Abstract
Psychological or physical stress causes an elevation of glucocorticoids in the circulating system. Glucocorticoids regulate a variety of physiological functions, from energy metabolism and biochemical homeostasis to immune response. Synthetic steroids are among the most prescribed drugs for immune suppression and chemotherapy. While glucocorticoids are best known for inducing apoptosis in a number of cell types, we have found that corticosteroids at stress relevant levels protect cardiomyocytes from apoptosis. Current study addresses whether glucocorticoids inhibit cardiac injury in vivo. Adult male C57BL6 mice were administered with dexamethasone (20 mg/kg, i.p.) or vehicle control 20 hours prior to left anterior descending coronary artery occlusion surgery. Myocardial infarction was measured by triphenyl tetrazoliumchloride staining in tissue slices and by levels of cardiac Troponin (cTn I) in the blood. Treatment of dexamethasone markedly reduced infarct size (19.6± 4.3%, vs. 29.2±4.9%, p< 0.01) and cTn I level in the blood (3.83±0.66 ng/ml vs. 5.62±0.37 ng/ml, p<0.01). In studying the mechanism of such protection, we found that dexamethasone induces the expression of Bcl-xL gene in the myocardium. With cardiomyocytes in culture, glucocorticoids increase transcription of Bcl-xL gene as evidenced by Bcl-xL mRNA increase and promoter activation. The glucocorticoid receptor antagonist mifepristone prevented dexamethasone from inducing cardiac protection or Bcl-xL expression. Our data suggest that activation of glucocorticoid receptor can prevent cardiac injury through transcriptional activation of Bcl-xL gene.
Keywords: Steroids, Glucocorticoid receptor, Bcl-xL gene, Transcription, Cytoprotection
1. Introduction
Psychological stress is an inevitable event of our daily life. Stress increases synthesis of glucocorticoids from the adrenal glands, causing an elevated level of circulating glucocorticoids from the baseline of 3–16 µg/dl to 25 µg/dl or higher. Such increase results from activation of hypothalamic-pituitary-adrenal axis. The major form of glucocorticoid is cortisol (i.e. hydrocortisone) in the human or corticosterone in rodents. Whereas overproduction of glucocorticoids suppresses the immune system and causes psychiatric disorders, metabolic disease and osteoporosis, glucocorticoids are well known for inducing apoptosis in a number of cell types, including lymphocytes, osteocytes and neuronal cells (Chen and Sun, 2009; Piazza and Le Moal, 1996; Sun et al., 2008a; Sun et al., 2008b; Sun et al., 2008c). Synthetic glucocorticoids have been widely used as anti-inflammatory agents and immune suppressants. A few examples of frequently prescribed synthetic glucocorticoids are dexamethasone, cortisone, prednisone and methylprednisolone. Dexamethasone has a higher efficacy and longer half life than endogenous glucocorticoids.
Although a large number of studies have been carried out on the function and pharmacological implication of glucocorticoids, the specific effect of these steroids has not been well studied on the heart. A randomized trial with 235 patients undergoing coronary artery or valvular heart surgery found that dexamethasone reduces postoperative fever and atrial fibrillation (Yared et al., 2007; Yared et al., 2000). Although the number of deaths or myocardial infarction incidence is small among the studied patient population, dexamethasone appears to be protective (Yared et al., 2007; Yared et al., 2000). A single dose of methylprednisolone before cardiopulmonary bypass surgery improves myocardial function (Liakopoulos et al., 2007). An early study with experimental dogs found that hydrocortisone administration reduced myocardial infarction size (Libby et al., 1973). With experimental rats, pretreatment of methylprednisolone protects the heart from ischemic reperfusion injury (Valen et al., 2000). In contrast to these observed protective effect, reducing corticosteroids by adrenalectomy impairs sarcoplasmic reticulum (SR) Ca2+ cycling due to reduction of SR-associated Ca2+−calmodulin kinase II protein (Rao et al., 2001). At the cellular level, dexamethasone regulates outward K+ current and L-type Ca2+ current to prolong action potential repolarization (Wang et al., 1999a). Overexpressing the glucocorticoid receptor gene specifically in cardiomyocytes causes benign electrocardiogram abnormalities without cardiac hypertrophy, fibrosis or mortality (Sainte-Marie et al., 2007). These are among the limited literature in the area of glucocorticoids’ effect on the heart.
Previous works from our laboratory have demonstrated that glucocorticoids elicit cytoprotective response in cultured cardiomyocytes (Chen et al., 2005). Microarray analyses reveal 140 upregulated genes and 108 downregulated gene in corticosterone treated rat cardiomyocytes (Chen et al., 2005), among which is upregulated Bcl-xL. We have also reported that corticosterone activates p38 MAP kinase, CREB, c/EBPβ and Sp3 transcription factors (Sun and Chen, 2008; Sun et al., 2008a; Sun et al., 2008b). This study addresses whether glucocorticoids protect cardiomyocytes in vivo. We have used left anterior descending coronary artery occlusion as a model to determine the effect of glucocorticoids on cardiac injury and whether or not corticosteroid administration reduces experimental myocardial infarct size.
2. Materials and methods
2.1. Induction of myocardial infarction
Laboratory animals were cared for according to National Institute of Health guideline for the Use of Laboratory Animals. Experimental protocols were reviewed and approval by University of Arizona Institutional Animal Care and Use Committee. Male C57BL/6 mice (Jackson Lab, ME; or Harlan, IN) at 8–11 weeks old were used for dexamethasone administration (i.p. 20 mg/kg) with vehicle control (vegetable oil) 20 h prior to surgery. A tracheotomy was performed to ventilate the animal through a Harvard Rodent Respirator (Harvard Apparatus, Boston, MA). A left lateral thoracotomy was performed at the 3th intercostal space with sufficient incision size to expose the pericardium. Upon exposure of the heart, an 8–0 silk suture was tightened around the proximal left anterior descending coronary artery after rapidly passing through the myocardium with a tapered needle, 1–3 mm from the tip of the left atrium. Occlusion of coronary artery results in a visible blanched area in the myocardium distal to the ligation site, serving as an indicator for successful coronary artery ligation. Sham-operated control animals were prepared in the same manner except the left anterior descending coronary artery was not ligated and therefore did not develop myocardial ischemia or infarction.
For ischemic preconditioning, after placing a 8–0 sterile suture through the myocardium underneath the left anterior descending artery 1–3 mm from the tip of the left atrium, both ends of the suture were passed through a piece of 1–2 mm PE50 hollow tube in opposite directions so that a cross was formed inside the tube. While pulling two ends of the suture in opposite directions to place the PE50 tube perpendicular to left anterior descending, ischemia was produced by clamping the sutures against the tube tightly. The success of ischemia is evidenced by the development of blanched area in the myocardium downstream of the ligation site. Following 5 mins of ischemia, the suture was loosened up for 5 mins allowing reperfusion. Reperfusion causes the return of a bright red color to the ischemic area. The cycle of 5 mins ischemia and 5 mins reperfusion was repeated 2 times before permanent occlusion of the left anterior descending coronary artery.
The chest cavity is closed by bringing together the second and third ribs with one 6–0 nylon suture, slight pressure was applied on the chest with the needle holder to reduce the volume of free air in the chest cavity while tying a knot. All layers of muscle and skin were closed with 6–0 continuous absorbable and nylon sutures, respectively. Upon recovering from anesthesia, the mice were removed from the ventilator and kept warm with heat lamps with pain management.
2.2. Triphenyl tetrazoliumchloride staining and measurement of infarct size
Upon euthanization by anesthetic overdose, the entire heart was excised. After removal of the great blood vessels, atria and right ventricle, the left ventricle was sectioned into 5 transverse slices even in thickness. The tissue slices were incubated in 1% triphenyl tetrazoliumchloride in phosphate buffered saline, pH 7.4, at 37°C for 20 mins followed by fixation in 10% formalin overnight at 2 − 8°C. Both sides of each stained tissue slice were photographed with a digital camera. The area of infarction for each slide was determined by computerized planimetry using NIH image J software.
2.3. Serum cardiac troponin I ELISA
The blood was collected via the abdominal vena cava and subsequently centrifuging for 10 minutes at 1500g or 3000 rpm for serum collection. Cardiac troponin assay was performed according to the manufacturer's instructions (Life diagnostics, Cat No.2010).
2.4. Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) assay
At 24 h after left anterior descending coronary artery occlusion, the mouse heart was excised for quick frozen in liquid nitrogen. The frozen hearts were used for transverse sections (5 mm thick) by a cryostat microtome. The tissue sections were fixed in acetone, digested with Proteinase K for 10 min at room temperature and incubated with a terminal deoxynucleotide transferase reaction mix in a humid atmosphere for 60 min at 37 °C. The reaction was s topped by 2 × Saline-Sodium Citrate (2× SSC) buffer and TUNEL positive staining shows green fluorescence under a fluorescent microscope. To determine the proportion of apoptotic nuclei within a region of the myocardium, the transverse sections were counterstained with fluorescent DNA binding dye 4’,6’-diamidino-2-phenylindole. Midventricular area was examined microscopically at 20 magnification. Fifteen tissue sections from 3 animals in each group were examined and at least 100 cells were counted per field for 8 or more slides to determine the percentage of apoptotic cells.
2.5. Cell culture
Cardiomyocytes were prepared from 1 to 2-days-old neonatal Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) as previously described (Chen et al., 2005). Cardiomyocytes were seeded at a density of 7.5 × 10 4 cells per well in 6-wells plates in low-glucose DMEM with 10% FBS. At 4 th day after plating, cells were placed in fresh DMEM containing 0.5% FBS for 24 h before experiments.
2.6. Western blot analysis
Frozen heart tissues were grinded into powder form in a liquid nitrogen bath and were dissolved in lysis buffer for electrophoresis after protein concentration measurements by the Bradford method (Bio-Rad, CA). After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane for incubation with antibodies against Bcl-xL (sc-8392, Santa Cruz Biotechnology) or vinculin (V9131, Sigma-Aldrich). Horseradish peroxidase-conjugated secondary antibodies (Zymed) bound to the primary antibodies were detected with an enhanced chemiluminescence reaction.
2.7. RT-PCR and real-time PCR
Total RNA (2 µg) was isolated with TRIzol (Invitrogen) for reverse transcription (RT) using the First Strand cDNA Synthesis kit (Roche). RT products (2 µl) were amplified in a reaction mixture (18 µl) containing 5× reaction buffer, 200 µmol dNTP, 20 pmol each of two primers (forward 5’-ATCGCTAAACACAGAGCAGAC, reverse 5’-TTCAAACTCATCGCCTGCCTC) and 0.4 ml of Phire DNA polymerase with 35 cycles of 98°C for 5’s, 60°C for 5’s and 72°C for 5’s. GAPDH was amplified in parallel PCR as a loading control with the primer pair of forward 5’-AACTTTGGCATTGTGGAAGG and reverse 5’-ACACATTGGGGGTAGGAACA.
For real-time PCR, hexanucleotide random primers were used for RT with 2 µg RNA in a 20 µl reaction mixture. Bcl-xL cDNA was amplified with SsoFast EvaGreen super mix (Bio-Rad) and Bcl-xL primers above. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene. The fluorescence for newly synthesized DNA was detected with Bio-Rad CFX96 real-time PCR system during 40 cycles of 95°C for 5’s and 60°C for 5’s after 30’s denaturation at 95°C. The relative difference between the levels of Bcl-xL cDNA among samples was calculated based on 2−ΔCt, where Ct is the threshold cycle.
2.8. Bcl-xL promoter construct transfection and luciferase assay
The −905 bp promoter sequence of human bcl-x gene was cloned into pGL3 firefly luciferase construct. Cardiomyocytes in 6-well plates were transfected with 0.5 µg bcl-x-luc plasmid and 0.05 µg pRL-TK plasmids per well by FuGene-6 liposomes. pRL-TK plasmid has a Renilla luciferase gene under the control of a thymidine kinase (TK) promoter and was used to correct for transfection efficiency. Cells were placed in 10% FBS/DMEM overnight before being placed in 0.5% FBS/DMEM for 24 h. After serum starvation, cells were treated with vehicle (DMSO, less than 0.05%) or µ mM of dexamethasone with or without 1 µM mifeprestone for 24 h. Dual Luciferase assay was performed according to manufacturer’s instruction (Promega).
2.9. Statistics
The student t test was used when means from two samples, control versus treated group, were compared. One-way analysis of variance (ANOVA) was used to compare groups of means followed by the Bonferroni Correction for multiple samples using Stata 8.2 software.
3. Results
3.1. Dexamethasone reduces cardiac injury
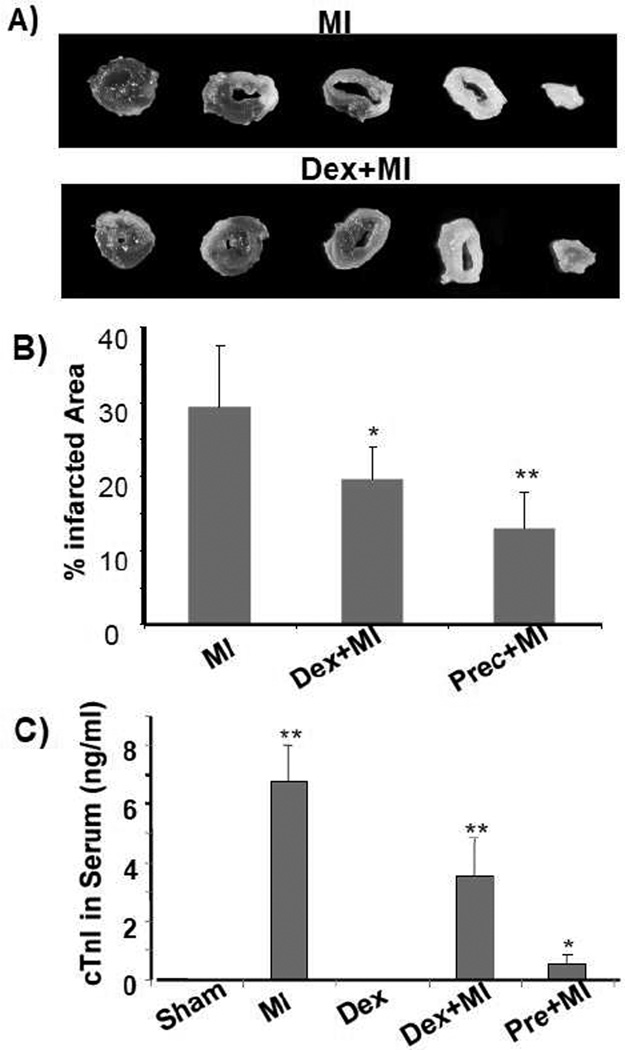
Left coronary artery occlusion induces regional ischemia and infarction occurs within 24 h (Michael et al., 1999; Michael et al., 1995). To demonstrate a protective effect of dexamethasone, we used ischemic preconditioning as a positive control. Preconditioning by brief cycles of ischemia and reperfusion is known to protect the heart from injuries due to prolonged ischemia. When the left anterior descending coronary artery was occluded 5 mins and released 5 mins for two cycles before permanent occlusion, this preconditioning protocol reduced infarction size about 50% (Fig 1A). When mice were pretreated with dexamethasone for 20 h prior to coronary artery occlusion, about 30% reduction in infarct size was observed (Fig 1A). In humans and experimental animals, myocardial infarction can be measured by release of cardiac troponin I (cTnI) from the myocardium into the blood. Elevated blood cTnI levels serve as a quantitative measurement of myocardial injury. While preconditioning of 2 cycles of 5 mins ischemia and 5 mins of reperfusion reduced the level of cTnI in the blood to a minimal, dexamethasone pretreatment caused a significant reduction of cTnI release (Fig 1B).
Fig. 1. Dexamethasone reduces myocardial infarct size and cTnI release.
C57BL6 mice at 8 weeks old were used for left anterior descending coronary artery occlusion surgery. For preconditioning, the coronary artery was occluded for 5 mins then released for 5 mins for two cycles. Dexamethasone (i.p. 20 mg/kg) was administered 20 h prior to the surgery. At 24 h after permanent occlusion, the hearts and plasma were collected for triphenyl tetrazoliumchloride staining to measure areas of infarction (A, B) and for serum cTnI concentration assay respectively. A serial of transverse sections of representative left ventricles downstream of ligature were shown (A). Total left ventricular areas or areas of infarct were quantified by tracing using NIH Image J software. cTnI was not detectable in sham operated or dexamethasone treated control groups. The data represent means + standard deviations from 3 animals in each group (B). * indicates p<0.05 and ** indicates p<0.01 when compared to MI (B) or sham operated control (C).
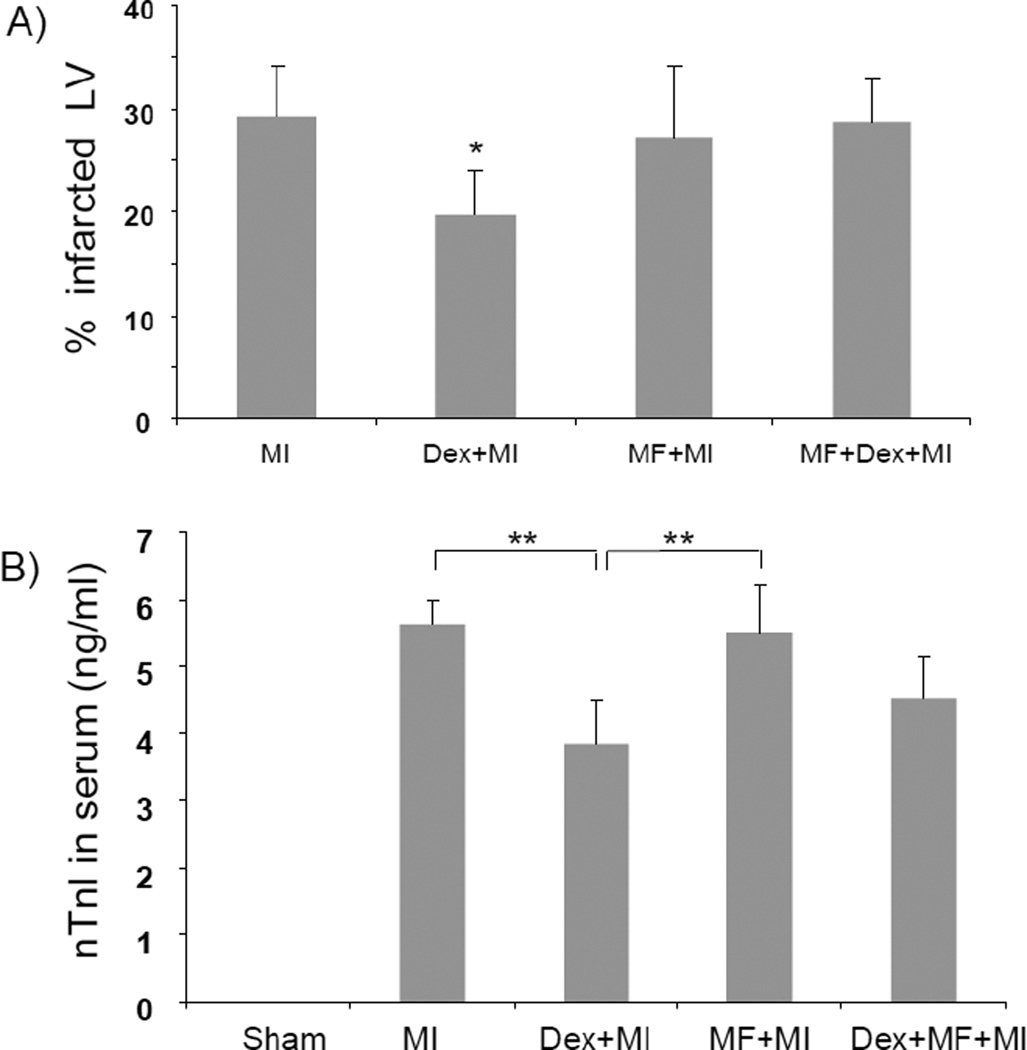
Glucocorticoids bind to their receptor in the cytosol after diffusing through the plasma membrane. Ligand binding causes glucocorticoid receptor to interact with co-factors and to translocate to the nuclei where it acts as a transcription factor or causes chromatin remodeling. Mifeprestone, an antagonist of glucocorticoid receptor, prevents nuclear translocation of glucocorticoid receptor (Cadepond et al., 1997). Mifeprestone was used to test the involvement of glucocorticoid receptor in cardiac protection. Measurements of infarct size and serum cTnI indicate that mifeprestone was able to reverse in part the cardiac protective effect of dexamethasone (Fig 2A & B).
Fig. 2. Mifeprestone attenuates dexamethasone induced cardiac protection.
C57BL6 mice at 8 wks of age were injected with vehicle (vegetable oil, 0.2 mL each mouse) or dexamethasone (20 mg/kg, i.p) in the absence or presence of mifeprestone (70 mg/kg, ip) for 20 h prior to left anterior descending coronary artery occlusion surgery. At 24 h after the surgery, the hearts and plasma were harvested for triphenyl tetrazoliumchloride staining and cTnI measurements respectively. The data represent means + standard deviations from 5–8 animals in each group (B). * indicates p<0.05 and ** indicates p<0.01 when compared to MI (A) or as labeled (B).
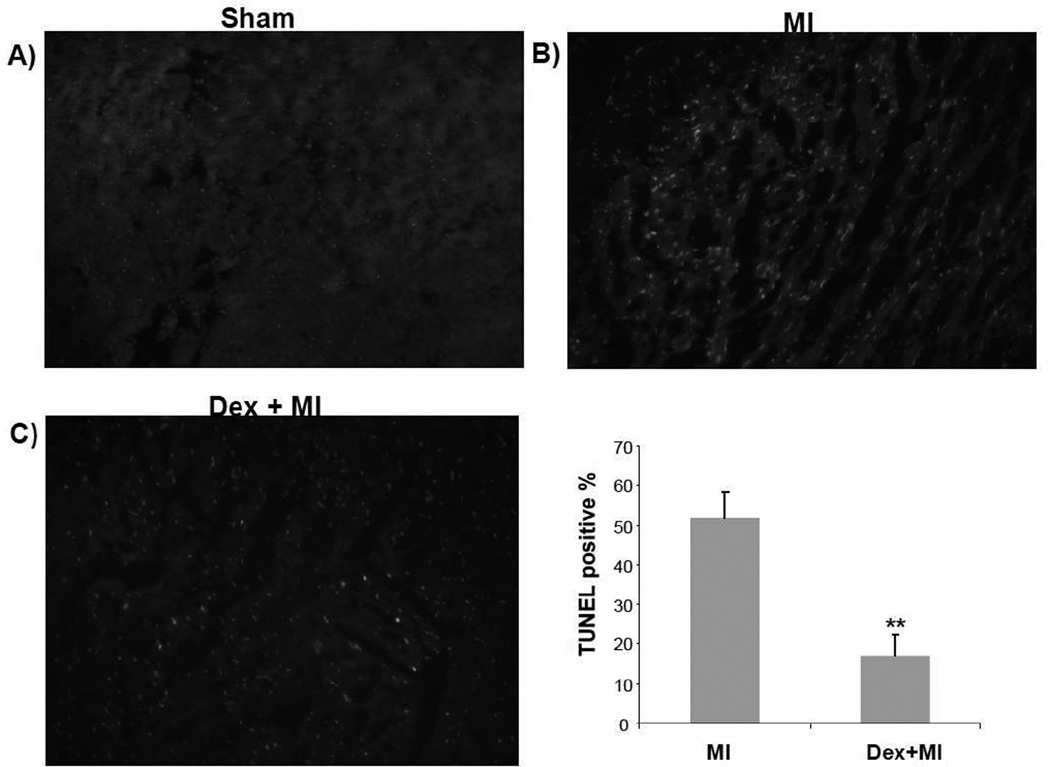
Myocardial infarction involves cell death. While necrosis is a main form of cell death in the infarct area, apoptosis has been detected around the border zone (Bialik et al., 1997). A long list of literature has documented that ischemic preconditioning protects the myocardium from apoptosis (Lazou et al., 2006; Maulik et al., 1999; Okamura et al., 1999; Okubo et al., 2004; Piot et al., 1997; Schwartz et al., 2001; Wang et al., 1999b; Wu et al., 2003; Zhang et al., 2010; Zhao and Vinten-Johansen, 2002). To test whether dexamethasone inhibits apoptosis in vivo, we performed TUNEL assay using the myocardium following left anterior descending coronary artery occlusion. TUNEL positive staining was not observed in sham operated animals but was prevalent and localized in the left ventricular free wall area (Fig 3A). Pretreatment with dexamethasone reduced the number of TUNEL positive cells (Fig 3A & B).
Fig. 3. TUNEL assay shows cardiac protection by dexamethasone.
At 24 h after left anterior descending coronary artery occlusion, the heart was excised for quick freezing in liquid nitrogen. The transverse sections (5 mm thick) were used for TUNEL staining. Nuclei were stained with DAPI florescent dye to establish the background. A representative image from sham, myocardial infarcted or an animal pretreated with dexamethasone before coronary artery occlusion is shown (A). The numbers of TUNEL-positive cells were counted against DAPI stained cells to determine the percentage of apoptotic cells (B). The data represent means ± standard deviations from three animals in each group (B). ** indicates p<0.01 when the means of Dex treated group is compared to that of myocardial infarct group.
3.2. Dexamethasone induces bcl-xL in the myocardium and cultured cardiomyocytes
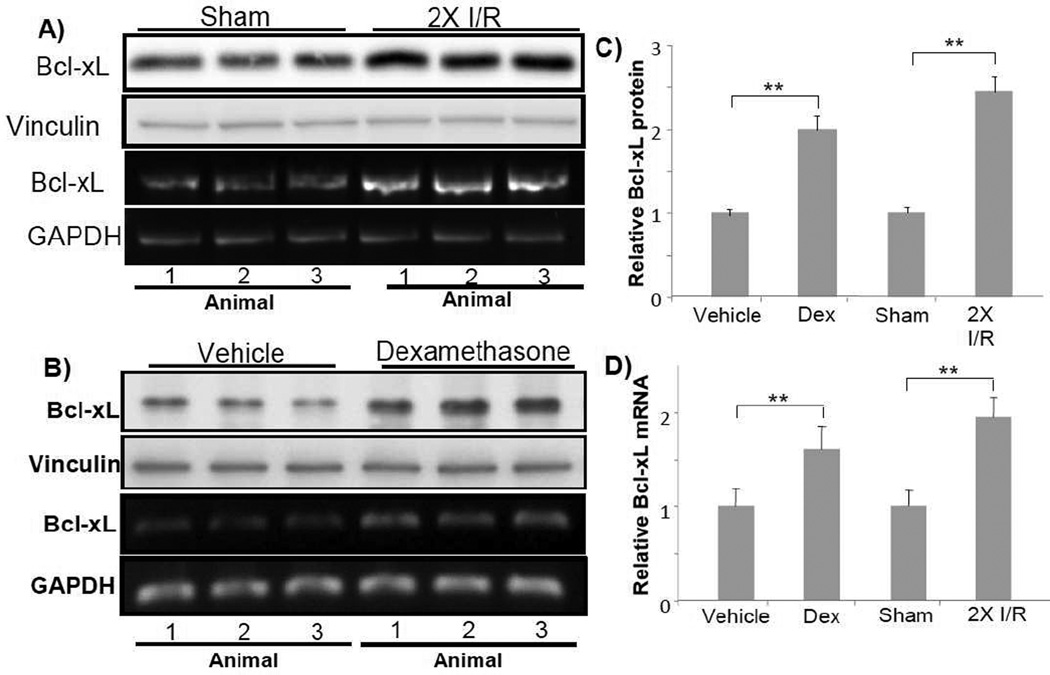
One mechanism of cell survival response is elevated expression of pro-survival members of bcl-2 family. With primary cultured cardiomyocytes, investigating corticosteroids induced cytoprotection using microarray technology lead to the discovery of Bcl-xL (Chen et al., 2005). Other members of bcl-2 family, such as bcl-2, bax, bak and bad did not change the level with corticosteroids treatment (Chen et al., 2005). Bcl-xL protects the heart from ischemic reperfusion injury by preventing mitochondrial release of cytochrome C (Huang et al., 2003; Huang et al., 2005; Kunisada et al., 2002). With ischemic preconditioning, an elevated level of Bcl-xL protein or mRNA was observed (Fig 4A & C). When Bcl-xL protein or mRNA was measured in the mouse ventricles following dexamethasone administration, increases were observed (Fig 4B & C).
Fig. 4. Dexamethasone causes elevated expression of Bcl-xL in the myocardium.
C57BL6 mice at 8 weeks old were used for left anterior descending coronary artery occlusion surgery or dexamethasone treatment. For preconditioning, the coronary artery was occluded for 5 mins then released for 5 mins for two cycles. Ventricular tissues were collected immediately at the end of the ischema-reperfusion cycles for Western blot analyses (80 µg protein/lane) or RT-PCR (A). Dexamethasone (i.p. 20 mg/kg) was administered 20 h prior to ventricular tissue collection (B). Each lane represents Bcl-xL protein or mRNA level from one animal. The quantitative Bcl-xL protein data (C) or mRNA (D) show means + standard deviations of band densities from three animals. Vinculin (A) or GAPDH (B) was used as a loading control. * indicates p<0.05 and ** indicates p<0.01.
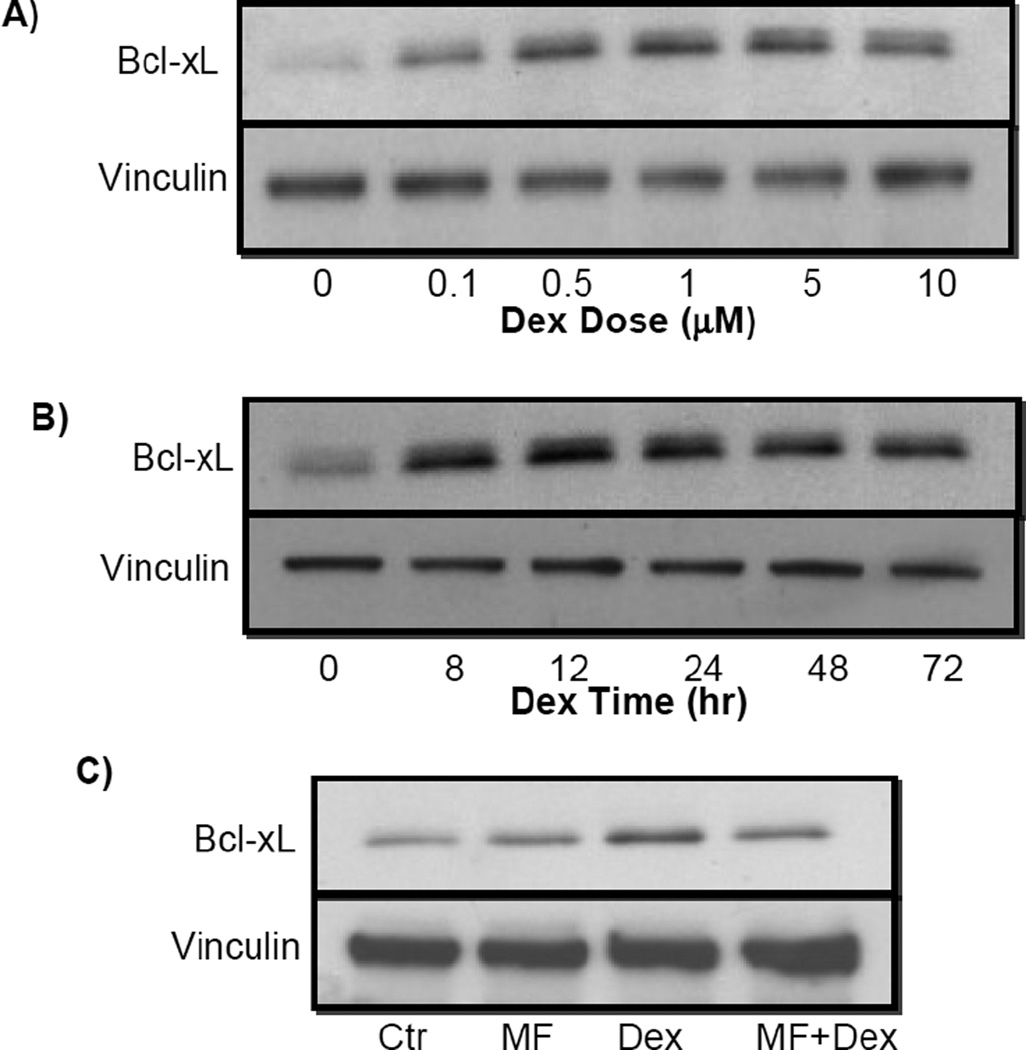
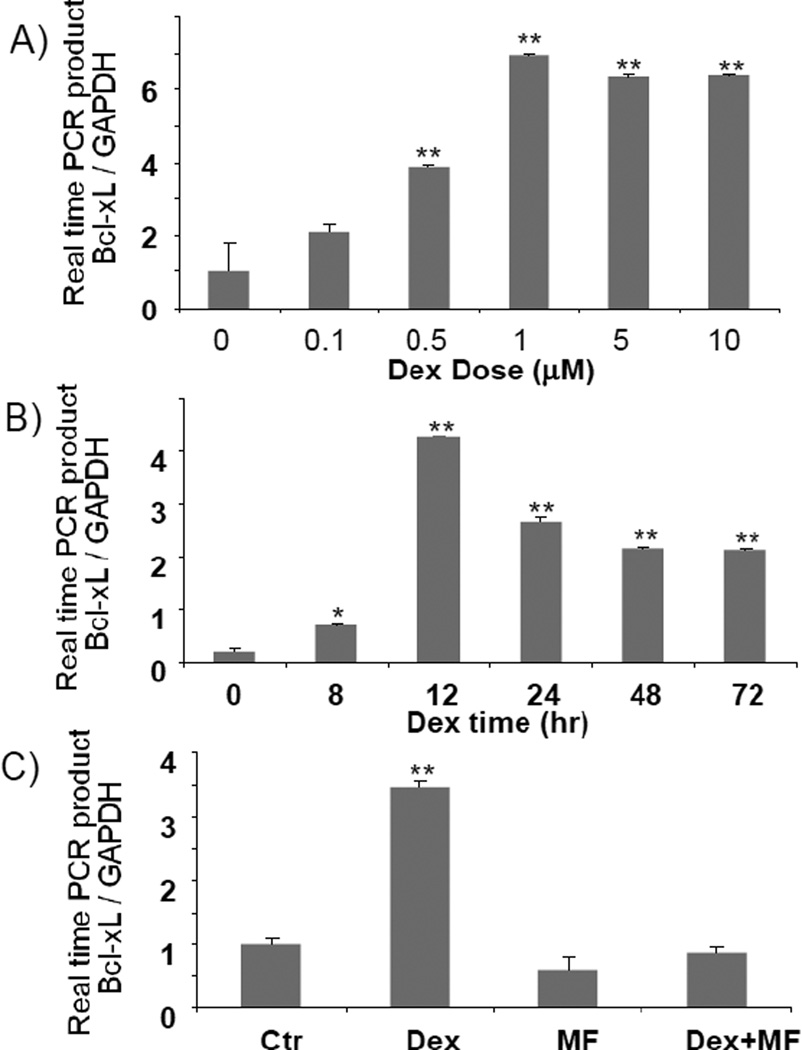
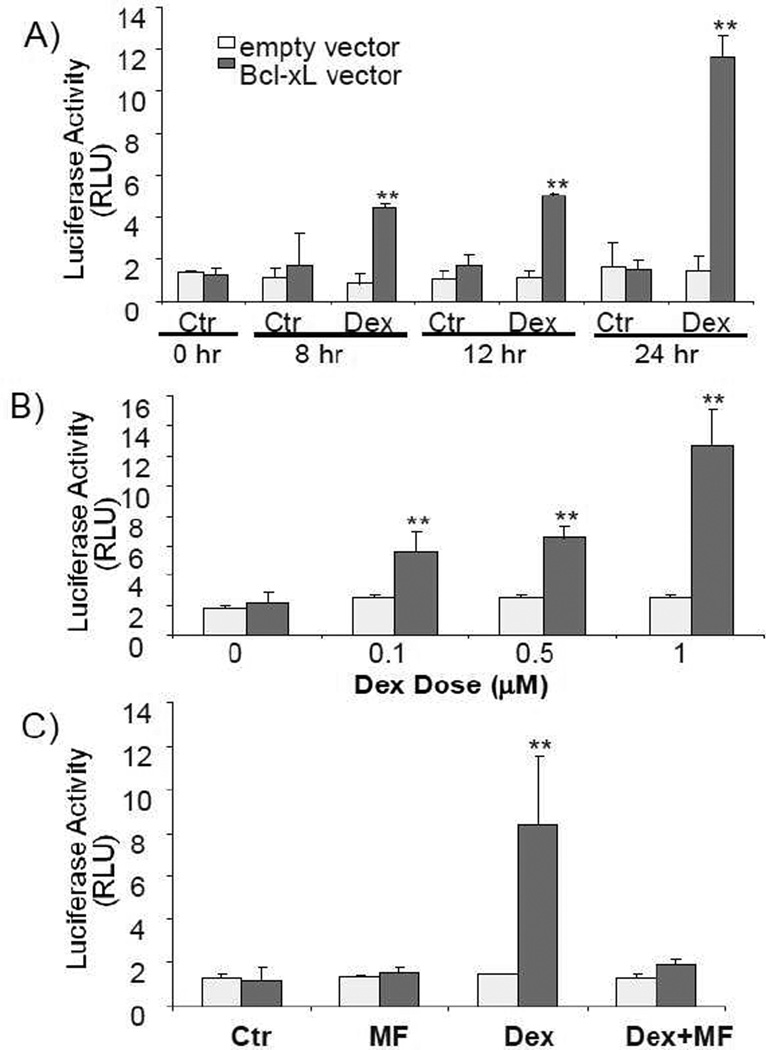
Cardiomyocytes in culture allow us to address whether elevated Bcl-xL results from transcriptional activation of bcl-x gene. A dexamethasone dose and time dependent induction of Bcl-xL protein was observed in primary cultured neonatal rat cardiomyocytes (Fig 5A & B). Induction of Bcl-xL protein by dexamethasone can be blocked by co-treatment with mifeprestone (Fig 5C). Bcl-xL mRNA also showed a dexamethasone dose and time dependent induction in cultured cardiomyocytes (Fig 6). When cardiomyocytes were transfected with a reporter construct under the control of 905 kb Bcl-xL promoter sequence, we found that dexamethasone induced a time and dose dependent activation of Bcl-xL promoter. The dose response and time course correlate with that for Bcl-xL mRNA or protein. These data suggest that dexamethasone induces transcriptional activation of Bcl-xL gene.
Fig. 5. Dexamethasone induces dose- and time- dependent elevation of Bcl-xL protein.
Cardiomyocytes isolated from neonatal rats were treated with various doses of dexamethasone for 24 h (A), or 1 µM dexamethasone for indicated time (B) or 24 h (C). Mifeprestone (1 µM) was added to cells 30 mins prior to addition of 1 µM dexamethasone (C). Cells were harvested for Western blot (30 µg protein/lane) to measure for levels of Bcl-xL protein with vinculin as a loading control.
Fig. 6. Dexamethasone induces Bcl-xL mRNA elevation.
Cardiomyocytes isolated from neonatal rats were treated with various doses of dexamethasone for 12 h (A), 1 µM dexamethasone for various time points (B) or 1 µM dexamethasone for 12 h with 30 mins pretreatment of 1 µM mifeprestone (C). Bcl-xL mRNA was determined by realtime PCR. * indicates p<0.05 and ** indicates p<0.01 when compared to untreated control group.
4. Discussion
In this study, we have found that dexamethasone pretreatment reduced infarct size, attenuated cTnI release and reduced apoptosis of cardiomyocytes following left anterior descending coronary artery occlusion. Correlating with the protective effect, dexamethasone administration caused elevated levels of Bcl-xL mRNA and protein in the myocardial tissue. With cardiomyocytes in culture, transcriptional activation of Bcl-xL gene by dexamethasone was evidenced with activation of Bcl-xL promoter and increases in Bcl-xL mRNA. Glucocorticoid receptor antagonist mifeprestone reduced the protective effect of dexamethasone in vivo and prevented Bcl-xL induction. Blocking Bcl-xL gene expression by siRNA led to a loss of cytoprotective effect of glucocorticoids in cultured cardiomyocytes (Chen et al., 2005). Therefore, transcriptional activation of Bcl-xL gene appears to play a central role in the observed protective effect of dexamethasone.
Glucocorticoids carry out biological functions through regulation of transcription after binding to the glucocorticoid receptor. The receptor has α (94 kD) and β (90 kD) isoforms (Bamberger et al., 1996; Funder, 1997; Yudt and Cidlowski, 2002). These two isoforms are encoded by one gene undergoing alternative splicing. Whereas the α isoform becomes active upon binding to glucocorticoids, the β isoform does not bind to the ligand and may serve as a dominant negative regulator. Upon ligand binding, the glucocorticoid receptor dissociates from the Hsp90 complex, translocating to the nucleus, where it forms a homodimer for binding to the Glucocorticoid Receptor Response Element, a palindromic sequence AGAACAnnnTGTTCT in the promoter region of targeted genes. Glucocorticoid receptor also regulates transcription through DNA binding independent mechanisms: 1) by forming a heterodimer to repress other transcription factors; 2) by modifying chromatin structure via altering histone acetyltransferase or deacetylase activity (Adcock, 2001; Deroo and Archer, 2001; Fryer and Archer, 1998), or interacting with the chromatin-remodeling factor BRG1 (Deroo and Archer, 2001; Fryer and Archer, 1998). 3) A large number of coregulators have been reported (Lonard and O'Malley B, 2007); (Jenkins et al., 2001). While some coordinate the assembly of glucocorticoid receptor–protein complexes, others mediate the interaction of the receptor with other transcription factors or chromatin. Some cofactors, such as E6-AP, an E3 ubiquitin ligase, catalyzes glucocorticoid receptor protein ubiqutination and degradation, while others such as the poly-C-RNA binding protein 1 (PCBP1), exhibit multiple functions, from translational repression or transcriptional coactivation to RNA splicing. It remains to be addressed which of these pathways regulating Bcl-xL gene transcription.
Our studies have found that dexamethasone activates bcl-x gene promoter, a 905 bp fragment that does not contain sequences of the Glucocorticoid Receptor Response Element. The mouse bcl-x gene has 5 promoters, P1 - P5, and is predicted to produce five mRNA species sharing the same translational start site with various lengths of 5’-untranslated region. P1 - P5 promoter is located from −151, −802, −1886, − 2721 and −3412 bp from the translational start site respectively (Viegas et al., 2004). Two Hormone Response Element -like sequences have been identified at positions − 3040 (TGgTgTGTCTGTTCc) and −3001 (aGcTCTCCAGcACA), upstream of P4 promoter. Glucocorticoid receptor is capable of binding to these sequences in vitro, contributing to Bcl-xL expression in mouse mammary epithelial cells (Viegas et al., 2004). In addition to the glucocorticoid receptor binding sites, several cis-elements have been identified for binding of transcription factors such as Sp1, AP-1, Oct-1, Ets, Rel/NF-kB, GATA-1 and STATs within −3.2 kb promoter region of mouse bcl-x gene (Grillot et al., 1997). Within −905 kb of human bcl-x gene, binding of Rel/NF-kB, Ets, STATs or AP-1 transcription factors has been shown to regulate transcriptional activation of bcl-x gene encoding Bcl-xL protein (Grad et al., 2000). Based on −905 bp sequence of human bcl-x gene promoter, Transfac software has predicted the binding sites for NF-Y, AP-2, and Forkhead Homolog Like1 transcription factors in addition to NF-kB, STATs and AP-1. The facts that glucocorticoid receptor exhibits multiple modes of transcriptional modulation, the presence of Hormone Response Element - like sequences upstreatm of P4 promoter and 905 bp Bcl-xL promoter sequence responsive to dexamethasone treatment suggest that multiple transcriptional mechanisms may mediate glucocorticoid induced Bcl-xL gene expression in cardiomyocytes.
Fig. 7. Dexamethasone activates bcl-x gene promoter.
Cardiomyocytes were transfected with either empty pGL3 luciferase vector or pGL3 luciferase under the control of 905 bp human bcl-x gene promoter. A thymidine kinase promoter driven Renilla luciferase construct was cotransfected to correct for transfection efficiency. At 48 h after transfection, cells were treated with vehicle or 1 µM dexamethasone for indicated time (A), for 24 h with various doses of dexamethasone (B) or in the absence or presence of 1 µM mifeprestone for 24 h (C) for luciferase assay. Data are presented as ratios of relative light unit (RLU) of Firefly versus Renilla luciferase. * indicates p<0.05 and ** indicates p<0.01 when compared to untreated control group.
Acknowledgements
This work was supported by NIH R01 HL 076530, Arizona Disease Control Research Commission (QMC) and Mark and Mary Anne Fay Investigator Awards (BX) from Sarver Heart Center University of Arizona. We appreciate the advice of Dr. Dean Billheimer for statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM. Glucocorticoid-regulated transcription factors. Pulmonary Pharm Therap. 2001;14:211–219. doi: 10.1006/pupt.2001.0283. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocrine Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, Lord EM, Koch CJ, Kitsis RN. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Ann Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Chen Q, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. Corticosteroids Inhibit Cell Death Induced by Doxorubicin in Cardiomyocytes: Induction of Anti-apoptosis, Antioxidant and Detoxification Genes. Mol Pharm. 2005;67:1861–1873. doi: 10.1124/mol.104.003814. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun H. Glucocortidoid Pharmacotoxicological Interactions and Modulation of Toxicity. In: Harvey P, Everett D, Springall C, editors. Adrenal Toxicology. New York: Informa; 2009. pp. 205–230. [Google Scholar]
- Deroo BJ, Archer TK. Glucocorticoid receptor-mediated chromatin remodeling in vivo. Oncogene. 2001;20:3039–3046. doi: 10.1038/sj.onc.1204328. [DOI] [PubMed] [Google Scholar]
- Fryer CJ, Archer TK. Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature. 1998;393:88–91. doi: 10.1038/30032. [DOI] [PubMed] [Google Scholar]
- Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Ann Rev Med. 1997;48:231–240. doi: 10.1146/annurev.med.48.1.231. [DOI] [PubMed] [Google Scholar]
- Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Grillot DA, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- Huang J, Ito Y, Morikawa M, Uchida H, Kobune M, Sasaki K, Abe T, Hamada H. Bcl-xL gene transfer protects the heart against ischemia/reperfusion injury. Biochem Biophys Res Comm. 2003;311:64–70. doi: 10.1016/j.bbrc.2003.09.160. [DOI] [PubMed] [Google Scholar]
- Huang J, Nakamura K, Ito Y, Uzuka T, Morikawa M, Hirai S, Tomihara K, Tanaka T, Masuta Y, Ishii K, Kato K, Hamada H. Bcl-xL gene transfer inhibits Bax translocation and prolongs cardiac cold preservation time in rats. Circulation. 2005;112:76–83. doi: 10.1161/CIRCULATIONAHA.105.535740. [DOI] [PubMed] [Google Scholar]
- Jenkins BD, Pullen CB, Darimont BD. Novel glucocorticoid receptor coactivator effector mechanisms. Trends Endocrin Metab. 2001;12:122–126. doi: 10.1016/s1043-2760(00)00357-x. [DOI] [PubMed] [Google Scholar]
- Kunisada K, Tone E, Negoro S, Nakaoka Y, Oshima Y, Osugi T, Funamoto M, Izumi M, Fujio Y, Hirota H, Yamauchi-Takihara K. Bcl-xl reduces doxorubicin-induced myocardial damage but fails to control cardiac gene downregulation. Cardiovasc Res. 2002;53:936–943. doi: 10.1016/s0008-6363(01)00506-5. [DOI] [PubMed] [Google Scholar]
- Lazou A, Iliodromitis EK, Cieslak D, Voskarides K, Mousikos S, Bofilis E, Kremastinos DT. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: the role of Bcl-2 family proteins and ERK1/2. Apoptosis. 2006;11:2195–2204. doi: 10.1007/s10495-006-0292-5. [DOI] [PubMed] [Google Scholar]
- Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, Dorge H. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thoracic Surgery. 2007;84:110–118. doi: 10.1016/j.athoracsur.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Libby P, Maroko PR, Bloor CM, Sobel BE, Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973;52:599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Maulik N, Engelman RM, Rousou JA, Flack JE, Deaton D, 3rd, Das DK. Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation. 1999;100:II369–II375. doi: 10.1161/01.cir.100.suppl_2.ii-369. [DOI] [PubMed] [Google Scholar]
- Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol. 1999;277:H660–H668. doi: 10.1152/ajpheart.1999.277.2.H660. [DOI] [PubMed] [Google Scholar]
- Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- Okamura T, Miura T, Iwamoto H, Shirakawa K, Kawamura S, Ikeda Y, Iwatate M, Matsuzaki M. Ischemic preconditioning attenuates apoptosis through protein kinase C in rat hearts. Am J Physiol. 1999;277:H1997–H2001. doi: 10.1152/ajpheart.1999.277.5.H1997. [DOI] [PubMed] [Google Scholar]
- Okubo S, Tanabe Y, Takeda K, Kitayama M, Kanemitsu S, Kukreja RC, Takekoshi N. Ischemic preconditioning and morphine attenuate myocardial apoptosis and infarction after ischemia-reperfusion in rabbits: role of delta-opioid receptor. Am J Physiol Heart Circ Physiol. 2004;287:H1786–H1791. doi: 10.1152/ajpheart.01143.2003. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Ann Rev Pharm Tox. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piot CA, Padmanaban D, Ursell PC, Sievers RE, Wolfe CL. Ischemic preconditioning decreases apoptosis in rat hearts in vivo. Circulation. 1997;96:1598–1604. doi: 10.1161/01.cir.96.5.1598. [DOI] [PubMed] [Google Scholar]
- Rao MK, Xu A, Narayanan N. Glucocorticoid modulation of protein phosphorylation and sarcoplasmic reticulum function in rat myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H325–H333. doi: 10.1152/ajpheart.2001.281.1.H325. [DOI] [PubMed] [Google Scholar]
- Sainte-Marie Y, Nguyen Dinh Cat A, Perrier R, Mangin L, Soukaseum C, Peuchmaur M, Tronche F, Farman N, Escoubet B, Benitah JP, Jaisser F. Conditional glucocorticoid receptor expression in the heart induces atrio-ventricular block. FASEB J. 2007;21:3133–3141. doi: 10.1096/fj.07-8357com. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Sebbag L, Jennings RB, Reimer KA. Duration and reinstatement of myocardial protection against infarction by ischemic preconditioning in open chest dogs. J Mol Cell Cardiol. 2001;33:1561–1570. doi: 10.1006/jmcc.2001.1426. [DOI] [PubMed] [Google Scholar]
- Sun H, Chen QM. Inhibitors of GSK-3 prevent corticosterone from inducing COX-1 expression in cardiomyocytes. Cardiovasc Tox. 2008;8:93–100. doi: 10.1007/s12012-008-9018-y. [DOI] [PubMed] [Google Scholar]
- Sun H, Sheveleva E, Chen QM. Corticosteroids induce cyclooxygenase 1 expression in cardiomyocytes: role of glucocorticoid receptor and Sp3 transcription factor. Mol Endocrinol. 2008a;22:2076–2084. doi: 10.1210/me.2007-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Sheveleva E, Xu B, Inoue H, Bowden TG, Chen QM. Corticosteroids induce COX-2 expression in cardiomyocytes: role of glucocorticoid receptor and C/EBP-beta. Am J Physiol Cell Physiol. 2008b;295:915–922. doi: 10.1152/ajpcell.90646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xu B, Inoue H, Chen QM. P38 MAPK mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell Signalling. 2008c;20:1952–1959. doi: 10.1016/j.cellsig.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Valen G, Kawakami T, Tahepold P, Dumitrescu A, Lowbeer C, Vaage J. Glucocorticoid pretreatment protects cardiac function and induces cardiac heat shock protein 72. Am J Physiol Heart Circ Physiol. 2000;279:H836–H843. doi: 10.1152/ajpheart.2000.279.2.H836. [DOI] [PubMed] [Google Scholar]
- Viegas LR, Vicent GP, Baranao JL, Beato M, Pecci A. Steroid hormones induce bcl-X gene expression through direct activation of distal promoter P4. J Biol Chem. 2004;279:9831–9839. doi: 10.1074/jbc.M312402200. [DOI] [PubMed] [Google Scholar]
- Wang L, Feng ZP, Duff HJ. Glucocorticoid regulation of cardiac K+ currents and L-type Ca2+ current in neonatal mice. Circ Res. 1999a;85:168–173. doi: 10.1161/01.res.85.2.168. [DOI] [PubMed] [Google Scholar]
- Wang NP, Bufkin BL, Nakamura M, Zhao ZQ, Wilcox JN, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J. Ischemic preconditioning reduces neutrophil accumulation and myocardial apoptosis. Ann Thorac Surg. 1999b;67:1689–1695. doi: 10.1016/s0003-4975(99)00305-7. [DOI] [PubMed] [Google Scholar]
- Wu ZK, Laurikka J, Saraste A, Kyto V, Pehkonen EJ, Savunen T, Tarkka MR. Cardiomyocyte apoptosis and ischemic preconditioning in open heart operations. Ann Thorac Surg. 2003;76:528–534. doi: 10.1016/s0003-4975(03)00432-6. [DOI] [PubMed] [Google Scholar]
- Yared JP, Bakri MH, Erzurum SC, Moravec CS, Laskowski DM, Van Wagoner DR, Mascha E, Thornton J. Effect of dexamethasone on atrial fibrillation after cardiac surgery: prospective, randomized, double-blind, placebo-controlled trial. J Cardiothoracic Vasc Anesth. 2007;21:68–75. doi: 10.1053/j.jvca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Yared JP, Starr NJ, Torres FK, Bashour CA, Bourdakos G, Piedmonte M, Michener JA, Davis JA, Rosenberger TE. Effects of single dose, postinduction dexamethasone on recovery after cardiac surgery. Ann Thoracic Surg. 2000;69:1420–1424. doi: 10.1016/s0003-4975(00)01180-2. [DOI] [PubMed] [Google Scholar]
- Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol. 2002;16:1719–1726. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, Zhang Y, Ji XP. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307–315. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438–455. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]