Abstract
Eukaryotic gene regulation is controlled, in part, by inducible transcription factors binding regulatory sequences in a tissue-specific and hormone-responsive manner. The development of methods for analysis of transcription factor interaction within native chromatin has been a significant advance for the systematic analyses of the timing of gene regulation and studies on the effects of chromatin modifying enzymes on promoter accessibility. Cross-linking-ChIP (XChIP) is a specific method involving formaldehyde mediated protein-chromatin fixation to preserve the interaction for subsequent target identification. However, the conventional single-step cross-linking technique does not preserve all protein-DNA interactions, especially for transcription factors in hyper-dynamic equilibrium with chromatin or for coactivator interactions. Here we describe a versatile, efficient “two-step” XChIP method that involves sequential protein-protein fixation followed by protein-DNA fixation. This method has been used successfully for analysis of chromatin binding for transcription factors (NF-κB, STAT3), polymerases (RNA Pol II), coactivators (CBP/p300, CDK9) and chromatin structural proteins (modified histones). Modifications of DNA extraction and sonication suitable for downstream target identification by quantitative genomic PCR and next generation sequencing are described.
Keywords: Chromatin immunoprecipitation (ChIP), nuclear factor-κB (NF-κB), polymerase chain reaction (PCR), next generation sequencing (NGS)
1. Introduction
Eukaryotic DNA is ordered in a dynamic nucleoprotein complex, “chromatin”, that is recognized to play an important mechanism of gene expression control in developmental and physiological stimulation. The first level of chromatin organization is at the nucleosome, a protein structure that regulates access of transcriptional regulators to gene regulatory sequences (1). Chromatin immunoprecipitation (ChIP) is a widely applied technique for the analysis of native- or cross-linked protein-chromatin interactions (XChIP). In XChIP, DNA-binding proteins are reversibly fixed to their target DNA. Subsequently, the DNA is sheared, and associated chromatin enriched by immunoprecipitation using specific antibodies directed to protein of interest. The cross-links are then reversed and enrichment of specific DNA is then detected by PCR, array hybridization, or next generation sequencing (NGS).
Although a number of successful studies have employed a XChIP method using a single-step DNA-protein crosslinking reagent (formaldehyde), some highly inducible transcription factors are in hyperdynamic exchange with target DNA and do not cross-link effectively to DNA (2;3). Similarly, transcriptional coactivators interact with chromatin through protein-protein interactions and therefore do not cross-link well to DNA. To this end, we have optimized a “two-step” XChip method, where protein-protein interactions are stabilized by cross-linking, followed by formaldehyde-mediated DNA-protein crosslinking.
This method describes our optimization of the two-step XChip method, a method that has been applied for the identification of NF-κB (4), STAT3 (5), CDK9, p300/CBP, RNA Pol II interactions both in cellulo and in vivo (6). Special modifications of the DNA extraction procedure are needed depending on the downstream method chosen for detection of target gene enrichment (Fig. 1). We focus on the popular quantitative genomic PCR and next generation sequencing, known as “ChIP-Seq” (7).
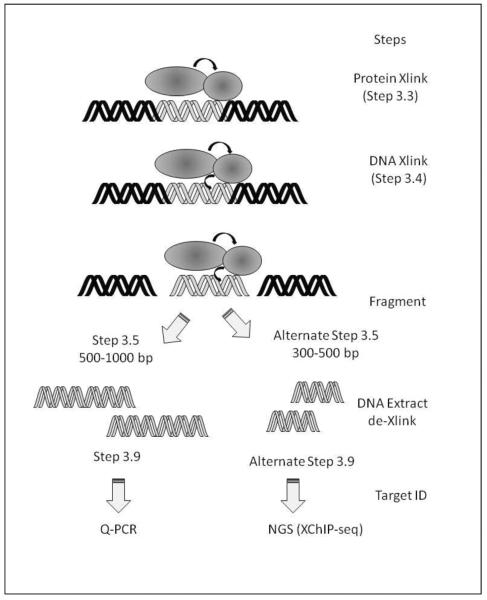
Figure 1. Overview of the two-step XChIP assay.
Shown is a schematic view of the major steps involved in the two-step XChIP assay. Abbreviations: DMA, Dimethyl adipimidate; DMP, Dimethyl pimelimidate•2HCI DSG, Disuccinimidyl glutarate; DSP, Dithiobis(succimidylpropionate) (Lomant's Reagent); EGS, Ethylene glycol bis(succinimidylsuccinate); TSAT, Tris-(succimimidyl aminotricetate) (Trifunctional).
2. Materials
2.1 Cell Culture and stimulation
Seed the cells 24 h prior to experiment. For epithelial cells, a density of 4–6 × 106 cells per 100 mm dish work well. This results in ~75 % confluence on the day of experiment for adherent cells.
Stimulate the cells. For factors requiring stimulation in the absence of serum, cells are changed to growth medium supplemented with 0.5% (wt/vol) Bovine Serum Albumin (Sigma Aldrich).
2.2 Preparation of Cross-linking reagents
0.25M Disuccinimidyl glutarate (DSG) stock. Dissolve 50 mg DSG (Cat No. 20593, Pierce, Rockford IL) in 100% Dimethylsulfoxide (DMSO) to a concentration of 0.25M (Table I). DSG solution is freshly used and excess is discarded. DSG is stored as a dry powder in a dessicated bottle at 4 °C.
Formaldehyde (FA)-PBS. FA (37%, Sigma Aldrich) is diluted to 1% final concentration in Phosphate Buffered Saline (PBS, 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4, Table II).
Table I.
Recipe for DSG solution.
| Number of plates | DSG (mg) | Vol DMSO (μL) |
|---|---|---|
| 2 | 13 | 160 |
| 4 | 25 | 320 |
| 6 | 38 | 480 |
| 12 | 72 | 960 |
Table II.
Recipe for FA-PBS solution.
| Number of plates | Volume FA | Vol PBS |
|---|---|---|
| 2 | 0.54 | 20 |
| 4 | 1.08 | 40 |
| 6 | 1.62 | 60 |
| 12 | 3.24 | 120 |
2.3 Immunoprecipitation reagents
Protease Inhibitor Cocktail: Sigma Aldrich Product No. P8340. Stock is stored at −20 °C; PIC is added to Lysis Buffer immediately before use.
DynaBeads: Protein A conjugated. No. 100.02, Dynal Inc. Store at 4 °C.
2.4 Buffers
TE 10 mM Tris-Cl, pH 7.5, 1 mM EDTA. Store at RT.
PBS/MgCl2. 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4, 1 mM MgCl2. Store at RT.
SDS-Lysis Buffer. 1% Sodium Dodecyl Sulfate (SDS), 10 mM EDTA, 50 mM Tris, pH 8.0.
Low Ionic Strength Chip dilution buffer. 50 mM NaCl, 10 mM HEPES, pH 7.4 1% IGEPAL-630 10 % glycerol. Store at RT. Add protease inhibitor cocktail immediately before use.
LiCl Wash Buffer. 0.25 M LiCl, 1% IGEPAL CA-630, 1% Sodium Deoxycholate, 1 mM EDTA 10 mM Tris-Hcl, pH 8.0. Store at RT
High Salt Wash Buffer. 500 mM NaCl, 0.1 % SDS, 1% IGEPAL CA-630, 2 mM EDTA, 20 mM Tris-Cl pH 8.0. Store at RT
Elution Buffer. 0.09 M NaHCO3, 1% SDS. Elution Buffer is prepared from adding 10% SDS to 0.1 M NaHCO3 immediately before use. Both are stored at RT.
Decross-linking Mixture. 0.2 M NaCl, 0.1M EDTA,0.4 M Tris-HCl, pH6.8, 0.4 mg/ml proteinase K (diluted from 20 mg/ml stock in H20). Decross-linking Mixture is prepared immediately before use.
3. Methods
3.1 Experimental Considerations
An overview of the two-step ChIP protocol is shown in Fig. 1. The selection of the cross-linker should be undertaken with some understanding of the chemistry and effective cross-linking length. We have had success with DSG, an irreversible cross-linking agent that cross-links NHS esters with an effective radius of approximately 7Å. This agent has been useful for highly efficient cross-linking of NF-κB, STAT3, p300/CBP, RNA Pol II and CDK9 where stimulus – inducible chromatin interactions can be seen (4;5). A summary of the types of useful cross-linkers, their chemistries, spacing arms, and methods for reversal is shown in Table III. Some demonstrations of their application have been previously reported (2;8).
Table III.
Properties of Cross-Linking Reagents.
| Crosslinker | Chemistry | Spacer arm (Å) | Reversible? | Working Cone (mM) | |
|---|---|---|---|---|---|
| Protein-protein | |||||
| DMA | imidoester | 8.6 | N | 10 | |
| DMP | imidoester | 9.2 | N | 10 | |
| DSG | NHS-ester | 7.7 | N | 2 | |
| DSP | NHS-ester | 12 | Thiols | 2 | |
| EGS | NHS-ester | 16.1 | Hydroxylamine | 2 | |
| TSAT | NHS-ester | 4.2 | N | 2 | |
| Protein-DNA | |||||
| Formaldehyde | methylene bridge | 2 | 65 C + 0.2 M NaCl | 1% |
Selection of the appropriate negative control for the immunoprecipitation is an important consideration. We typically include a tube of chromatin immunoprecipitated using pre-immune IgG. This control is important in some approaches for quantification using quantitative real-time genomic PCR (Q-RT-gPCR).
Another critical parameter in the design of the experimental protocol is to decide which type of target identification assay will be employed (Schematically diagrammed in Fig. 1). For downstream analysis using qualitative- or quantitative real-time genomic PCR (Q-RT-gPCR), fragmentation of the chromatin into 500–1000 bp fragments is optimal. However, for downstream analysis using next generation sequencing, fragmentation into smaller 300–500 bp fragments is preferred. These fragmentation methods are described as alternate protocols (Section 3.5). Similarly, the method of decross-linking is different for ChIP-Seq applications, and requires selection of one of the alternate protocols (Section 3.9).
3.2 Protein Cross-linking
Wash cells with PBS at RT three times. Tip plate to the side and remove residual solution gently with vacuum aspirator.
Add 10 ml of PBS/MgCl2 to each plate. Direct pipette spray to side of tissue culture dish to avoid disrupting adherent cells (see Note 1).
Add 80 μL of freshly prepared 0.25 M DSG solution to each plate with a p200 pipetteman. Rapidly swirl PBS to get the DSG into solution (see Note 2). The final working DSG crosslinking concentration is 2 mM.
Incubate at room temperature for 45 min. Under microscopic examination, the cells may become vacuolated, but still should be adherent to plate.
3.3 DNA Cross-linking
-
1.
Wash with PBS 3X. Tip plate to the side and remove residual solution gently with vacuum aspirator (see Note 3).
-
2.
Add 10 ml of FA-PBS solution to each plate.
-
4.
Incubate cells for 15 min at RT. Formadelhyde reactivity is temperature-dependent; this step is done at 22 °C (RT); do not use 37 °C.
-
5.
Wash cells three times with PBS (Note 4).
-
6.
Harvest cells by addition of 1 ml PBS/10 cm2 plate and scraping with a spatula. Transfer to labeled 1.7 mL Eppendorf tubes.
-
7.
Spin 3K, 1 min at RT. Remove PBS carefully to not disturbe the cellular pellet.
3.4 Cell lysis
Resuspend pellets in 500 μL of SDS Lysis Buffer/Protease inhibitor cocktail mix at room temp.
3.5 DNA fragmentation for quantitative real time genomic PCR (Q-RT-gPCR)
Sonicate the cross-linked cellular lysate 4 times, 15 sec at setting 4 with 10 sec break on ice in-between each pulse (Branson sonifier 150, obtained from Branson Ultrasonics, Danbury, CT). Immerse the tip into the middle of the lysate; do not sonicate the surface, as this will make the sample foam. If this occurs, spin sample in microfuge and repeat sonication.
Spin 10 K, 10 min at 4 °C. Transfer supernatant to a clean, 1.7 ml Eppendorf tube. Be careful not to disturb the pellet.
3.5 (Alternate Protocol) DNA fragmentation for ChIP-Seq
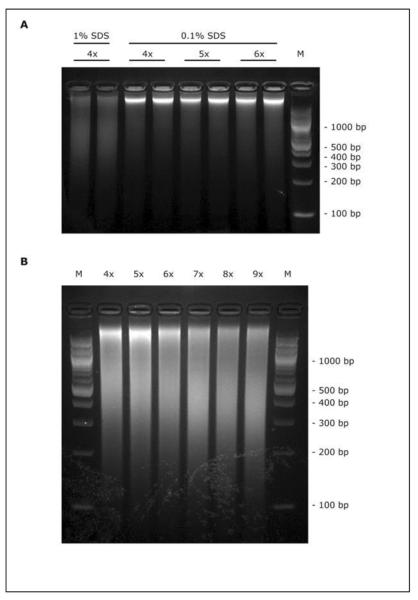
The duration and intensity of sonication must be empirically optimized in order to shearing cross-linked chromatin to a size range of 200–500 bp which is ideal for ChIP-seq. It is important to note that use of two crosslinking agents can make chromatin more resistant to sonication. The concentration of SDS in sonication buffer also has dramatic influence on DNA shearing efficiency. As an example in Fig. 2, we compared DNA fragments obtained from A549 cells after sonication under different SDS concentration (0.1% vs 1% SDS, Fig. 2A) or different sonication rounds (Fig. 2B). Likewise, over-sonication of chromatin can increase the yield of DNA fragments at right size, but might cause denaturation of proteins and may not produce good signal for ChIP.
Figure 2. Dynamic Range of Q-RT-gPCR Assay.
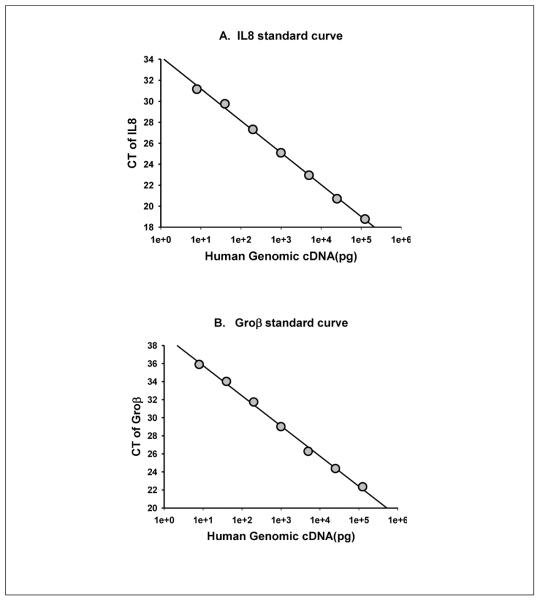
To check the Q-RT-gPCR dynamic range of primers designed in this protocol, each primer pair can be used to amplify a series of diluted human genomic DNA. Plotted is the mean Ct value of duplicate samples for each log-transformed concentration. Note the wide range of linear response. A. IL8 primers, B. Groβ primers
3.6 Sizing of sonicated DNA fragments
Remove 20 μL of sonicated lysate, and add to 200 μL of (200 mM NaCl, 0.5% SDS. Add 1 μL of proteinase K (stock 20 mg/ml in H20). Heat at 65 °C for 1 h in a heating block.
Phenol-chlorofoform extract the DNA. Add 100 μL of PIC, vortex, spin. Add 1 mL of 100% ETOH, vortex, spin. Dry, run on 1% Agarose gel with Ethium Bromide.
3.7 Quantification of DNA recovery
Dilute supernatant 1:50 into 100 μL of TE. Read absorbance at 260 nM (A260) in a UV spectrophotometer relative to blank solution (2 μL SDS-Lysis buffer diluted into 100 μL TE; see Note 5).
3.8 Immunoprecipitation
Use equal amounts of lysate (adding equal ODs of DNA) from each treatment for the immunoprecipitation. The maximum amount of lysate added should be no more than 100 μL.
Bring total volume up to 900μL with Low Ionic Strength Chip dilution buffer. Add 4 μgm of affinity isolated antibody and rotate tubes overnight at 4°C.
Dilute herring sperm DNA to a concentration of 100 μg/ml in Low Ionic Strength Chip dilution buffer. (Herring Sperm DNA is made at 10 mg/ml in H20). Sonicate 3 times, 15 sec each pulse. Add 0.5 ml to 40 μL of 50% slurry of pre-washed Dynal magnetic beads. Rotate overnight at 4°C.
In the morning, wash beads twice with Low Ionic Strength Chip Buffer, capturing beads on a magnetic stand after each addition of washing buffer. Resuspend to original 50% slurry, and add 40 μL immunoprecipitation tube. Rotate 1–4 h, 4°C.
Spin the immunoprecipitates at 5 K rpm in microfuge for 1 min at RT. Save 50 μL for input control.
Wash beads 2X with 500 μL Low Ionic Strength ChIP Dilution Buffer (invert tubes before spin.). After first wash, transfer to fresh prelabeled 1.7 ml Eppendorf tube.
Wash beads once with 500 μL high salt buffer.
Wash beads once with 500 μL LiCl buffer.
Wash beads 2X with 500 μL TE buffer.
Add 250 μL of Elution Buffer, vortex, incubate 15 min at RT
Transfer supernatant to clean Eppendorf tube. Repeat elution with another 50 μL of elution buffer, 15 min at RT. Combine eluates.
3.9 Decross-Link Sample for Q-RT-gPCR
Add 51 μL of Decross-Linking Mixture to eluates and incubate at 65 °C for 1–4 h.
3.9 (Alternate Protocol) Decross-Link Sample for ChIP-Seq
A high background signal of ChIP-seq may originate from nondigested RNA since the Klenow fragment used for generating ChIP-seq sequencing library can also react with RNA. Therefore it is better to include RNase A treatment before proteinase K digestion in order to potentially reduce the RNA caused background (9).
To do this,
Add RNase A (Sigma) to the eluate at a final concentration of 20–50ug/ml. Mix and incubate at 37°C for 30 min.
Add Proteinase K to a final concentration of 0.4mg/ml and incubate at 65 °C for 1–4 h.
Generally ChIP DNA as obtained by standard ChIP protocol is of adequate purity for quantitative PCR analysis but often contains contaminants that interfere with subsequent steps required for ChIP-seq analysis. DNA purification through commercially available clean-up kits such as Qiagen QiaQuick spin columns typically eliminates these contaminants thus ensures a success.
3.10 Decross-linking input
To process input decrosslink in 200 mM NaCl 65 °C for 1 h with proteinase K.
3.11 DNA Extraction
Add 100 μL of phenol/chloroform to each sample (see Note 9).
Vortex/ Spin at 14K for 3–5min. (If solution becomes white, add 100 μL TE, vortex and spin again).
Transfer top layer to clean Eppendorf tube.
Add 100 μL of Chloroform to each sample/ Vortex/Spin, Transfer to fresh tube.
Add 1 ml of 100% ETOH and 100 μL of 7.5 M ammonium acetate and 3 μL glycogen (20 mg/ml in H20; see Note 10).
Incubate −80 °C for 30 min
Spin 14 K in a benchtop microfuge for 10 min, 4 °C
Wash pellets with 1ml of 100% ETOH
Spin 14 K for 10 min, 4 °C
Resuspend pellets in 20–50 μL of TE or H20.
3.12 Target Analysis by Quantitative Real-Time Genomic PCR (Q-RT-gPCR)
Q-RT-gPCR is the standardly accepted assay used to accurately measure the amount of enrichment of a particular DNA sequence by ChIP in this protocol. The principle of Q-RT-gPCR is based on detection of a fluorescent signal produced proportionally during amplification of a PCR product. The use of Q-RT-gPCR has several advantages: short turnaround time for data acquisition and analysis; reliable results compared to traditional PCR methods; and it is quantitative. However, as the procedure is very sensitive, there is an increased chance of variability of the results. There are several aspects of Q-RT-gPCR that need to be considered for reliable and reproducible results. They are: (i) primer design using dedicated softwares, some of which are freely available on the Internet; (ii) deciding on positive and negative controls; (iii) technical and biological repeats, and (iv) analyses of the data.
3.12.1. Designing Q-RT-gPCR primers for genomic DNA regions of interest
-
1
Primers are designed encompassing the predicted Transcription Factor binding site if the latter information is available. As a control, we use primers designed to amplify the 3′-untranslated region (3′-UTR) of the target gene.
-
2
We regularly use NCBI/Primer-BLAST in NCBI website for designing real-time PCR primer pairs that are typically 20–25 mers with a Tm range of 58–63 °C (maximum Tm difference 2 °C) and that amplify a product of 50–150 bp. The melting temperature is calculated based on the Nearest-Neighbor thermodynamic parameters. The GC content is set between 40 and 55%.
-
3
Order primers with standard desalting purification.
-
4.
The Q-RT-gPCR primers are dissolved in TE buffer at 8 μM of stock concentrations. Each pair of primers was examined by making standard curve of CT using a series of diluted human genomic cDNA (Fig. 3). Only the primers that its CT value showed excellent correlation with the concentrations of human genomic cDNA are used for Q-RT-gPCR in this protocol.
Figure 3. Sonication efficiency under different conditions.
(A) Human alveolar epithelial A549 cells were lysed and sonicated in 0.1% or 1% SDS containing buffer. Using 40% duty cycle, sonicate chromatin for indicated 4 to 6 rounds, each round with 15 second pulses. DNA was reverse crosslinked and separated on a 2% agarose gel. 1kb ladder has been used as a marker of DNA size. Average size of DNA fragments revealed that performing the sonication in a 0.1% SDS-containing buffer compromises shearing efficiency. (B) A549 cells were lysed in 1% SDS buffer and sonicated for indicated 4 to 9 rounds. Here, 7 rounds of sonication seems better for fragmentation at a size range of 200–500bp.
3.12.2. Equipment and CHIP-Q-RT-gPCR reagents
-
1
Bio-Rad MyiQ Real Time PCR Detection System is used in this protocol.
-
2
Bio-Rad iQ SYBR Green Supermix- a prepackaged PCR mix containing dNTPs, 50 U/ml iTaq DNA polymerase, 6 mM MgCl2, SYBR Green I, and 20 nM fluorescein.
-
3
Mix each primer pair (forward and reverse of experimental or control) in the same tube and prepare a stock concentration of 8 μM each with 10 mM Tris-Cl pH 8.5.
-
4
We use purified human genomic cDNA as positive control and negative control is chromatin immunoprecipitated with IgG.
-
5
Bio-Rad 96-well clear multiplate PCR plate are used; the plate is sealed by Bio-Rad Microseal B film before putting into PCR machine.
3.12.3. Real-time PCR Set up
Set up real-time PCRs in triplicate with the diluted input DNA, as shown in Table IV. The total reaction volume in each tube is 20 μL(see Notes 6,7, 8).
Select the two Step-Melting PCR protocol from iQ5 software window to run Q-RT-gPCR.
Table IV.
Recipe for Q-RT-gPCR reaction mixture.
| Component | Amount/reaction (μL) | Final |
|---|---|---|
| Primer mix (stock 8 μM each) | 1 | 400 nM |
| DNA sample (ChIP or diluted “Input” DNA) | 3 | |
| Nuclease-free water | 6 | |
| SYBR Green Supermix | 10 | |
| Total Volume | 20 |
3.12.4. Data Analysis
After the cycling is complete, first visualize the Dissociation plot. The Dissociation plot for a primer should produce a single sharp peak. More than one peak represents additionally amplified products and the amplification data should not be used.
The next step is to adjust the baseline. The default baseline is set from cycles 3 to 15. To find out whether the baseline needs to be adjusted, it is important to find out which reaction emerges before the default baseline. For this, change the plot to linear view. For reactions that emerge after 15 cycles, no changes have to be made. For highly abundant targets, the end point of the baseline (15 is the default) needs to be adjusted. Usually, the baseline is adjusted to one or two cycles before the earliest amplification.
The threshold cycle (Ct; the fractional cycle number at which the fluorescence passes the threshold) value is determined in the experimental report.
Export the results into Microsoft Excel for analysis. The Ct values for the technical repeats should show minimal variation (ideally be within half a Ct value of each other).
Calculate the ΔCt value (normalized to the input samples) for each sample: ΔCt [Ct (sample) − Ct (input)]. Next, calculate the ΔΔCt (ΔCt (experimental sample) −ΔCt (negative control)).
Calculate the fold difference between experimental sample and negative control using the equation 2−ΔΔCt. As indicated earlier, the negative control is DNA immunoprecipitated with control IgG.
3.13 Target Analysis by Next Generation Sequencing (NGS)
ChIP coupled with NGS is known as ChIP-seq, enables the unbiased identification of protein-chromatin interactions on a global basis. When compared with microarray analysis of ChIP (ChIP-chip), ChIP-seq is less expensive, has higher signal/noise ratios and requires lower amounts of ChIP DNA for genome-wide analysis (7). This process is initiated by adding sequencing adapters to blunt-ended ChIP DNA. Subsequently, PCR is performed for limited number of cycles to generate the sequencing library which is ready to be sequenced, e.g., using the procedure developed for the Illumina Genome Analyzer (10). In general, approximately 10 to 20 million sequence reads (for 36 cycles of sequencing) are obtained for each sample per run. The sequence of ChIP DNA fragments is then mapped back to the reference genome and further visualized using a genome browser to identify the binding sites.
Although commercial services are currently available for ChIP-seq analysis starting from purified ChIP DNA, many considerations on ChIP DNA preparation have to be taken into account in order to achieve successful ChIP-seq results.
3.13.1. Materials
ChIP DNA sample from Section 3.9 (approx.10 ng in 42.5 ul water), the quality should always be validated (e.g., by Real-Time PCR) before performing the library preparation steps for high throughput sequencing.
NEBNext™ DNA Sample Prep Master Mix Set 1 (New England Biolabs).
T4 DNA ligase (Enzymatics).
QIAquick PCR purification Kit (Qiagen).
Adapter oligonucleotides, Illumina PE PCR primers 1.0 and 2.0.
E-Gel® SizeSelect™ 2% Starter kit (Invitrogen).
Agilent Technologies 2100 Bioanalyzer.
Illumina Genome Analyzer II system and associated equipment, reagents, kits, and software.
3.13.2. End repair
- Prepare the following reaction mix:
- ChIP enriched, qPCR verified DNA (42.5ul)
- NEBNext End Repair Reaction Buffer (5ul)
- NEBNext End Repair Enzyme Mix (2.5ul)
Incubate in a thermal cycler for 30–45 minutes at 20°C.
Purify DNA on QIAquick column and elute in 42ul elution buffer.
3.13.3. dA-tailing
- Prepare the following reaction mix:
- End repaired, blunt DNA (42ul)
- NEBNext dA-Tailing Reaction Buffer (5ul)
- Klenow exo- (3ul)
Incubate for 30 minutes at 37°C.
Purify the reaction product on QIAquick column and elute in 34ul elution buffer.
3.11.4. Adapter ligation
- Prepare the following reaction mix:
- dA-tailed DNA (34 μL)
- Enzymatics 2× Rapid Ligation Buffer (40 μL)
- 2.5uM Adapter Oligo mix (1 μL)
- Enzymatics T4 DNA ligase (5 μL)
Incubate for 15 minutes at room temperature.
Purify DNA on QIAquick column and elute in 50 μL elution buffer.
3.13.5. Gel-purification of ligation products
-
1.
On E-Gel® SizeSelect™ 2% Agarose (Invitrogen), load 100 – 250 ng of DNA ladder diluted in 5–10 μL into the middle well (1:50, 10 μL).
-
2.
load 25 μL samples into every other lane of the gel.
-
3.
load 25 μL deionized water into all empty wells.
-
5.
Run SizeSelect 2% (program 8) and set time according to the Estimation Table.
-
6.
Extract DNA fragment at approximately 400 bp and/or 600 bp in size.
3.13.6. PCR enrichment
- Prepare the following reaction mix on ice:
- Gel extracted DNA (24 μL)
- 25uM PCR Primers 1.0 and 2.0 Mix (1 μL)
- 2× Phusion™ High-Fidelity PCR Master Mix (25 μL)
- Amplify using the following PCR protocol:
- 30 seconds at 98°C
- 6–18 cycles of 10 sec, 9 8°C; 30 sec, 65 °C; 30 sec, 72 °C
- 5 minutes at 72°C
Purify DNA on QIAquick column and elute in 50ul elution buffer.
3.13.7. Library validation
Load 1 μL of the resuspended construct and 1ul of the negative control on an Agilent Technologies 2100 Bioanalyzer using the high-sensitivity DNA1000 kit.
Check the size, purity, and concentration of the sample.
3.13.8. Sequencing and data analysis
Sequence adapter ligated ChIP DNA using the Illumina Genome Analyzer II.
The sequence reads obtained after base-calling can be aligned to a reference genome and further visualized using for example the UCSC or Ensembl genome browser.
Several algorithms can be utilized to analyze ChIP-seq data and identify the enriched regions across the whole genome, thus harbor the protein-chromatin interaction in vivo.
4. Notes
Inclusion of divalent cations is important to keep cells adherent during fixation. CaCl2 can be substituted for MgCl2 if necessary.
Addition of DSG solution will become milky immediately on contact with PBS, but should become clear with rapid mixing. There should be no precipitate under the microscope.
DSG must be efficiently washed prior to addition of formaldehyde; DSG will react with formaldehyde and reduce its effective concentration.
We do not specifically neutralize FA; SDS-Lysis buffer contains Tris; the free amines in Tris neutralizes the cross-linking reaction..
A260 readings of 0.1–0.8 are generally within the linear range; if the chromatin is outside of this range, adjust the dilutions accordingly and repeat the A260 measurement.
Eliminate bubble before running PCR because it will affect the final Ct results.
Make mixtures for PCR setup to reduce the differences among reactions.
If the initial set of 400 nM primer doesn't work, optimize the primer concentration.
During phenol-chloroform extraction, Phase Lock Gel Light tubes (Flowgen Bioscience, FPR5101) can be used easily to eliminate interphase-protein contamination and increase the yields of up to 30%.
GlycoBlue (Ambion, AM9516), a glycogen covalently linked to a blue dye, can be used instead of glycogen as carrier for the ethanol precipitation. It substantially facilitates nucleic acid precipitation while increasing the size and visibility of the pellet.
There are two common normalization methods for ChIP analysis using qPCR - the percent input method and the fold enrichment method. With the percent input method, signals obtained from the ChIP are divided by signals obtained from an input sample. This input sample represents the amount of chromatin used in the ChIP. Typically, 1% of starting chromatin is used as input. In this case, 6.644 cycles (i.e., log2 of 100) must be subtracted from the Ct value of diluted input before calculating delta Ct. The fold enrichment method is also called 'signal over background' method. With this method, the ChIP signals are divided by the mock antibody signals, representing the ChIP signal as the fold increase in signal relative to the background signal. The assumption of this method is that the level of background signal is reproducible between different primer sets, samples, and replicate experiments. Combined these two methods together, another method aims to end up with a signal over noise ratio normalized to total input DNA signal.
Acknowledgements
This work was supported, in part, by NIH grants AI062885 (ARB), NIAID Contract NHLBI contract BAA-HL-02-04 (ARB), and ES06676 (to K. Elferink, UTMB).
References
- 1.Kornberg RD, Lorch Y. Twenty five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 2.Nowak DE, Tian B, Brasier AR. Two-Step Cross-linking method for Identification of NF-κB Gene Network by Chromatin Immunoprecipitation. Biotechniques. 2005;39:715–725. doi: 10.2144/000112014. [DOI] [PubMed] [Google Scholar]
- 3.Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kappaB-dependent gene activity. EMBO J. 2006;25:798–810. doi: 10.1038/sj.emboj.7600977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR. RelA Ser276 Phosphorylation Is Required for Activation of a Subset of NF-{kappa}B-Dependent Genes by Recruiting Cyclin-Dependent Kinase 9/Cyclin T1 Complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou T, Ray S, Brasier AR. The functional role of an IL-6 inducible CDK9-STAT3 complex in human ã-fibrinogen gene expression. J. Biol. Chem. 2007;282:37091–37102. doi: 10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 6.Ramadoss P, Chiappini F, Bilban M, Hollenberg AN. Regulation of Hepatic Six Transmembrane Epithelial Antigen of Prostate 4 (STEAP4) Expression by STAT3 and CCAAT/Enhancer-binding Protein +¦. J. Biol. Chem. 2010;285:16453–16466. doi: 10.1074/jbc.M109.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-Wide Mapping of in Vivo Protein-DNA Interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 8.Zeng PY, Vakoc CR, Chen Z-C, Blobel GA, Berger SL. In vivo dual cross-linking for identification of indirect DNA-associate proteins by chromatin immunoprecipitation. Biotechniques. 2006;41:694698. doi: 10.2144/000112297. [DOI] [PubMed] [Google Scholar]
- 9.Waldminhaus T, Skarstad K. ChIP on Chip: surprising results are often artifacts. BMC Genomics. 2010;11:414. doi: 10.1186/1471-2164-11-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. METHODS. 2009;48:240–248. doi: 10.1016/j.ymeth.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]