Abstract
Background/aims
Kangaroo care (KC) has possible benefits for promoting physiological stability and positive developmental outcomes in preterm infants. The purpose of this study was to compare bradycardia and desaturation events in preterm infants in standard incubator care (SC) versus KC.
Methods
Thirty-eight infants 27 to 30 weeks gestational age were randomly assigned to 2 hours of KC daily between days of life (DOL) 5 to 10 or to continuous SC. Infants were monitored for bradycardia (heart rate <80) or oxygen desaturation (<80%). Analysis of hourly events was based on three sets of data: SC group 24 hours daily, KC group during incubator time 22 hours daily, and KC group during holding time 2 hours daily.
Results
The KC group had fewer bradycardia events per hour while being held compared to time spent in an incubator (p=0.048). The KC group also had significantly fewer oxygen desaturation events while being held than while in the incubator (p=0.017) and significantly fewer desaturation events than infants in standard care (p=0.02).
Conclusion
KC reduces bradycardia and oxygen desaturation events in preterm infants, providing physiological stability and possible benefits for neurodevelopmental outcomes.
Keywords: infant, premature, kangaroo care, bradycardia, oxygen desaturation
Introduction
The care of preterm infants is a major national health issue, with the rate of preterm births in the United States reported as 12.3 percent overall and a rate of 2.87 percent for infants less than 34 weeks gestational age. [1] The March of Dimes (2012) reported that approximately half a million infants were born prematurely.[2] Each year about 64,000 preterm infants are born weighing ≤1500 grams. [3] Respiratory care of these preterm infants continues to be a challenge because ventilatory control, involving central respiratory rhythmogenesis and central and peripheral chemoreception, is immature in this population.[4] The introduction of prenatal steroids and improved surfactant treatments at birth has greatly reduced the incidence of respiratory distress syndrome (RDS) and the need for mechanical ventilation in preterm infants.[5] However, preterm infants remain at risk for apnea, bradycardia and oxygen desaturation events. A few neonates, especially if not exposed to prenatal steroids or if exposed to chorioamnionitis, may even require continued endotracheal intubation and mechanical ventilation. [6,7] Although these interventions are life-saving, they can in turn lead to chronic lung disease [8] and subsequent adverse neurodevelopmental effects. [9–15] Safe and effective treatments are needed to prevent adverse cardiorespiratory events and to promote physiologic stability and positive developmental outcomes in preterm infants.
This study was part of a larger study that determined (a) the effects of two hours of kangaroo care (KC) on stress and pain management in preterm infants 27 – 30 weeks gestational age and (b) the safety of KC in this population. One purpose of the safety arm of the study was to determine whether KC affects the stability of cardiorespiratory parameters in preterm infants. A more complete secondary analysis of cardiorespiratory data was carried out after completion of the study to determine specifically whether KC might prevent cardiorespiratory events and promote physiologic stability in 27–30 week infants. The hypothesis was that there would be a decreased incidence of bradycardia and desaturation events in preterm infants during KC compared to events during standard incubator care (SC).
Methods
Study Design
This study on cardiorespiratory parameters of KC was one arm of a randomized, controlled trial to determine various effects of KC on 27–30 week gestational age infants undergoing incubator care in a neonatal intensive care unit (NICU). Parents gave consent to randomize infants to either KC or SC starting on day of life (DOL) 5 and continuing for 5 days. In the KC group, mothers (or fathers) held their infants skin-to-skin in an upright position on the chest for 2 hours daily. Infants in the SC group were cared for in the incubator as usual, but could be held skin-to-skin for a maximum of 15 minutes daily at the parent’s request. The 15 minute time period was chosen a priori to allow a short time of KC for parents who desired KC but were not randomized to the KC arm of the study.
Patient Population
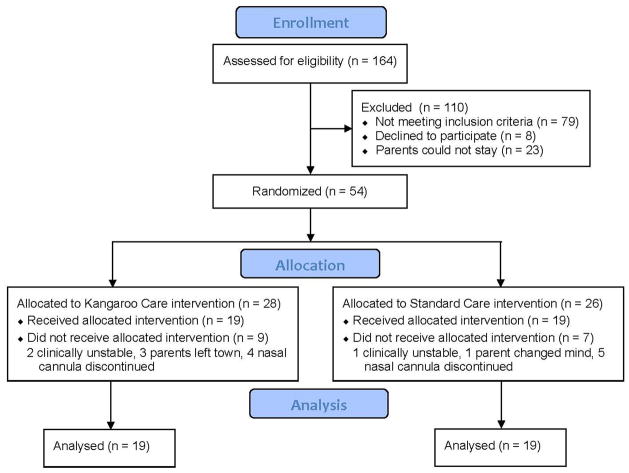
A total of 38 infants were enrolled in the study, 19 infants assigned to the KC group and 19 to the SC group (Figure 3). A power analysis indicated that 26 infants would be required for 80% power using a p value of 0.05. Infants hospitalized in the neonatal intensive care unit (NICU) who were 27–30 weeks gestational age at birth, weighed at least 1000 grams, and were receiving nasal continuous positive airway pressure (CPAP) or nasal cannula flow were eligible for the study. Three infants were on CPAP for a short period of time, otherwise all infants were on nasal cannula flow. These infants received nasal cannula flow the remainder of the time, as did all other infants in the study. Although no infants in the study required intubation, they would have been reintubated for recurrent apnea, bradycardia or desaturation events per unit protocol. In case of transient adverse events, infants were managed per unit protocol including stimulation, suctioning, or increased oxygen as needed. All infants enrolled in the study were receiving caffeine, 6mg/kg per unit protocol. All infants were being fed per unit protocol, which included trophic feeds (10–20 ml) of expressed breast milk (EBM) or formula per orogastric tube every three hours. As participants in the study, parents agreed to be able to hold their infant for 2 hours daily for 5 consecutive days if randomized to KC. Eight mothers in the SC group chose to hold their infants for 15 minutes on some of the days that infants were enrolled in the study. Infants were excluded from the study if there was clinical instability, severe congenital defects or history of major surgery, Apgar score of 3 or less at 5 minutes of age, or a cord blood pH of <7.0 or base deficit <−15, or severe intraventricular hemorrhage (grade 3 or 4). Infants were also excluded if there was documented maternal opiate use prior to delivery.
Figure 3.
Enrollment flow diagram for infants in study to examine cardiorespiratory effects of kangaroo care
Intervention and Control Group Process and Data Collection
Beginning on DOL 5 and continuing for 5 days, infants followed a positioning and holding protocol depending on randomization to the KC or SC group. In the KC group a parent, usually the mother, held the infant for 2 hours daily at a time convenient to the family schedule. The parent sat at the side of the infant’s incubator in a rocking chair and received the infant who was placed skin-to-skin on the parent’s chest in an upright position facing the parent. Infants wore knitted caps, were covered over the back with blankets to maintain warmth, and were monitored for temperature stability by taking an axillary temperature every 30 minutes during holding. There was continuous monitoring of heart rate, respiratory rate and oxygen saturation levels during the process. Parents could interact and talk to the infant as they chose. The NICU in which the study took place provides private rooms for all infants. Infants received routine positioning in an incubator during the remaining 22 hours of the day. Routine positioning was defined as turning and repositioning infants from prone, supine or side-laying positions in the incubator every 2 hours according to unit protocol. In the SC group, infants received routine positioning for the entire study period. If desired, the parent could request 15 minutes of holding time daily as described above.
Data were recorded from a Philips Intellivue MP 70 neonatal monitor used to monitor heart rate, respiratory rate, frequency and duration of apnea events per unit routine over the 5 days of the study. Nellcor pulse oximetry was used to monitor oxygen saturation levels. Bradycardia (heart rate<80) and desaturation events (oxygen saturation<80%) were noted by registered nurses assigned to the infants, with observations based on monitor alarms and clinical assessment. Events were documented on flow sheets per unit protocol and counted over the 120 hours (5 days) of the study.
Analyses
Data were collected on gender, race, gestational age, birth weight, and number of days on the ventilator. Because of the differences in duration of time that KC infants spent being held (2 hours) versus incubator care (22 hours), the frequency of bradycardia and oxygen desaturation events were summarized using the mean number of events per hour. A repeated measures analysis of variance was used to analyze main or overall effects of infant positioning on bradycardia or oxygen desaturation events.
Tukey post hoc analysis for multiple comparisons was carried out to determine if there were significant differences in frequency of bradycardia or oxygen desaturation events (a) between the KC and SC groups or (b) within infants in the KC group depending on whether they were being held or were in the incubator. The Welch two sample t-test was used to determine any differences between infants assigned to KC and SC groups with regard to birth weight, gestational age, number of daily painful procedures that might affect heart rate and oxygen levels, number of days on the ventilator before the study started and number of days between administration of maternal prenatal steroids and delivery.
Results
The mean gestational age for infants in the KC group was 29 weeks, and 28.5 weeks for infants in the SC group. All infants weighed < 1500 grams. Table 1 presents demographic data and other infant characteristics. There were no significant differences between the two groups with regard to birth weight, gestational age, number of daily painful procedures, number of days on the ventilator before the study started and timing of maternal prenatal steroids.
Table 1.
Characteristics of infants in kangaroo care (KC) (n=19) and standard care (SC) (n=19) groups
| KC Group | SC Group | |
|---|---|---|
|
| ||
| Mean (SD) | Mean (SD) | |
| Birth weight (grams) | 1311.5 (216.6) | 1213.2 (169.8) |
| Gestational age | 29 (0.8) | 28.5 (1) |
| Total # painful procedures | 13.6 (4) | 17.9 (6.8) |
| Days on vent before study | 1.95 (1.5) | 3 (1.58) |
| * Maternal prenatal steroids | 14 (11) | 9.6 (9.5) |
number of days between administration of maternal steroids and delivery
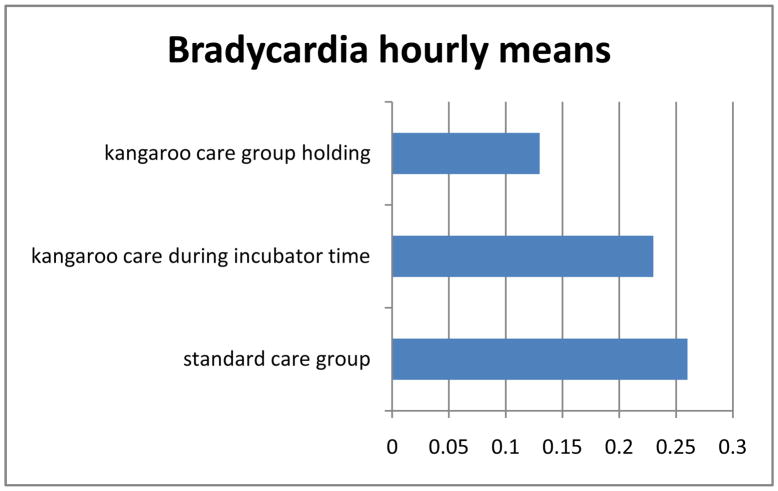
Analysis of variance showed a significant main or overall effect of treatment group on bradycardia (p=0.02) and oxygen desaturation (p=0.0015). There were no significant differences between the KC or SC groups in frequency of bradycardia or oxygen desaturation events. However, there were significant differences in adverse events for infants within the KC group depending on whether they were being held or were in the incubator. There were significantly fewer bradycardia events (p=0.048) and oxygen desaturation events (p=0.017) for infants in the KC group while they were being held skin-to-skin. Figures 1 and 2 illustrate differences in bradycardia and oxygen desaturation events per hour.
Figure 1.
Hourly means of bradycardia over five days.
*main effect of treatment group p=0.02
Figure 2.
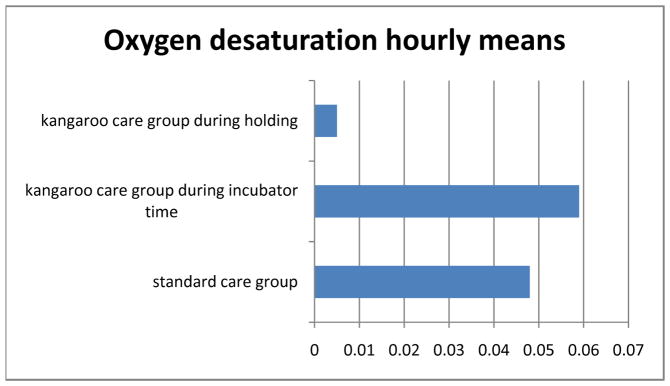
Hourly means of oxygen desaturation over five days.
*main effect of treatment group p=0.0015
There were no changes in temperature stability, loss of IV access, feeding intolerance, or the need for enhanced respiratory support during skin-to-skin holding. Additionally, there were no differences in infection rates between SC and KC groups, although this study was not powered to detect those differences.
Comment
Our work is similar to that of Ludington-Hoe [16] who used a pretest-posttest control group design to compare cardiorespiratory and thermal parameters during KC versus SC in 24 healthy preterm infants who were 33 – 35 weeks gestational age at birth, but were preparing for discharge. Infants were monitored for one day. Apnea and bradycardia were absent in both the KC and SC groups, although heart rates approached bradycardia in the KC group during pre and post testing but then rose a mean of 8 beats during KC (p=0.01). Oxygen saturation dropped from 95.3% to 94.3% during KC (p=0.04), but this decrease was not clinically significant and may have been related to a slight increase in temperature from 36.33 to 36.99°C. Their population was older (KC group 17.82 days; SC group 13 days), weighed more (KC group 2062.73 grams; SC group 2085 grams) and were more stable than our study population.
Bosque et al. found no significant differences in rates of bradycardia and desaturation events during KC or SC in their NICU population; however, their infants were significantly older (43 days) and larger (weight 1914 grams at study completion) than infants in our study. [17] On the other hand Bohnhorst [18] found an increased number of bradycardia and desaturation events during KC in a similar population of preterm neonates. Their population was older (mean age 26 days) than the babies in our study and there was concern that the reason for the increased events was an elevated temperature in the KC patients. Additionally, their patients were held at a 15–30 degree angle unlike the neonates in our study who were held skin to skin at approximately a 45 degree angle. The higher head and chest elevation could possible decrease obstructive apnea. A follow-up study from the same group demonstrated a continued increase in adverse events during KC with neutrothermia with a similar population and urged caution during KC in neonates with pre-existing cardiorespiratory events. [19] Other differences in our population were that all of our neonates were receiving caffeine and some form of respiratory assistance, whereas not all of the infants in Bohnhorst’s study received these treatments.
There are several possible mechanisms to explain our findings. First, the act of holding may reduce stress and thereby promote autonomic stability. Cong [20] and Johnston [21] reported a reduction of stress and promotion of autonomic stability in preterm infants during maternal contact. Bellieni [22] and Lago [23] have documented the calming benefits of being held by the mother and being made aware of the mother’s heart-beat, breathing, aroma, touch and voice. It is possible that all of these factors play a part in improved cardiorespiratory parameters.
Second, the upright positioning of the infant during KC, especially if the angle is high enough (45 – 60 degrees), may reduce obstructive apnea, but additional research is needed to verify this hypothesis. There is a higher incidence of obstructive sleep apnea in preterm infants than in term infants. [24,25] [26] The results of studies that have examined the effects of positioning on cardiorespiratory parameters have been inconclusive and possibly confused by extraneous variables [27–29]. Reher et al. [27] investigated the effect of three positions on bradycardia and desaturation events in 18 preterm infants <32 weeks: prone horizontal, head elevated 15 degrees, and the three-level position. In the three-level position, folded infant blankets were used to place the head and chest on a higher level than the abdomen, and both chest and abdomen were raised more than the legs, resulting in a 15-degree head elevation but without the whole body being at a tilt. All infants in this study received nasal CPAP and oral caffeine, and 15 infants received supplemental oxygen. There were no significant differences in bradycardia and desaturation events among the three positions (combined event rate p=0.486). The authors propose that differences in positioning may not make a difference in apnea, bradycardia, and desaturation in infants who were already being treated with caffeine and nasal CPAP. Poets [30] suggested that the head-up position may serve as a first-line intervention for apnea of prematurity but agrees with Reher et al. (2008) that this intervention may be most effective if infants have not already been treated with caffeine or CPAP. He suggested that positioning may have less effect on cardiorespiratory parameters if these other treatments were already in place. All infants enrolled in our study were receiving caffeine, and three infants received CPAP for brief periods of time. Thus we cannot comment on the interaction of caffeine or the effects of respiratory support.
One limitation of the current study is the small sample size that was not powered to detect outcomes such as feeding patterns, length of hospital stay or infection rates. A second limitation was the data collection method of recording vital signs directly from the monitor with documentation by registered nurses. It is possible that some events could have been overlooked or misinterpreted because neonates were not under direct and constant observation. However, since the alarm signals were preset by nursery guidelines to alert staff and since caregivers responded to every alarm, bias should be limited. Further research using high-resolution monitors that incorporate software designed to define apnea, bradycardia, and oxygen desaturation events should be done to determine any associations between these events. Specific information on whether therapeutic interventions were required during bradycardia or desaturation events would also be helpful. A third limitation was that nasal airflow was not assessed during this study. Measurement of nasal airflow in association with respiratory movements could help to distinguish central from obstructive apnea. Finally, long-term outcomes of KC were not assessed. Future research should focus on possible long-term benefits of KC on mothers and babies in this vulnerable population. While we did not assess long-term neurodevelopmental outcomes, we were reassured that practicing KC, at least in the manner in which it was applied in this study, was safe in preterm neonates, even those experiencing adverse cardiorespiratory events.
Given the many advantages of KC in parental bonding and apparent safety, we believe it should continue to be offered as a means of promoting development and encouraging family centered care to preterm neonates. The results from our study indicate that KC with upright positioning of the infant leads to decreased bradycardia and desaturation in preterm neonates.
Acknowledgments
This project was supported by grants from the National Center for Research Resources (5P20RR020146-09) and the National Institute of General Medical Sciences (8 P20 GM103425-09) from the National Institutes of Health
Footnotes
Conflict of Interest
The authors declare no conflict of interest for this study
Human Research Statement
Parents of the infants who participated in the study provided written informed consent. The study was approved by the Institutional Review Board and conducted in accordance with the ethical standards of all applicable national and institutional committees and the Helsinki Declaration.
Contributor Information
Anita J. Mitchell, Email: AMitchell@uams.edu, University of Arkansas for Medical Sciences (UAMS), College of Nursing, 4301 West Markham, Slot 529, Little Rock, AR 72205, Phone: 501-266-1551, Fax: 501-686-8350.
Charlotte Yates, Email: cyates@uca.edu, University of Central Arkansas Department of Physical Therapy, University of Arkansas for Medical Sciences, Center for Translational Neuroscience.
Keith Williams, Email: WilliamsDavidK@uams.edu, University of Arkansas for Medical Sciences, College of Medicine, Department of Biostatistics.
Richard W Hall, Email: HallRichardW@uams.edu, University of Arkansas for Medical Sciences, College of Medicine, Department of Pediatrics and Neonatology.
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- 2.March of Dimes. March of Dimes 2012 Premature Birth Report Card. March of Dimes; 2012. Available from: URL: www.marchofdimes.com/prematurity_reportcord.html. [Google Scholar]
- 3.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 4.Carroll JL, Agarwal A. Development of ventilatory control in infants. Paediatr Respir Rev. 2010;11:199–207. doi: 10.1016/j.prrv.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics. 2009;123:137–42. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–89. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: What’s new? Pediatric Pulmonology. 2008;43:937–44. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- 8.Lopez ES, Rodriguez EM, Navarro CR, Sanchez-Luna M. Initial respiratory management in preterm infants and bronchopulmonary dysplasia. Clinics (Sao Paulo) 2011;66:823–7. doi: 10.1590/S1807-59322011000500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity - implications for neurodevelopment. Neonatology. 2007;91:155–61. doi: 10.1159/000097446. [DOI] [PubMed] [Google Scholar]
- 10.Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100:303–10. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas CW, Meinzen-Derr J, Hoath SB, Narendran V. Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants Ventilated with Continuous Positive Airway Pressure vs. Mechanical Ventilation Indian J Pediatr. 2012;79:218–23. doi: 10.1007/s12098-011-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrianakos-Hoobler AI, Msall ME, Huo D, Marks JD, Plesha-Troyke S, Schreiber MD. Predicting school readiness from neurodevelopmental assessments at age 2 years after respiratory distress syndrome in infants born preterm. Dev Med Child Neurol. 2010;52:379–85. doi: 10.1111/j.1469-8749.2009.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 14.Jeng SF, Hsu CH, Tsao PN, Chou HC, Lee WT, Kao HA, et al. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev Med Child Neurol. 2008;50:51–7. doi: 10.1111/j.1469-8749.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 15.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludington-Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Netw. 2004;23:39–48. doi: 10.1891/0730-0832.23.3.39. [DOI] [PubMed] [Google Scholar]
- 17.Bosque EM, Brady JP, Affonso DD, Wahlberg V. Physiologic measures of kangaroo versus incubator care in a tertiary-level nursery. J Obstet Gynecol Neonatal Nurs. 1995 Mar;24:219–26. doi: 10.1111/j.1552-6909.1995.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 18.Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr. 2001 Feb;138:193–7. doi: 10.1067/mpd.2001.110978. [DOI] [PubMed] [Google Scholar]
- 19.Bohnhorst B, Gill D, Dordelmann M, Peter CS, Poets CF. Bradycardia and desaturation during skin-to-skin care: no relationship to hyperthermia. J Pediatr. 2004 Oct;145:499–502. doi: 10.1016/j.jpeds.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Cong X, Ludington-Hoe SM, McCain G, Fu P. Kangaroo Care modifies preterm infant heart rate variability in response to heel stick pain: Pilot study. Early Human Development. 2009;85:561–7. doi: 10.1016/j.earlhumdev.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston CC. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. Pediatrics. 2008;8:13. doi: 10.1186/1471-2431-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellieni CV, Bagnoli F, Perrone S, Nenci A, Cordelli DM, Fusi M, et al. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatr Res. 2002 Apr;51:460–3. doi: 10.1203/00006450-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Lago P, Garetti E, Merazzi D, Pieragostini L, Ancora G, Pirelli A, et al. Guidelines for procedural pain in the newborn. Acta Paediatr. 2009 Jun;98:932–9. doi: 10.1111/j.1651-2227.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma PB, Baroody F, Gozal D, Lester LA. Obstructive sleep apnea in the formerly preterm infant: an overlooked diagnosis. Front Neurol. 2011;2:73. doi: 10.3389/fneur.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calhoun SL, Vgontzas AN, Mayes SD, Tsaoussoglou M, Sauder K, Mahr F, et al. Prenatal and perinatal complications: is it the link between race and SES and childhood sleep disordered breathing? J Clin Sleep Med. 2010;6:264–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics. 2006;118:101–7. doi: 10.1542/peds.2005-1873. [DOI] [PubMed] [Google Scholar]
- 27.Reher C, Kuny KD, Pantalitschka T, Urschitz MS, Poets CF. Randomised crossover trial of different postural interventions on bradycardia and intermittent hypoxia in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2008;93:F289–F291. doi: 10.1136/adc.2007.132746. [DOI] [PubMed] [Google Scholar]
- 28.Heimann K. Impact of skin to skin care, prone and supine positioning on cardiorespiratory parameters adn thermoregulation in premature infants. Neonatology. 2010;97:311–7. doi: 10.1159/000255163. [DOI] [PubMed] [Google Scholar]
- 29.Bauschatz AS, Kaufmann CM, Haensse D, Pfister R, Bucher HU. A preliminary report of nursing in the three-stair-position to prevent apnoea of prematurity. Acta Paediatr. 2008;97:1743–5. doi: 10.1111/j.1651-2227.2008.00989.x. [DOI] [PubMed] [Google Scholar]
- 30.Poets CF. Interventions for apnoea of prematurity: a personal view. Acta Paediatr. 2010;99:172–7. doi: 10.1111/j.1651-2227.2009.01604.x. [DOI] [PubMed] [Google Scholar]