N-terminal FLAG-tagged RDH9 construct (FLAG-RDH9) flanked by 5′ Bam1H and 3′ XhoI restriction endonuclease sequences was generated by PCR and then cloned into the pDNR-1r plasmid (Clontech Laboratories). The FLAG-RDH9 fusion construct was subcloned into the pLP-TRE2 acceptor vector (Creator Vector; Clontech Laboratories) by Cre-mediated recombineering to generate the pLP-TRE2-FLAG-RDH9 expression construct. The sequence and orientation of FLAG-RDH9 insert were confirmed by restriction endonuclease digestion and DNA sequencing. Doxycycline-inducible expression of FLAG-RDH9 was confirmed in the Tet-ON U2OS cell line (Clontech Laboratories). The 4.6-kb ApaLI-ApaLI DNA fragment of pLP-TRE2-FLAG-RDH9 was used for microinjection to generate the TRE2-FLAG-RDH9 transgenic mice in FVB/N background.
Animal Genotyping
TRE2-FLAG-RDH9 transgenic mice were genotyped by PCR using the following set of primers: 5′-ACG CCA TCC ACG CTG TTT TG-3′ and 5′-AAG TCC TGT TTC TTC ATC CAC TCG-3′ (amplicon size 557 bp). The genotyping for the podocin-rtTA mice was performed as previously described.33 All animal studies were performed in accordance with the guidelines of and approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai.
Podocyte Cell Culture
A conditionally immortalized murine podocyte cell line was cultured as described.34 Cells were cultured on rat tail collagen I–coated plates and maintained at 33°C (5% CO2, 90% humidity). For induction of differentiation, cells were cultured at 37°C up to 7 days.
Antibodies
Antibodies to RHD9 were custom made by GenoScript. Rabbit antibody to nephrin was a gift from Dr. Lawrence Holzman (University of Pennsylvania). Mouse antibody to synaptopodin was a gift from Dr. Peter Mundel (Massachusetts General Hospital).
Measurements of Serum Urea Nitrogen And Creatinine
Serum BUN content was measured using a commercially available kit (BioAssay Systems). Serum creatinine was measured by a HPLC-based method as previously described35 with slight modifications: 5 µl of serum per mouse was processed as described and then assayed using a Shimadzu Prominence HPLC unit connected with a Hamilton cation exchange column (PRP-X200).
Western Blot Analyses
Western blotting was performed using specific antibodies for proteins of interest as previously described.36
Histopathology and Immunohistochemistry Staining of Kidney Tissue
Kidney samples were fixed in 10% formalin, embedded in paraffin, and sectioned to 4-µm thickness. Periodic acid Schiff–stained sections were used for assessment of kidney histology.
Renal histologic abnormalities were scored as previously described.9 The three parameters scored included the percentage of glomeruli exhibiting collapse and segmental or global sclerosis, the percentage of total renal parenchyma occupied by tubular casts and/or microcysts, and the severity of podocyte hypertrophy and hyperplasia (graded on a semiquantitative scale from 0 to 3+). For the latter parameter, podocyte hypertrophy and hyperplasia was graded as 0 (absent), 1+ (involving 1%–25% of all glomeruli sampled), 2+ (involving 26%–50% of glomeruli), and 3+ (involving >50% of glomeruli) as described. Frozen sections were used for immunofluorescence staining for nephrin and synaptopodin and images were taken by using a Zeiss Axioplan 2 IE microscope as previously described.37
Urine Albumin and Creatinine
Urine creatinine was quantified using commercial kits from BioAssay Systems. Urine albumin was determined using a commercial assay from Bethyl Laboratory, Inc. Urine albumin excretion was expressed as the UACR .
Coomassie Blue Staining of Urinary Proteins
Five microliters of urine was denatured with Laemmli sample buffer and then resolved on a 10% SDS-PAGE. Proteins on the gel were detected by Coomassie blue staining.
Isolation of Glomeruli
Glomeruli were isolated by Fe3O4 perfusion as previously described.38 Mice were perfused with sterile HBSS containing 2.5 mg/ml Fe3O4 (Sigma-Aldrich) and 1% BSA. At the end of perfusion, kidneys were digested with 100 μg/ml of collagenase A and 1 μ/ml of DNase I in HBSS at 37°C for 30 minutes. Digested samples were washed and passed through a 100-μm cell strainer. After centrifugation, the pellets were resuspended in HBSS and glomeruli were collected using a magnet (Invitrogen). The purity of the glomeruli was verified under light microscopy and by Western blot analysis for podocyte-specific markers as described.13,39
Quantitative Real-Time PCR
Primers for RT-PCR were designed by using the National Center for Biotechnology Information Primer-BLAST tool (see Table 4). Gene expression was normalized to Gapdh and fold change in expression relative to the control group was calculated using the 2−∆∆Ct method. Two technical replicates per gene were used.
Table 4.
Real-time PCR primers
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-GCCATCAACGACCCCTTCAT | 5′-ATGATGACCCGTTTGGCTCC |
| RDH9 | 5′-TCTTGGGCCGAGTATCTTTG | 5′-ACTACTGGTCACACCGGTCA |
| Nphs1 | 5′-GTGCCCTGAAGGACCCTACT | 5′-CCTGTGGATCCCTTTGACAT |
| Pphs2 | 5′- CTTGGCACATCGATCCCTCA | 5′-CGCACTTTGGCCTGTCTTTG |
| Synpo | 5′-CTTTGGGGAAGAGGCCGATTG | 5′-GTTTTCGGTGAAGCTTGTGC |
| Ki67 | 5′-GTTCCTTCAGCAAGCCTGAG | 5′-TGTCCACCAAAGGATACACG |
| Gsta4 | 5′- ACTTTAATGGCAGGGGACGG | 5′-TACTTGGCCGAAAAGCAGGT |
| Irfd1 | 5′-AGAGACGTTCTGAGGGCTGT | 5′-TGTCTGCAAGTGGTACTGCAT |
| Gdnf | 5′-GATTCGGGCCACTTGGAGTT | 5′-GACAGCCACGACATCCCATA |
| Klf15 | 5′-AGAGCAGCCACCTCAAGGCCCA | 5′-TCACACCCGAGTGAGATCGCCGGT |
| Ccnd2 | 5′-GCCAAGATCACCCACACTGA | 5′-GCGTTATGCTGCTCTTGACG |
| Wisp1 | 5′-TGCGATTACAGTGGGGATCG | 5′-GCAGTTGGGTTGGAAGGACT |
Statistical Analyses
Data are expressed as the mean±SEM. For comparison of means between three or more groups, two-way ANOVA with the Bonferroni post-test was applied. For comparisons of means between two groups, two-tailed, unpaired t tests were performed. GraphPad Prism 5 software was used for statistical analyses.
Disclosures
None.
Supplementary Material
Acknowledgments
J.C.H. and P.Y.C. are supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (1R01-DK078897, 1R01-DK088541, and P01-DK56492 to J.C.H.; 5K08-DK082760 to P.Y.C.). J.C.H. is also supported by the Chinese 973 fund (2012CB517601).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111150/-/DCSupplemental.
References
- 1.Evans TR, Kaye SB: Retinoids: Present role and future potential. Br J Cancer 80: 1–8, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlet-Bénichou C, Vilar J, Lelièvre-Pégorier M, Gilbert T: Role of retinoids in renal development: Pathophysiological implication. Curr Opin Nephrol Hypertens 8: 39–43, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE: HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem 283: 8173–8182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ: ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int 68: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, de la Serna J, León A, Esteve J, Milone G, Debén G, Rivas C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S, Amutio E, Brunet S, Lowenberg B, Sanz MA: Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: Characteristics, outcome, and prognostic factors. Blood 113: 775–783, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Parés X, Farrés J, Kedishvili N, Duester G: Medium- and short-chain dehydrogenase/reductase gene and protein families: Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci 65: 3936–3949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhinn M, Dollé P: Retinoic acid signalling during development. Development 139: 843–858, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Siddikuzzaman G, Guruvayoorappan C, Berlin Grace VM: All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol 33: 241–249, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Ratnam KK, Feng X, Chuang PY, Verma V, Lu TC, Wang J, Jin Y, Farias EF, Napoli JL, Chen N, Kaufman L, Takano T, D’Agati VD, Klotman PE, He JC: Role of the retinoic acid receptor-α in HIV-associated nephropathy. Kidney Int 79: 624–634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starkey JM, Zhao Y, Sadygov RG, Haidacher SJ, Lejeune WS, Dey N, Luxon BA, Kane MA, Napoli JL, Denner L, Tilton RG: Altered retinoic acid metabolism in diabetic mouse kidney identified by O isotopic labeling and 2D mass spectrometry. PLoS ONE 5: e11095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Hendry BM, Maden M, Lu H, Wong YF, Rankin AC, Noor M, Kopp JB: Kidneys of Alb/TGF-beta1 transgenic mice are deficient in retinoic acid and exogenous retinoic acid shows dose-dependent toxicity. Nephron, Exp Nephrol 114: e127–e132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P, Chuang PY, He JC: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287: 19122–19135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui CC, Shankland SJ, Pierchala BA: Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J Am Soc Nephrol 17: 1543–1552, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Buanne P, Incerti B, Guardavaccaro D, Avvantaggiato V, Simeone A, Tirone F: Cloning of the human interferon-related developmental regulator (IFRD1) gene coding for the PC4 protein, a member of a novel family of developmentally regulated genes. Genomics 51: 233–242, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Micheli L, Leonardi L, Conti F, Buanne P, Canu N, Caruso M, Tirone F: PC4 coactivates MyoD by relieving the histone deacetylase 4-mediated inhibition of myocyte enhancer factor 2C. Mol Cell Biol 25: 2242–2259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, Ballerini L, Sagrinati C, Wanner N, Huber TB, Liapis H, Lazzeri E, Lasagni L, Romagnani P: Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol 24: 1756–1768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, Klotman PE: Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol 18: 93–102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE: Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int 64: 1695–1701, 2003 [DOI] [PubMed] [Google Scholar]
- 22.He JC, Husain M, Sunamoto M, D’Agati VD, Klotman ME, Iyengar R, Klotman PE: Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest 114: 643–651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berschneider B, Königshoff M: WNT1 inducible signaling pathway protein 1 (WISP1): A novel mediator linking development and disease. Int J Biochem Cell Biol 43: 306–309, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lehrke I, Schaier M, Schade K, Morath C, Waldherr R, Ritz E, Wagner J: Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol Renal Physiol 282: F741–F751, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Ito T, Imai E, Yamato M, Iwatani H, Kawachi H, Hori M: Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol 14: 981–991, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Pérez de Lema G, Lucio-Cazaña FJ, Molina A, Luckow B, Schmid H, de Wit C, Moreno-Manzano V, Banas B, Mampaso F, Schlöndorff D: Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int 66: 1018–1028, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR: Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol 82: 568–576, 2004 [DOI] [PubMed] [Google Scholar]
- 28.D’Ambrosio DN, Clugston RD, Blaner WS: Vitamin A metabolism: An update. Nutrients 3: 63–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H: A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315: 820–825, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Molotkov A, Fan X, Duester G: Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3. Eur J Biochem 269: 2607–2612, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Chithalen JV, Luu L, Petkovich M, Jones G: HPLC-MS/MS analysis of the products generated from all-trans-retinoic acid using recombinant human CYP26A. J Lipid Res 43: 1133–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Zhuang R, Lin M, Napoli JL: cis-Retinol/androgen dehydrogenase, isozyme 3 (CRAD3): A short-chain dehydrogenase active in a reconstituted path of 9-cis-retinoic acid biosynthesis in intact cells. Biochemistry 41: 3477–3483, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA: A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116–F1119, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Chuang PY, Dai Y, Liu R, He H, Kretzler M, Jim B, Cohen CD, He JC: Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS ONE 6: e23566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, Ross MJ, Chen N, Ma’ayan A, He JC: A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med 18: 580–588, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baelde JJ, Bergijk EC, Hoedemaeker PJ, de Heer E, Bruijn JA: Optimal method for RNA extraction from mouse glomeruli. Nephrol Dial Transplant 9: 304–308, 1994 [PubMed] [Google Scholar]
- 39.Chuang PY, Yu Q, Fang W, Uribarri J, He JC: Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.