Abstract
In this issue of Cancer Cell, Mason et al. describe development of a Polo-like kinase 4 (Plk4) inhibitor (CFI-400945) with promising activity against tumors formed in mice from patient-derived tumor tissue. A clinical trial has initiated, but questions remain over whether Plk4 is the only relevant therapeutic target.
Protein kinases are prime targets for cancer therapeutics due to their druggability and central role in cellular growth pathways. Reported in this issue of Cancer Cell, Mason et al., perform an siRNA screen for kinases and related genes whose reduction suppresses the proliferation of breast cancer or immortalized cell lines in vitro(Mason et al., 2014). One candidate that emergedwasPolo-like kinase 4 (Plk4). Suppression of Plk4 levels by siRNA significantly reduced cellular proliferation in about half of the breast cancer cell lines tested, fueling a screen for novel Plk4inhibitors that identified a potent ATP competitive inhibitor known as CFI-400945.
Plk4 activity is essential for duplication of the centrosome, the cells major microtubule organizing center (Nigg and Raff, 2009). Centrosomes are present asa single copy at the beginning of the cycle and duplicate once during S phase, thereby yielding precisely two copies, each of which becomes a spindle pole in mitosis (Figure 1A). At the core of the centrosome lies a pair of centriolesthatact as the centrosome organizer and tight regulation of their replication controls centrosome duplication (Nigg and Raff, 2009). Polo-like kinase 4 (Plk4) has emerged as a central regulator of centriole duplication. Suppression of Plk4 levels causes a failure of centriole and centrosome duplication, while overexpressionleads to supernumarycentrioles, a feature sufficient to drive centrosome amplification and subsequent genome instability.
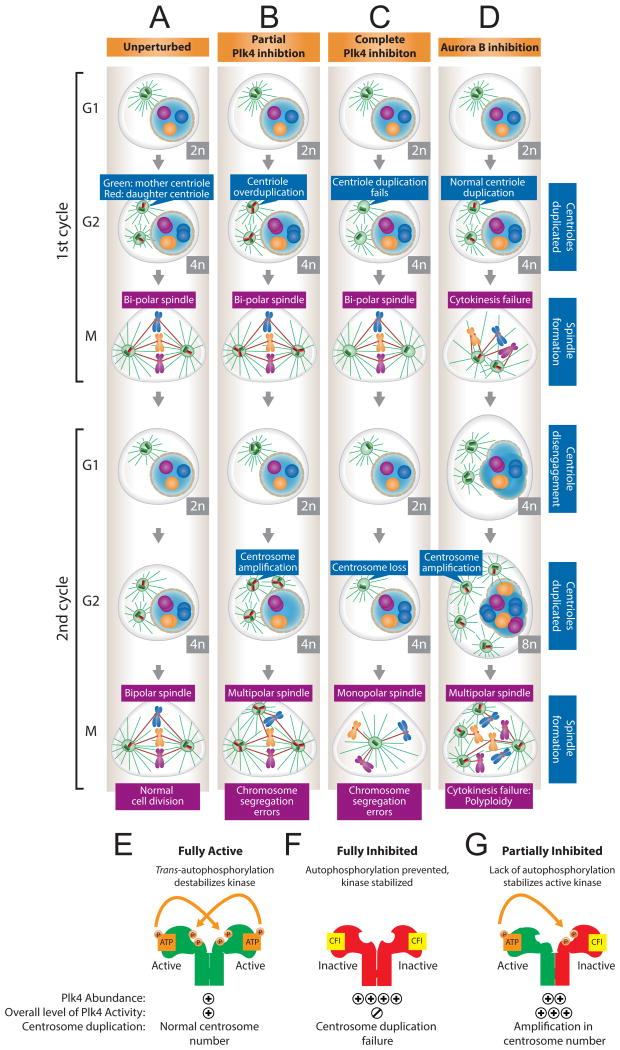
Figure 1. Effect of Plk4 or Aurora B inhibition on centriole number.
(A) Unperturbed. At the beginning of the cell cycle, cells contain a single centrosome comprised of a pair of centrioles. One new daughter centriole (red) is created next to each existing mother centriole (green). The two pairs of centrioles form two centrosomes that separate and guide the formation of the bipolar mitotic spindle upon which chromosomes are segregated. Like chromosomes, the centrosomes are equally divided into the daughter cells by the mitotic spindle. The two centrioles undergo disengagement in G1 to “license” centriole duplication in the next cell cycle. (B) Partial Plk4 inhibition. Partial inhibition of Plk4 leads to the formation of supernumerary centrioles (centriole overduplication). In the first cell cycle, extra centrioles remain cohesed as functional units and enable normal bipolar spindle assembly. In the second cell cycle, supernumerary centrioles undergo disengagement leading to the creation of multiple centrosomes. This results in the formation of a multipolar mitotic spindle that can promote chromosome segregation errors. (C) Complete Plk4 inhibition. Complete inhibition of Plk4 results in a failure of centriole duplication. In the first cell cycle, cells can form bipolar spindles with a single centriole at each pole. In the second cell cycle, a further failure of centriole duplication leads to cells entering mitosis with a single centrosome. This results in the formation of an aberrant mitotic spindlethat can promote chromosome segregation errors. (D) Aurora B inhibition. Aurora B inhibition causes rapid progression through mitosis and a failure of cytokinesis. Cells enter the second cell cycle with twice the normal number of chromosomes and centrosomes. Successive failed cell divisions lead to further polyploidization. (E) Plk4 forms homodimers with two molecules phosphorylating intrans to target the kinase for destruction. This auto-catalyzed destruction places Plk4 stability under direct control of its own activity (Holland et al., 2010). (F) Treatment with high doses of CFI-400945 (CFI) inhibits Plk4 activityand prevents auto-catalyzed destruction. This results in a failure of centriole duplication and an increase in Plk4 abundance. (G) Low doses of CFI-400945 generate heterodimers ofinhibited and activePlk4. Kinase inactive Plk4 cannot phosphorylate and destabilize the active Plk4, leading to an increase in kinase activity that results in centriole overduplication.
While sequencing of cancer cell genomes has not revealed recurrent/driver mutations in Plk4, bothincreased and decreased expression of the kinase have been reported in a variety of tumor types. Although the significance of these expression changes remains untested, Plk4 heterozygous mice are predisposed to tumorigenesis (Ko et al., 2005) and overexpression of Plk4 in Drosophila neuroblasts promotes transformation (Basto et al., 2008). These observations have made identification of a selective Plk4 inhibitor highly desirable, both as a potential therapeutic agent and for dissecting the function(s) of this kinase.
Plk4 is a low abundance enzyme that phosphorylates itself to promote its own destruction (Figure 1E). This self-regulation occurs through the formation of Plk4 homodimers that trans-autophosphorylate a conserved phosphodegron to target the kinase for proteasomal degradation(Figure 1F) (reviewed in (Zitouni et al., 2014)). As anticipated, complete inhibition of Plk4 withCFI-400945 led to an increase in Plk4 abundance and a failure of centriole duplication(Mason et al., 2014). Unexpectedly, however, lower doses of CFI-400945 drove the opposite: an increase in centriole number.
The bimodal effect of CFI-400945 concentration on centriole number is surprising. However, stable overexpression of a kinase inactive Plk4in the presence of the endogenous protein causes centriole overduplication(Guderian et al., 2010). This situation is thought to arise from the formation of heterodimers between kinase inactive and catalytically-active Plk4. Under these conditions kinase inactive Plk4 is unable to trans-autophosphorylate anddestabilize wild type Plk4, leading to an increase in the abundance of the wild type kinase that results in centriole overduplication. It is plausible thatdoses of CFI-400945 that partially inhibit Plk4 activity generate mixed heterodimers in which active Plk4 is stabilized, thereby increasing overall kinase activity (Figure 1G).
Mason et al. examined the long-term effect of CFI-400945 in a wide panel of breast cancer cell lines (Mason et al., 2014). Plk4mRNA levels were elevated in breast cancer cell lines relative to non-transformed cells. Nevertheless, the level of Plk4 expression was not predictive of CFI-400945 sensitivity and thus it remains unclear whether alterations in Plk4 levels are causal events in tumor initiation or progression. More surprisingly, cell lines that showed the greatest reduction in growth after Plk4 depletion by siRNAwere among the most resistant to the effects of Plk4 inhibition withCFI-400945. One explanation is that loss of Plk4 protein and inhibition of Plk4 kinase activity have different effects on cellular growth. An alternative possibility is that the cytostatic effect of CFI-400945 is caused, at least in part, by inhibition of targets in addition to Plk4 (see below).
A synthetic interaction screen previously reported that PTEN deficient breast cancer cell lines are particularly sensitized to Plk4 depletion (Brough et al., 2011). Consistently, Mason et al. report that loss of the tumor suppressor PTEN increases sensitivity to CFI-400945 (Mason et al., 2014). Given that several of the breast cancer cell lines tested are relatively resistant to CFI-400945 in vitro, it will be important to establish the genetic lesions that confer increased sensitivity so that responsive tumors may be pre-selected.
Testing of CFI-400945 in miceshowed this agent to be well tolerated with efficient anti-tumor activity in both cancer cell lines and patient derived xenograft tumors, including tumors derived from carboplatin resistant primary human breast cancers. This promising discovery has led to CFI-400945 entering Phase I clinical trials. While the preclinical studies provide optimism for the use of CFI-400945 as a novel anti-cancer therapy, some caution would also be advised. A first consideration here are the well documented limitations of using xenograft models to predict a clinical response, with several drugs that show dramatic tumor killing in xenograftmodels failing to yield clinical benefits (Sharpless and Depinho, 2006).
Additionally, it remains unclear if Plk4 is the only, or primary, therapeutic target of CFI-400945. All kinases share a similar catalytic core and achieving target specificity with ATP competitive inhibitors remains challenging. Plk4 is the most divergent member of the Polo-like kinase family and correspondingly CFI-400945 had no inhibitoryactivity towards Polo-like kinases 1-3. Nevertheless, CFI-400945 hadsignificant inhibitory activity towardsseveral other kinases, including Aurora B both in vitro and in cells (Mason et al., 2014). Consistently, CFI-400945 treatment accompanied by partial or complete Plk4 inhibition led tocytokinesis failure and subsequent polyploidization (Figure 1B,C). This outcome is what would be expected for inhibition of Aurora B (Keen and Taylor, 2009), which has been well documented to resultin rapid progression through mitosis, a failure of cytokinesis, and a corresponding increase in centrosome number (Figure 1D). Given that Aurora B inhibitors exhibit excellent anti-tumor activity in xenograftsof human tumor cell lines (and some are now in clinical evaluation) (Keen and Taylor, 2009), it seems likelythatinhibition of Aurora Bis at least partly responsible for the therapeutic response to CFI-400945. Perhaps inhibition of Plk4 and Aurora B will prove to be a particularly efficacious anti-cancer strategy.
Plk4 is a highly unusual therapeutic target, as partial inhibition of kinase activity leads to a phenotype consistent with increased protein function. This highlights a dichotomy when using Plk4 inhibitors therapeutically: depending on the effective dose achieved in the tumor, Plk4 inhibitors could partially inhibit Plk4 resulting incentrosome amplification or completely inhibit Plk4 and promote centrosome loss (Figure 1F,G). Since centrosome amplification has been linked with tumorigenesis, the in vivo consequence(s) of using Plk4 inhibitors may vary with the degree of target inhibition.
CFI-400945 is the first potent Plk4 inhibitor to be described and experiments in mice indicate this agent may be a useful oncology drug. However, the inhibition of Aurora B by CFI-400945 complicates understanding the drugs mechanism of action (Mason et al., 2014). Recognizing that, the question of whether inhibition of Plk4 alone is avaluable therapeutic strategy will have to await development of additional Plk4 kinase inhibitors.
Acknowledgments
We apologize to colleagues whose work was not discussed or cited owing to space constraints. Kevin Clutario is gratefully acknowledged for help preparing the figures. A.J.H is funded by a Leukemia & Lymphoma Society special fellowship, a Leukemia Research Foundation Research Grant, a W.W. Smith Charitable Trust Research Grant, a March of Dimes Basil O'Conner Scholar Award, a Pew Scholar Award and a Kimmel Scholar Award.D.W.C. is funded by a grant (R01-GM29513) from the National Institutes of Health and receives salary support from the Ludwig Institute for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew J. Holland, Email: aholland@jhmi.edu.
Don W. Cleveland, Email: dcleveland@ucsd.edu.
References
- Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough R, Frankum JR, Sims D, Mackay A, Mendes-Pereira AM, Bajrami I, Costa-Cabral S, Rafiq R, Ahmad AS, Cerone MA, et al. Functional viability profiles of breast cancer. Cancer Discov. 2011;1:260–273. doi: 10.1158/2159-8290.CD-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian G, Westendorf J, Uldschmid A, Nigg EA. Plk4 trans-autophosphorylation regulates centriole number by controlling betaTrCP-mediated degradation. J Cell Sci. 2010;123:2163–2169. doi: 10.1242/jcs.068502. [DOI] [PubMed] [Google Scholar]
- Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. The Journal of Cell Biology. 2010;188:191–198. doi: 10.1083/jcb.200911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N, Taylor S. Mitotic drivers--inhibitors of the Aurora B Kinase. Cancer metastasis reviews. 2009;28:185–195. doi: 10.1007/s10555-009-9184-9. [DOI] [PubMed] [Google Scholar]
- Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, Swallow CJ. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- Mason JM, Lin DCC, Wei X, Che Y, Yao Y, Kiarash R, Cescon DW, Fletcher GC, Awrey DE, Bray MR, et al. Functional Characterization of CFI-400945, a Polo-like Kinase 4 Inhibitor, as a Potential Anticancer Agent. Cancer Cell. 2014 doi: 10.1016/j.ccr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature reviews Drug discovery. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias M. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol. 2014;15:433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]