Abstract
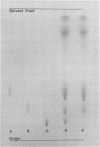
Relapsing fever borreliae require lipid compounds for growth in vitro. In this study, the major pathways of lipid catabolism in three species of tick-borne relapsing fever borreliae were investigated. Thin-layer chromatography was used to compare chloroform-methanol extracts of fresh culture media with extracts of exhausted culture media after organisms were removed by centrifugation. The chromatographic data demonstrated that lysolecithin was removed from the culture media during growth of the spirochetes, whereas lecithin, sphingomyelin, triglycerides, and cholesterol esters were not affected by growth of the organisms. Sonic extracts of the organism were tested for the presence of specific enzymes of lipid catabolism. Lysolecithinase, glycerophosphorylcholine diesterase, and acid phosphatase activities were demonstrated. Thus, these organisms can sequentially dissimilate lysolecithin to fatty acids, choline, inorganic phosphate, and glycerol. Assays for phospholipases A, C, and D, α-glycerophosphate dehydrogenase, alkaline phosphatase, and lipase were negative.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Johnson R. C., Peterson D. Metabolism of Common Substrates by the Reiter Strain of Treponema pallidum. Infect Immun. 1971 Jun;3(6):727–734. doi: 10.1128/iai.3.6.727-734.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. J., Cornatzer W. E. Rat kidney glycerylphosphorylcholine diesterase. Biochim Biophys Acta. 1968 Oct 22;164(2):195–204. doi: 10.1016/0005-2760(68)90146-x. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. Liver glycerylphosphorylcholine diesterase. Biochem J. 1956 Apr;62(4):689–693. doi: 10.1042/bj0620689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELSENFELD O., DECKER W. J., WOHLHEITER J. A., RAFY A. STUDIES IN BORRELIAE. II. SOME IMMUNOLOGIC, BIOCHEMICAL AND PHYSICAL PROPERTIES OF THE ANTIGENIC COMPONENTS OF BORRELIA TURICATAE. J Immunol. 1965 May;94:805–817. [PubMed] [Google Scholar]
- FULTON J. D., SMITH P. J. Carbohydrate metabolism in Spirochaeta recurrentis. 1. The metabolism of spirochaetes in vivo and in vitro. Biochem J. 1960 Sep;76:491–499. doi: 10.1042/bj0760491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., KORNBERG A. Metabolism of phospholipides by bacterial enzymes. J Biol Chem. 1954 Feb;206(2):647–663. [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of phosphomonoesterases. Arch Biochem Biophys. 1954 Jul;51(1):139–146. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. Beta-oxidation of fatty acids by Leptospira. Can J Microbiol. 1970 Jan;16(1):41–45. doi: 10.1139/m70-007. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Eggebraten L. M. Fatty Acid Requirements of the Kazan 5 and Reiter Strains of Treponema pallidum. Infect Immun. 1971 Jun;3(6):723–726. doi: 10.1128/iai.3.6.723-726.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G., Walby J. K. Characterization of leptospires according to fatty acid requirements. J Gen Microbiol. 1969 Mar;55(3):399–407. doi: 10.1099/00221287-55-3-399. [DOI] [PubMed] [Google Scholar]
- KUO M. H., BLUMENTHAL H. J. Purification and properties of an acid phosphomonoesterase from Neurospora crassa. Biochim Biophys Acta. 1961 Sep 2;52:13–29. doi: 10.1016/0006-3002(61)90899-x. [DOI] [PubMed] [Google Scholar]
- Kasarov L. B., Addamiano L. Metabolism of the lipoproteins of serum by leptospires: degradation of the triglycerides. J Med Microbiol. 1969 May;2(2):165–168. doi: 10.1099/00222615-2-2-165. [DOI] [PubMed] [Google Scholar]
- Kelly R. Cultivation of Borrelia hermsi. Science. 1971 Jul 30;173(3995):443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- Kăsarov L. B., Addamiano L. Degradation of the phospholipids of the serum lipoproteins by leptospirae. J Med Microbiol. 1969 Aug;2(3):243–248. doi: 10.1099/00222615-2-3-243. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., TAKEMORI A. E., LARDY H. Enhanced oxidation of alpha-glycerophosphate by mitochondria of thyroid-fed rats. J Biol Chem. 1959 Nov;234:3051–3054. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Leibovitz Z., Gatt S. Isolation of lysophospholipase, free of phospholipase activity, from rat brain. Biochim Biophys Acta. 1968 Oct 22;164(2):439–441. doi: 10.1016/0005-2760(68)90173-2. [DOI] [PubMed] [Google Scholar]
- MAGEE W. L., THOMPSON R. H. The estimation of phospholipase A activity in aqueous systems. Biochem J. 1960 Dec;77:526–534. doi: 10.1042/bj0770526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARPLES E. A., THOMPSON R. H. The distribution of phospholipase B in mammalian tissues. Biochem J. 1960 Jan;74:123–127. doi: 10.1042/bj0740123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van Deenen L. L. The effect of alterations in fatty acid composition and cholesterol content on the permeability of Mycoplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1970;219(1):245–247. doi: 10.1016/0005-2736(70)90083-0. [DOI] [PubMed] [Google Scholar]
- PATEL V., GOLDBERG H. S., BLENDEN D. CHARACTERIZATION OF LEPTOSPIRAL LIPASE. J Bacteriol. 1964 Oct;88:877–884. doi: 10.1128/jb.88.4.877-884.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajković A. D. Cultivation of the Noguchi strain of Treponema pallidum in a lipid medium in the absence of serum. Z Med Mikrobiol Immunol. 1967;153(4):297–312. doi: 10.1007/BF02123751. [DOI] [PubMed] [Google Scholar]
- Roemer G. B., Baltzersen E. Die wachstumsfördernde Wirkung von Lipiden in Kulturen von Reiterspirochäten. Z Hyg Infektionskr. 1965;151(3):204–210. [PubMed] [Google Scholar]
- SHAPIRO B. Purification and properties of a lysolecithinase from pancreas. Biochem J. 1953 Mar;53(4):663–666. doi: 10.1042/bj0530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Smith P. J. Carbohydrate metabolism in Spirochaeta recurrentis. 2. Enzymes associated with disintegrated cells and extracts of spirochaetes. Biochem J. 1960 Sep;76(3):500–508. doi: 10.1042/bj0760500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATTRIE N. H., MCARTHUR C. S. The preparation of L-alpha-glycerylphosphorylcholine from lecithins. Can J Biochem Physiol. 1955 Sep;33(5):761–766. [PubMed] [Google Scholar]
- TSUBOI K. K., HUDSON P. B. Acid phosphatase. VI. Kinetic properties of purified yeast and erythrocyte phosphomonoesterase. Arch Biochem Biophys. 1956 Mar;61(1):197–210. doi: 10.1016/0003-9861(56)90332-0. [DOI] [PubMed] [Google Scholar]