Abstract
Because combined administration of intramuscular and intravenous interferon has been partially successful in the incubationary treatment of rabies, the effect of direct interferon administration into the cerebrospinal fluid space was tested. After injecting 1,800 U of interferon into the cisterna magna or the lateral ventricle, periodic samples, obtained by cisternal taps, showed that 1 to 5% remained after 24 h, as opposed to the known clearance of interferon from the bloodstream to this level within minutes. The distributions of interferon and 131I-labeled albumin were similar as demonstrated by kinetics of clearance monitored over 24 h. Beginning with and after experimental infection of rabbits, daily intraventricular injections of one million units of interferon were given for as long as 3 weeks. Interferon was prepared from cell culture fluids after pressure dialysis and chromatography on Sephadex G-100. This intensive treatment did not prevent encephalitis, but prolonged the length of the incubation period by one- to two-thirds. The outcome after intraventricular administration was not as favorable as when one million units equally divided between intramuscular and intravenous injections were given at the time of challenge. Interferon administered in the subarachnoid space in this fashion is apparently inadequate to protect the rabbit against rabies. Its role as an adjunct measure, or other methods of administration in the nervous system, remains to be examined.
Full text
PDF

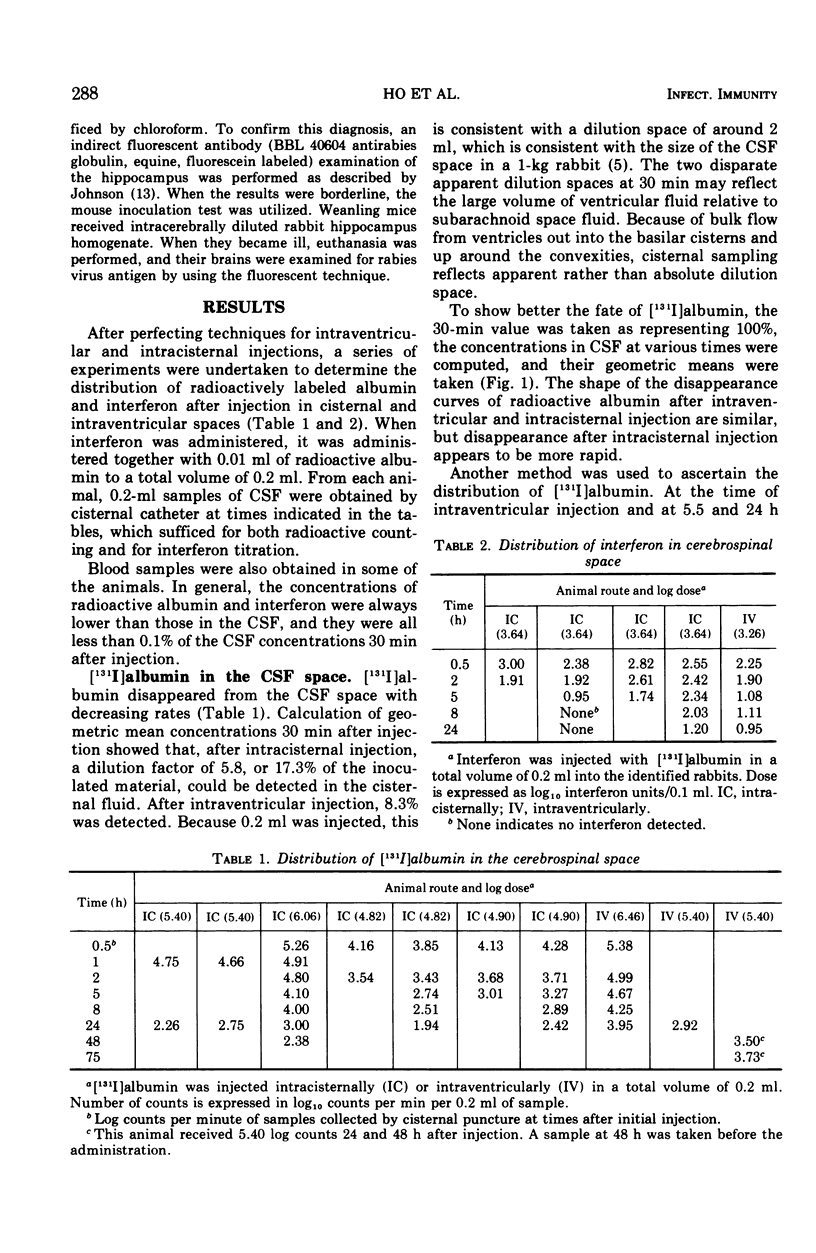
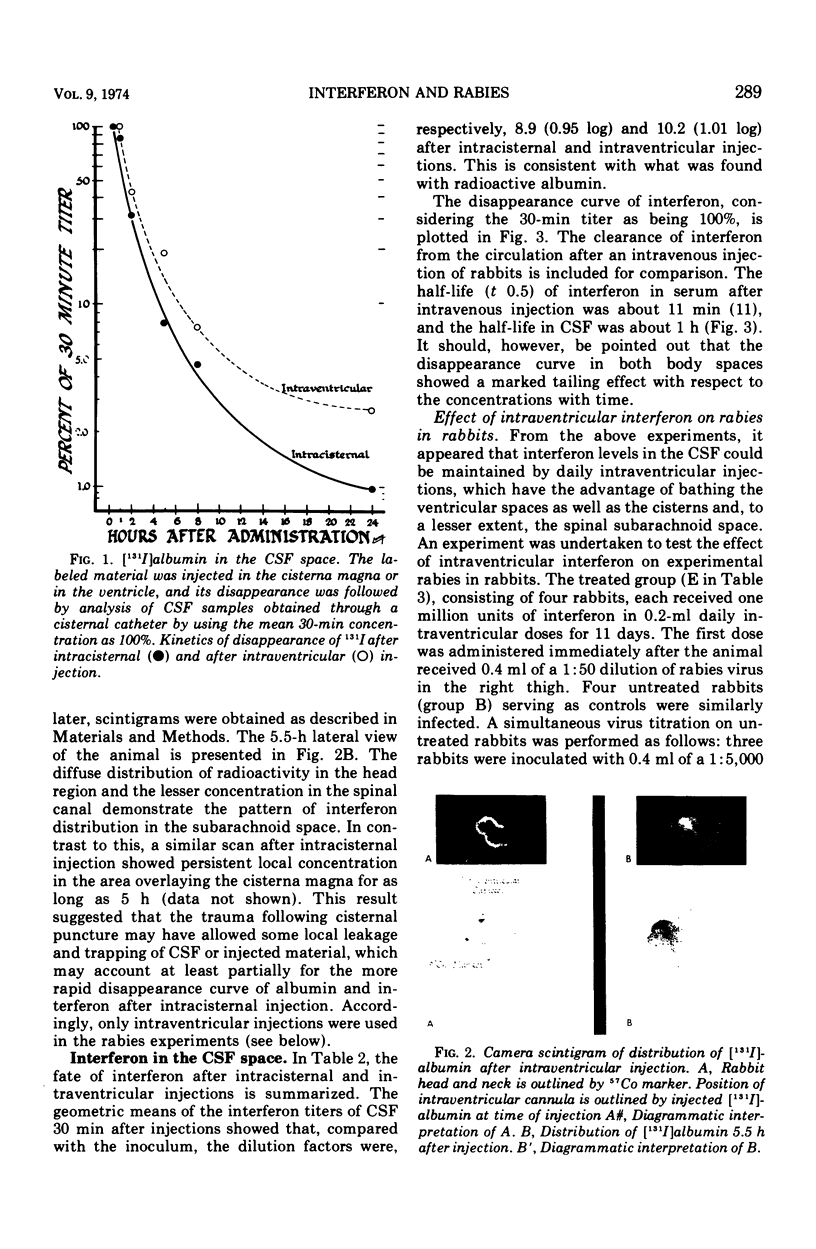
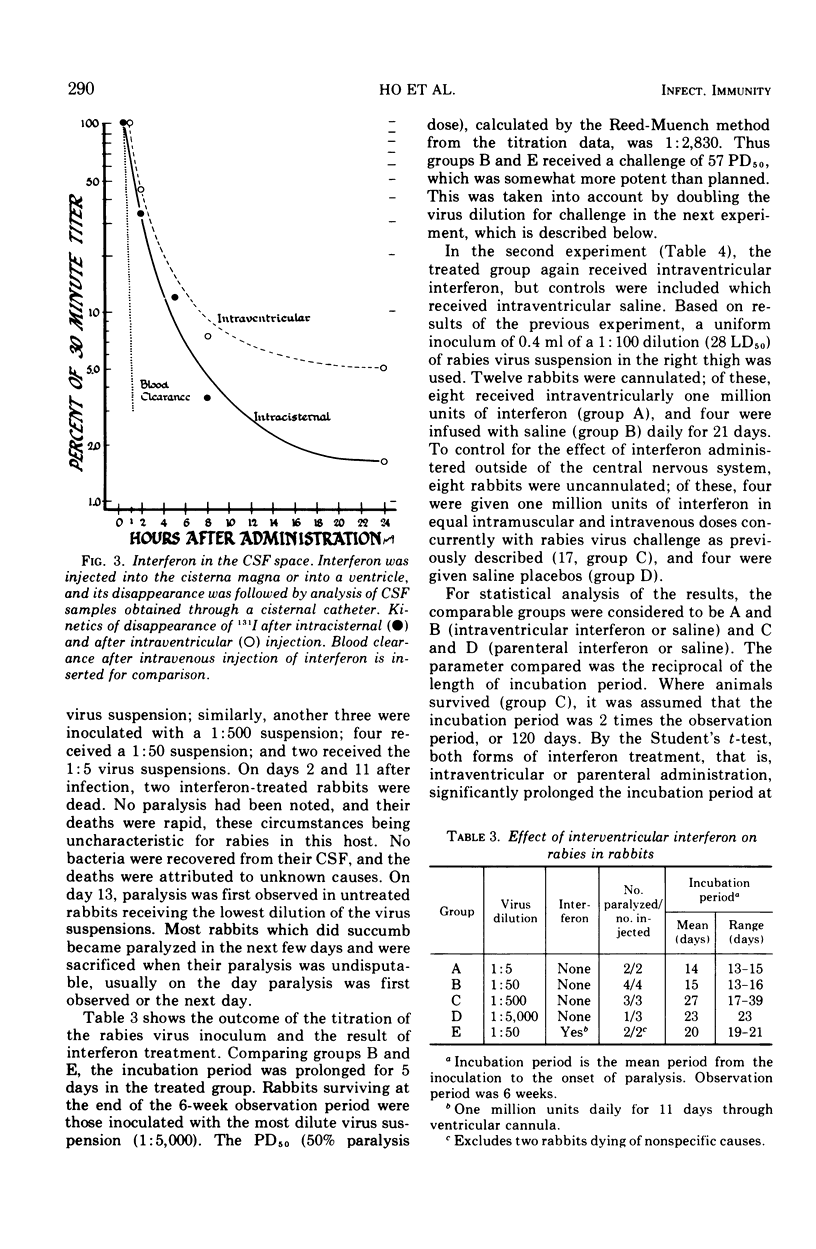


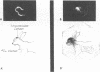
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasiu P., Barroeta M., Tsiang H., Favre S. Inhibition in vivo de la multiplication du virus rabique par un interféron endogène. Ann Inst Pasteur (Paris) 1970 Dec;119(6):767–777. [PubMed] [Google Scholar]
- Baer G. M., Cleary W. F. A model in mice for the pathogenesis and treatment of rabies. J Infect Dis. 1972 May;125(5):520–527. doi: 10.1093/infdis/125.5.520. [DOI] [PubMed] [Google Scholar]
- Cathala F., Baron S. Interferon in rabbit brain, cerebrospinal fluid and serum following administration of polyinosinic-polycytidylic acid. J Immunol. 1970 Jun;104(6):1355–1358. [PubMed] [Google Scholar]
- Cserr H. F. Physiology of the choroid plexus. Physiol Rev. 1971 Apr;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- Fenje P., Postic B. Prophylaxis of experimental rabies with the polyriboinosinic-polyribocytidylic acid complex. J Infect Dis. 1971 Apr;123(4):426–428. doi: 10.1093/infdis/123.4.426. [DOI] [PubMed] [Google Scholar]
- Fenje P., Postic B. Protection of rabbits against experimental rabies of poly I-poly C. Nature. 1970 Apr 11;226(5241):171–172. doi: 10.1038/226171a0. [DOI] [PubMed] [Google Scholar]
- Goodrich C. A., Greehey B., Miller T. B., Pappenheimer J. R. Cerebral ventricular infusions in unrestrained rats. J Appl Physiol. 1969 Jan;26(1):137–140. doi: 10.1152/jappl.1969.26.1.137. [DOI] [PubMed] [Google Scholar]
- Janis B., Habel K. Rabies in rabbits and mice: protective effect of polyriboinosinic-polyribocytidylic acid. J Infect Dis. 1972 Apr;125(4):345–352. doi: 10.1093/infdis/125.4.345. [DOI] [PubMed] [Google Scholar]
- Ke Y. H., Ho M. Characterization of virus- and endotoxin-induced interferons obtained from the serum and urine of rabbits. J Virol. 1967 Oct;1(5):883–890. doi: 10.1128/jvi.1.5.883-890.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Reed S. E., Hall T. S., Tyrrell D. A. Inhibition of respiratory virus infection by locally applied interferon. Lancet. 1973 Mar 17;1(7803):563–567. doi: 10.1016/s0140-6736(73)90714-9. [DOI] [PubMed] [Google Scholar]
- Postic B., Fenje P. Effect of administered interferon on rabies in rabbits. Appl Microbiol. 1971 Sep;22(3):428–431. doi: 10.1128/am.22.3.428-431.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Postic B., Ho M., Koprowski H. Role of interferon induction in the protective activity of rabies vaccines. J Infect Dis. 1972 Oct;126(4):408–418. doi: 10.1093/infdis/126.4.408. [DOI] [PubMed] [Google Scholar]
- Young C. W. Interferon induction in cancer: with some observations on the clinical effects of poly 1:C. Med Clin North Am. 1971 May;55(3):721–728. doi: 10.1016/s0025-7125(16)32513-5. [DOI] [PubMed] [Google Scholar]