SUMMARY
Cell therapy can improve cardiac function in animals and humans after injury, but the mechanism is unclear. We performed cell therapy experiments in genetically engineered mice that permanently express green fluorescent protein (GFP) only in cardiomyocytes after a pulse of 4-OH-tamoxifen. Myocardial infarction diluted the GFP+ cardiomyocyte pool, indicating refreshment by non-GFP+ progenitors. Cell therapy with bone marrow-derived c-kit+ cells, but not mesenchymal stem cells, further diluted the GFP+ pool, consistent with c-kit+ cell-mediated augmentation of cardiomyocyte progenitor activity. This effect could not be explained by transdifferentiation to cardiomyocytes by exogenously delivered c-kit+ cells or by cell fusion. Therapy with c-kit+ cells but not mesenchymal stem cells improved cardiac function. These findings suggest that stimulation of endogenous cardiogenic progenitor activity is a critical mechanism of cardiac cell therapy.
INTRODUCTION
Adult mammalian cardiomyocytes retain some capacity for renewal (Beltrami et al., 2003; Bergmann et al., 2009; Hsieh et al., 2007), but this response is inadequate for prevention of heart failure after cardiac injury (Lopez et al., 2006). Demonstrations of cardiomyocyte transdifferentiation by a variety of progenitor cells have provided the rationale for cell-therapy experiments in animal models and in humans with heart failure (Segers and Lee, 2008). Although some trials have shown improvements in cardiac function after cellular therapies, the therapeutic effect has been modest in most studies, and the mechanism of action remains incompletely elucidated (Abdel-Latif et al., 2007).
A wide variety of mechanistic explanations for functional benefits observed with cardiac cell therapy have been proposed. The direct transdifferentiation into cardiomyocytes by exogenous cells represents the most obvious explanation for cell therapy-mediated improvements in cardiac function, yet the capacity for direct transdifferentiation by exogenously delivered cells remains unclear (Balsam et al., 2004; Murry et al., 2004; Orlic et al., 2001. Additional proposed mechanisms of cell therapy include inflammatory modulation, transdifferentiation into endothelial or smooth muscle cells, or paracrine stimulation of angiogenesis or endogenous cardiomyocyte progenitors. Defining the contributions of these mechanisms represents a fundamental prerequisite to the future optimization of cardiac regenerative therapies.
Genetically engineered mice enable lineage mapping experiments to determine the role of progenitors in regenerative processes. We previously described a double-transgenic MerCreMer-ZEG mouse for genetic lineage mapping; the cardiomyocytes of MerCreMer-ZEG mice irreversibly express GFP upon treatment with 4-OH-tamoxifen, allowing for “pulse” labeling of existing cardiomyocytes (Hsieh et al., 2007). This lineage-mapping approach has demonstrated that myocardial infarction or pressure overload results in precursor-dependent replenishment of the cardiomyocyte pool. Here we used genetic fate mapping to determine the effect of exogenously delivered progenitor cells on endogenous cardiomyocyte refreshment.
RESULTS
Bone Marrow-Derived c-kit+ Cell Therapy Stimulates Endogenous Cardiac Progenitors after Myocardial Infarction
We generated an in-bred transgenic colony of MerCreMer-ZEG mice over a 4 year period to test the hypothesis that cell transplantation regulates endogenous progenitor activity. We assessed the regenerative properties of a purified bone marrow-derived cell population (lineage−/c-kit+ or c-kit+ cells) previously reported to improve cardiac function (Figure 1A; Orlic et al., 2001; Rota et al., 2007). MerCreMer-ZEG female mice were pulsed with 4-OH-tamoxifen to induce cardiomyocyte-specific GFP expression, after which they were subjected to myocardial infarction by coronary ligation. Mice were randomized to receive vehicle control or c-kit+ cells (6 × 105) freshly isolated from a wild-type male mouse (Figure S1 available online) in two divided 5 μL intramyocardial injections to the medial and lateral infarct borders of the left ventricle. After an 8-week chase, histologic sections were stained for GFP and β-galactosidase as previously described (Hsieh et al., 2007). An observer unaware of treatment group captured photographs from the infarct border zone and remote areas, after which a second blinded observer counted the positive and negative cardiomyocytes.
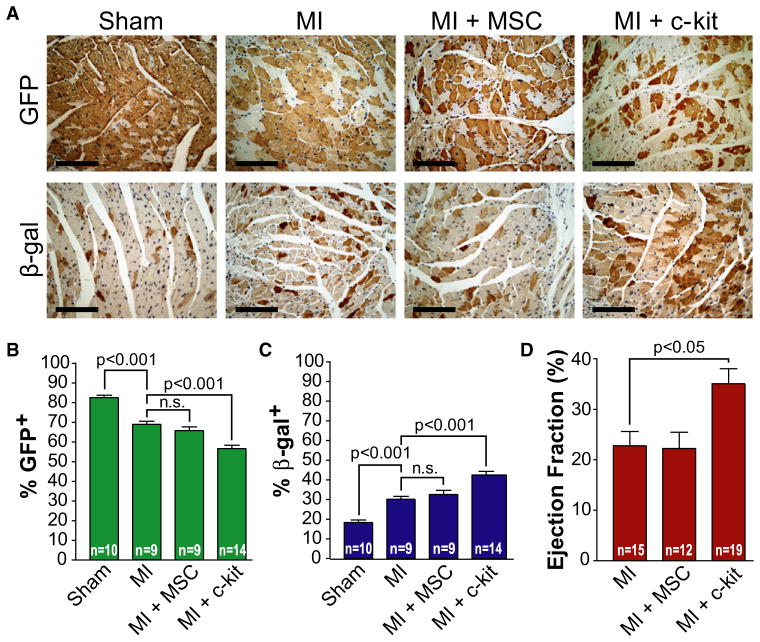
Figure 1. Intramyocardial Delivery of Bone Marrow-Derived c-kit+ Cells after Myocardial Infarction Stimulates Endogenous Cardiomyocyte Regeneration.
(A) Experimental protocol. MCM+/ZEG+ bitransgenic female mice were treated for 2 weeks with 4-OH-tamoxifen (OH-Tam) to induce a cardiomyocyte-specific switch from β-galactosidase (β-gal) to GFP expression. Experimental MI was performed by coronary ligation. Bone marrow c-kit+ cells, isolated from wild-type (WT) male mice, were then injected into the peri-infarct region. The hearts were fixed and stained 8 weeks post-MI. Also see Figures S1–S3.
(B) Hearts were stained (brown) for GFP (top) or β-galactosidase (bottom). Representative staining from the MI border zone. The percentage of GFP+ cardiomyocytes decreased after MI and decreased further after delivery of c-kit+ cells. Decrements in the percentage of GFP+ cardiomyocytes were matched by increases in the percentage of β-gal+ cardiomyocytes, consistent with cardiomyocyte repletion by endogenous β-gal+ progenitors. Scale bars represent 100 μm. (C and D) Graph representing the percentage of GFP+ (C) and β-gal+ (D) cardiomyocytes in the MI border zone and remote areas. Data expressed as mean ± SEM.
As expected, the percentage of GFP+ cardiomyocytes in the border zone (60.3% ± 1.2%; p < 0.0001) and remote area (73.3% ± 1.5%; p < 0.01) decreased after myocardial infarction compared with the sham (80.8% ± 1.8%), consistent with an increase in progenitor activity stimulated by the injury. However, c-kit+ cell therapy produced a significant further dilution in the GFP+ cardiomyocyte pool at the infarct border zone (49.4% ± 2.3%; p < 0.0001) (Figures 1B and 1C), a finding not observed in either the right ventricle (Figure S2) or regions of the left ventricle remote to the MI. We also studied β-galactosidase+ cardiomyocytes, because reduction in the GFP+ pool caused by transdifferentiation of exogenously delivered wild-type c-kit+ cells into mature cardiomyocytes would not result in a complimentary increase in β-galactosidase+ cardiomyocytes. The decrease in GFP+ cardiomyocytes was balanced by a corresponding increase in the percentage of β-galactosidase+ cardiomyocytes (Figures 1B and 1D), consistent with c-kit+ cell-mediated activation of endogenous progenitors and not transdifferentiation of the exogenous c-kit+ cells.
To determine the time frame of the c-kit+ cell-mediated reduction in GFP+ cardiomyocytes, we also analyzed a cohort of mice 2 weeks after myocardial infarction. We found a trend toward a reduction in the percentage of GFP+ cardiomyocytes 2 weeks after MI (Figure S2; sham = 80.7% ± 0.5% versus MI = 71.0% ± 3.9%, n.s.). At this time point, however, there was no additional reduction in the percentage of GFP+ cardiomyocytes in c-kit+ cell-treated mice, suggesting that exogenously delivered c-kit+ cells do not exert their stimulatory effects in the acute post-MI phase.
We performed a study in which c-kit+ cell- or vehicle control-treated mice also received BrdU (20 μg/hr) by osmotic minipump for 1 week after MI. Eight weeks after MI, we found that the majority of BrdU+/troponin+ cardiomyocytes were GFP−, a finding consistent with the proliferation and differentiation of β-galactosidase+ progenitors (Figure S3). We also detected a significant increase in the number of BrdU+/troponin+ cardiomyocytes in c-kit+ cell-treated mice compared to vehicle control, which is consistent with a c-kit+ cell-stimulated increase in the generation of new cardiomyocytes.
We also considered the possibility that the reduction in the percentage of GFP+ cardiomyocytes was due to selective proliferation of β-galactosidase+ cardiomyocytes, as might be expected if the observed inefficiency in β-galactosidase-to-GFP recombination was due to a subset of immature cardiomyocytes with higher proliferative capacity. To address this possibility, we performed morphometric analyses of β-galactosidase+ and GFP+ cardiomyocytes, finding that there was no baseline difference in the size of the two populations (Figure S3). After injury, however, the GFP+ pool of pre-existing cardiomyocytes was larger, consistent with compensatory hypertrophy. These data are consistent with the concept that there are no baseline differences in the maturity of the β-galactosidase+ and GFP+ cardiomyocytes pools, but after injury, the mean size of the β-galactosidase+ pool is smaller because it contains newly differentiated cardiomyocytes.
Cell Therapy with Mesenchymal Stem Cells after Myocardial Infarction Does Not Stimulate Endogenous Progenitors
We next sought to test whether the observed stimulation of progenitors resulted from properties specific to c-kit+ cells. We used an identical protocol of inducing cardiomyocyte-specific GFP expression in MerCreMer-ZEG mice and conducted a randomized, blinded, vehicle-controlled study comparing c-kit+ cells to bone marrow-derived mesenchymal stem cells (MSCs), a cell population used in clinical trials and demonstrated to differentiate into cardiomyocytes in vitro (Hare et al., 2009; Pijnappels et al., 2008). MSCs were enriched from bone marrow of wild-type male mice via a standard culture protocol (Beltrami et al., 2007); the MSC-specific marker profile was reconfirmed by flow cytometry prior to experimental delivery (Figure S1). A total of 6 × 105 freshly isolated c-kit+ cells or MSCs were injected immediately after coronary ligation in MerCreMer-ZEG female mice.
After an 8 week chase, cell therapy with c-kit+ cells again led to a significant further dilution of the GFP+ cardiomyocyte pool (56.6% ± 1.7% versus 68.8% ± 1.5%; p < 0.001) and a parallel increase in the percentage of β-galactosidase+ cardiomyocytes (42.5% ± 1.8% versus 30.1% ± 1.4%; p < 0.001) compared to control. However, the delivery of MSCs did not lead to reductions in GFP+ cardiomyocytes or increases in β-galactosidase+ cardiomyocytes, suggesting that MSCs lacked the ability of c-kit+ cells to stimulate endogenous progenitor activity (Figures 2A and 2B). Therapy with c-kit+ cells also resulted in a significant improvement in ejection fraction, a standard measure of cardiac function (as determined by a blinded cardiac catheterization) 8 weeks after MI (n > 12 mice in each group; p < 0.05; Figure 2C; Figure S4), consistent with prior rodent and human studies (Abdel-Latif et al., 2007; Rota et al., 2007). In contrast, MSC therapy, which did not stimulate endogenous progenitor activity, did not improve the ejection fraction. The differences in cardiac function could not be explained by intergroup differences in infarct size (Figure S4). Although it is possible that the observed stimulation of endogenous progenitors accounts for the associated improvements in ventricular function, we cannot exclude the possibility that additional mechanisms are also involved. Because of concerns of cardiotoxicity after high-dose tamoxifen in MerCreMer mice (Koitabashi et al., 2009), we also studied the effects of our 4-OH-tamoxifen dosing protocol on cardiac function. Although we were able to induce transient cardiotoxicity with high-dose 4-OH-tamoxifen, we did not observe a similar effect with our 14-day low-dose protocol (Figure S5). These experiments suggest that improvements in cardiac function from specific types of cells, such as c-kit+ cells, may be due to the ability of these cells to activate endogenous cardiogenic progenitors.
Figure 2. Intramyocardial Delivery of Bone Marrow c-kit+ Cells but Not Bone Marrow-Derived Mesenchymal Stem Cells Stimulates Endogenous Cardiomyocyte Regeneration.
(A) Data are derived from a randomized, blinded study with the same protocol depicted in Figure 1A and designed to compare the regenerative properties of c-kit+ cells versus mesenchymal stem cells (see Figure S1). After 8 weeks, hearts were fixed and stained (brown) for GFP (top) or β-galactosidase (bottom). Representative staining from the MI border zone. The percentage of GFP+ cardiomyocytes was again reduced by c-kit+ cells but not MSCs. All decrements in the percentage of GFP+ cardiomyocytes were matched by increases in the percentage of β-gal+ cardiomyocytes (bottom), consistent with cardiomyocyte repletion by endogenous β-gal+ progenitors. Scale bars represent 100 μm.
(B and C) Graph representing the percentage of GFP+ (B) and β-gal+ (C) cardiomyocytes in the MI border zone. Data expressed as mean ± SEM.
(D) Graph representing the mean ejection fraction as measured by cardiac catheterization 8 weeks after MI. Data expressed as mean ± SEM.
See Figures S4 and S5.
Cardiomyocyte Transdifferentiation Cannot Explain the c-kit+ Cell-Mediated Stimulation of Cardiomyocyte Renewal
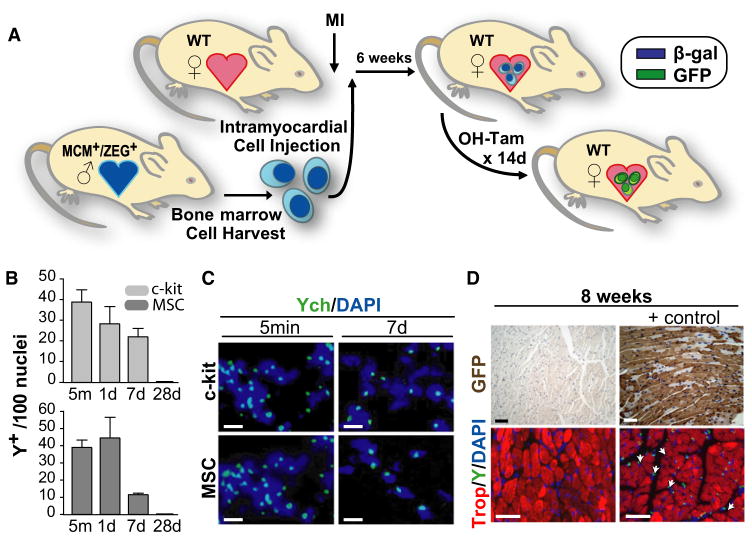
We next directly addressed the fate of injected cells to determine whether the dilution of GFP+ cardiomyocytes could be explained by either transdifferentiation of exogenously delivered c-kit+ cells or by their fusion with endogenous cells. We performed sex-mismatched cell transplantation after MI, delivering c-kit+ cells or MSCs derived from male MerCreMer-ZEG mice into female wild-type recipients (Figure 3A). Cell isolation, purification, and delivery protocols were identical to prior experiments; 4-OH-tamoxifen was administered between weeks 6 and 8 after MI to induce GFP expression by any exogenously delivered MerCreMer-ZEG cells that had transdifferentiated into cardiomyocytes. Eight weeks after MI, we screened for transdifferentiated cardiomyocytes by performing GFP and β-galactosidase immunohistochemistry, with 4-OH-tamoxifen-injected MerCreMer/ZEG mice used as a positive control. Despite screening multiple sections per mouse and >10 mice per experimental group, representing more than 7.5 × 105 border zone cardiomyocytes, we found no GFP+ or β-galactosidase+ cardiomyocytes (Figure 3C). Although this approach cannot exclude rare cardiomyocyte transdifferentiation by exogenously administered cells, this analysis excludes with a high degree of certainty (chi-square, p < 0.0001) that the reduction in the GFP+ cardiomyocyte pool observed in two independent experiments (Figures 1 and 2) is explained by transdifferentiation of exogenously administered c-kit+ cells. For additional confirmation, we performed FISH analysis on hearts from three independent studies (MSC, 21 mice; c-kit+, 34 mice) and found no Y chromosome-positive nuclei (Figure 3C), suggesting minimal survival of exogenously delivered cells in the heart 8 weeks after MI. To confirm successful cell delivery and further determine the fate of exogenously delivered cells, we analyzed mice at early time points. Y chromosome-positive donor cells were easily detected in all hearts 5 min, 24 hr, and 1 week after injection with c-kit+ cells or MSCs (n = 3 mice/group; Figures 3B and 3C). By 4 weeks after MI, we no longer could detect Y chromosome+ cells (n = 3 mice), suggesting substantial loss of exogenously delivered cells between weeks 1 and 4. Therefore, via multiple independent methods, the observed stimulation of progenitor activity in c-kit+ cell-treated mice could not be explained by transdifferentiation of exogenously delivered cells.
Figure 3. Cell Therapy-Mediated Cardiomyocyte Regeneration Cannot Be Explained by Transdifferentiation of Exogenously Delivered Bone Marrow Stem Cells.
(A) Experimental protocol. WT female mice underwent experimental MI by coronary ligation prior to the intramyocardial delivery of bone marrow cells isolated from bitransgenic MCM+/ZEG+ male mice. 6 weeks later, mice were treated for 2 weeks with 4-OH-tamoxifen (OH-tam) prior to sacrifice to induce a β-gal to GFP switch in expression in any exogenously administered cells that had transdifferentiated into CMs.
(B) Time course of the fate of injected Y chromosome+ c-kit+ cells and MSCs at the MI border zone. Data expressed as Y chromosome+ cells per 100 nuclei. Data expressed as mean ± SEM.
(C) Representative Y chromosome FISH analysis (green) revealed successful intramyocardial delivery of c-kit+ cells and MSCs in all hearts harvested 5 min or 1 week after intramyocardial injection (12/12). Scale bars represent 10 μm.
(D) GFP and Y chromosome stains from WT mice after MI and intramyocardial delivery of bone marrow cells isolated from bitransgenic mice. The absence of GFP+ (brown) CMs (top left) suggests that the observed c-kit+ cell-stimulated increase in CM progenitor activity cannot be explained by transdifferentiation of exogenously delivered cells. Sections from bitransgenic MCM+/ZEG+ mice used as positive controls. By 8 weeks post-MI, Y chromosome+ cells (green) were no longer detected (bottom left); Y chromosome staining of hearts from male mice used as positive controls (bottom right). Scale bars represent 40 μm.
Cell Fusion between Donor and Recipient Cells Cannot Explain the c-kit+ Cell-Mediated Stimulation of Cardiomyocyte Renewal
Because prior reports have demonstrated fusion between bone marrow cells and cardiomyocytes (Nygren et al., 2004), we considered the possibility that cell fusion could explain our findings. Although the absence of Y chromosome+ cardiomyocytes 8 weeks after sex-mismatched cell transplantation made it unlikely that fusion could account for our findings, we also performed independent experiments to address this possibility. Either c-kit+ cells or MSCs from MerCreMer−/ZEG+ male mice were delivered to MerCreMer+/ZEG− female mice with MI. In these animals, only cell fusion between donor cells and recipient cardiomyocytes should result in cells capable of 4-OH-tamoxifen-induced Cre-mediated recombination and subsequent GFP expression. Six weeks after MI, mice were treated with 2 weeks of 4-OH-tamoxifen; we did not identify any borderzone GFP+ cardiomyocytes, nor did we detect Y chromosome-positive nuclei in more than 10,000 analyzed cells (n = 4 mice per group). Although this analysis does not exclude very rare fusion events, it does exclude cell fusion as an explanation for the observed progenitor activity stimulated by c-kit+ cells (chi-square p < 0.0001).
Cell Therapy with c-kit+ Cells Increases the Number of Endogenous Cells Demonstrating Transcriptional Activity Consistent with Cardiac Progenitors
If exogenously delivered c-kit+ cells stimulate endogenous progenitors, we hypothesized that one manifestation would be an increase in the number of cells displaying progenitor characteristics at the infarct border. We performed a study in which female mice, undergoing experimental myocardial infarction, were randomized to receive vehicle control or c-kit+ cells (6 × 105) isolated from male mice, by intramyocardial injection. Osmotic minipumps delivering bromodeoxyuridine (20 μg/hr) were implanted subcutaneously at the time of myocardial infarction to allow the identification of proliferating cells. Mice were sacrificed after 1 week and the tissues were processed for histologic analysis.
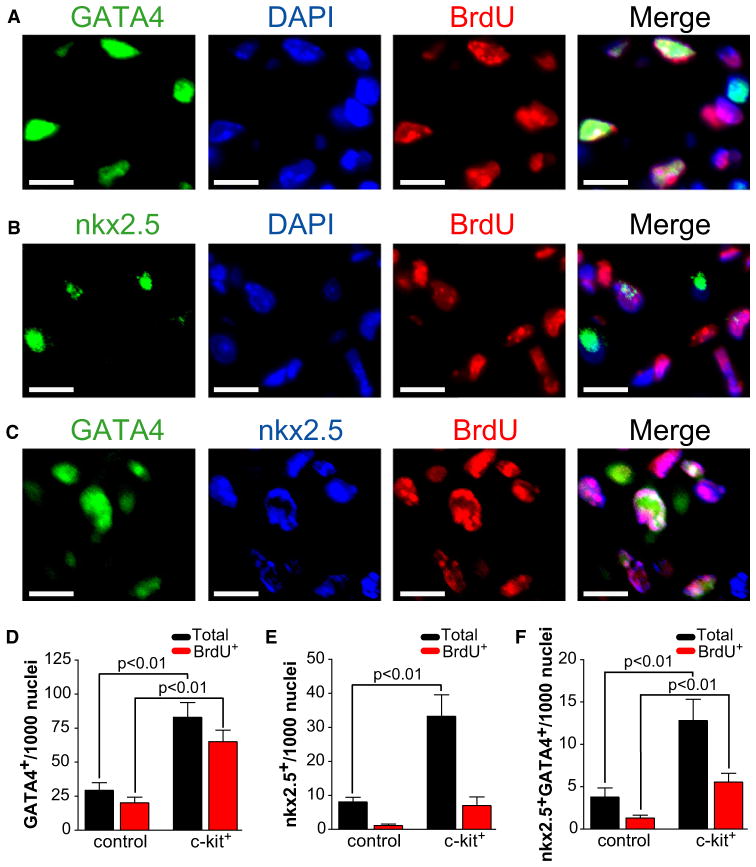
We assessed the expression of nkx2.5, GATA4, TBX5, and Mef2c with immunofluorescent staining of the infarct border zone, because these transcription factors have been associated with cardiogenic commitment (Ieda et al., 2010; Molkentin et al., 1997; Wu et al., 2006; Xin et al., 2006). We excluded troponin T-positive cardiomyocytes from the analysis, because we were specifically interested in identifying cells in the progenitor stage (data not shown). We found a significant increase in the total number of noncardiomyocyte nkx2.5+ and GATA4+ cells in mice having been administered c-kit+ cells, compared to vehicle (Figure 4; nkx2.5, 33.3 ± 6.3 versus 8.1 ± 1.3 per 1000 nuclei; GATA4, 82.9 ± 10.9 versus 29.3 ± 5.6 per 1000 nuclei, n = 3 mice per group, p < 0.01). We also found a significant increase in the number of cells coexpressing nkx2.5 and GATA4 (Figure 4; 12.8 ± 2.8 versus 3.8 ± 1.1 per 1000 nuclei, p < 0.01). In contrast, we found no difference in the frequency of TBX5- or Mef2c-expressing cells (data not shown). In order to exclude the possibility that cells expressing nkx2.5 and GATA4 were derived from exogenously delivered c-kit+ cells, we also performed FISH analysis to identify Y chromosomes. Although we identified donor-derived Y chromosome-positive cells in all analyzed sections, the nkx2.5- or GATA4-positive cells were not Y chromosome positive (data not shown). These findings are most consistent with a c-kit+ cell-mediated increase in cardiac progenitor number 1 week after myocardial infarction, a finding that is congruent with the observed c-kit+ cell stimulation of cardiomyocyte refreshment.
Figure 4. c-kit+ Cell Therapy Leads to Increased Numbers of Cardiac Progenitors after Myocardial Infarction.
(A and B) Immunofluorescent staining with a representative cluster of proliferating GATA4+ (A) and nkx2.5+ (B) cells.
(C) Immnofluorescent staining representing a subset of coexpressing nkx2.5+ and GATA4+ cells.
Scale bars represent 10 μm.
(D) Bar graph depicting an increase in the number of infarct border zone GATA4+ cells in mice treated with c-kit+ cells compared to vehicle control. The BrdU+ fraction is denoted by red bars. Data are expressed as total number of noncardiomyocyte GATA4+ cells per 1000 nuclei (n = 3 animals per group). Only troponin T-negative noncardiomyocytes were counted. Data expressed as mean ± SEM.
(E) Bar graph depicting an increase in the number of infarct border zone nkx2.5+ cells in mice treated with c-kit+ cells compared to vehicle control. The BrdU+ fraction is denoted by red bars. Data are expressed as total number of noncardiomyocyte nkx2.5+ cells per 1000 nuclei (n = 3 animals per group). Only troponin T-negative noncardiomyocytes were counted. Data expressed as mean ± SEM.
(F) Bar graph depicting an increase in the number of infarct border zone coexpressing nkx2.5+/GATA4+ cells in mice treated with c-kit+ cells compared to vehicle control. The BrdU+ fraction is denoted by red bars. Data are expressed as total number of noncardiomyocyte GATA4+/nkx2.5+ cells per 1000 nuclei (n = 3 animals per group). Only troponin T-negative noncardiomyocytes were counted. Data expressed as mean ± SEM.
Because resident cardiac c-kit+ cells have been shown to contribute to endogenous cardiomyocyte regeneration (Beltrami et al., 2003), we reasoned that if exogenously administered bone marrow c-kit+ cells were stimulating resident cardiac c-kit+ cells, there would be a detectable increase in the frequency of proliferating endogenous c-kit+ cells. We performed c-kit staining at the 1 week time point. We excluded exogenously administered Y chromosome+/c-kit+ cells from the analysis and found no increase in the frequency of endogenous border zone c-kit+ cells (data not shown). We also performed a cell therapy experiment with cardiac c-kit+ cells isolated from MerCreMer-ZEG mice delivered by intramyocardial injection to wild-type mice after MI. 4-OH-tamoxifen was administered for 2 weeks prior to sacrificing the mice a total of 4 weeks after MI. We detected no β-galactosidase+ or GFP+ cardiomyocytes (data not shown). Taken together, these data argue against the concept that cell therapy with bone marrow c-kit+ cells exerts proregenerative effects by stimulating resident c-kit+ cells.
Local Delivery of SDF-1 Does Not Stimulate Cardiomyocyte Progenitors
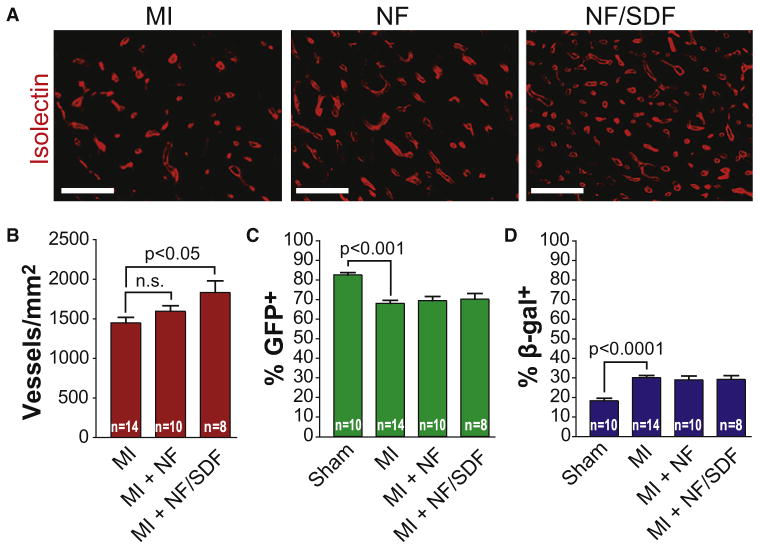
Because paracrine factors have been proposed as a mechanism of benefit of cardiac cell therapy, and because c-kit+ cell-mediated production of a proregenerative paracrine factor could account for the observed activation of endogenous progenitors, we asked whether local administration of the stem cell chemokine, SDF-1α, can stimulate endogenous progenitor activity. To test this hypothesis, we used a protease-resistant form of SDF-1α that we previously demonstrated to facilitate sustained local delivery after intramyocardial delivery (Segers et al., 2007). We performed a randomized, blinded, vehicle-controlled study in MerCreMer-ZEG mice to test the hypothesis that sustained delivery of SDF-1α can activate cardiomyocyte progenitors. Although SDF-1α stimulated neovascularization as predicted (Figures 5A and 5B), the percentage of GFP+ or β-galactosidase+ cardiomyocytes was unchanged (Figures 5C and 5D), suggesting that SDF-1α cannot recapitulate the progenitor-activating effect of c-kit+ cells. It is possible that c-kit+ cells deliver many paracrine signals or that SDF-1α can benefit cardiac function through a mechanism distinct from cell therapy.
Figure 5. c-kit+ Cell-Mediated Stimulation of Endogenous Cardiomyocyte Progenitor Activity Cannot Be Recapitulated by Local Delivery of the Stem Cell Chemoattractant, Stromal-Derived Factor-1α.
(A) Local delivery of SDF-1 stimulates neovascularization at the MI border zone. Representative images of isolectin staining in the MI border zone 8 weeks after MI and the administration of vehicle control (top), nanofibers (middle), or nanofibers and SDF-1 (bottom). Scale bars represent 20 μm.
(B) Graph representing the number of isolectin+ vessels in the MI border zone per mm2. Representative of two independent, randomized, double-blinded experiments. Data expressed as mean ± SEM.
(C) Graph representing the percentage of GFP+ cardiomyocytes in the MI border zone. Local delivery of SDF-1 does not change the percentage of GFP+ CMs in the MI border zone compared to nanofibers alone or vehicle control. Data expressed as mean ± SEM.
(D) Graph representing the percentage of β-gal+ cardiomyocytes in the MI border zone. Data expressed as mean ± SEM.
DISCUSSION
There have been intense efforts to develop cellular therapies to treat human heart failure, but the mechanism of action of cardiac cell therapies has remained unclear (Hansson et al., 2009). Many possible explanations for cell therapy-mediated improvements in cardiac function have been proposed, including direct transdifferentiation of exogenously delivered cells into cardiovascular cells, cell therapy-mediated cardioprotection, and paracrine stimulation of angiogenesis or endogenous progenitors (Dimmeler et al., 2005). The current study was prompted by the recognition that optimization of cell therapies will require an understanding of the mechanisms responsible for cardiac functional improvements. Here, we used a genetic lineage-mapping approach to determine the effect of bone marrow-derived cell therapy on the endogenous regenerative response. We chose to study bone marrow-derived c-kit+ cells and mesenchymal stem cells because these cell types have been attributed cardiogenic properties and similar preparations have been used in human clinical trials (Abdel-Latif et al., 2007).
The central finding of this study is that cell therapy with c-kit+ bone marrow progenitors after experimental myocardial infarction activates endogenous cardiac progenitors. The MerCreMer-ZEG transgenic mouse provides an indirect measure of progenitor activity when the pre-existing GFP-labeled cardiomyocyte pool is diluted by differentiation of unlabeled cardiomyocyte progenitors. An important advantage of this approach is that it detects cumulative progenitor activity and is not dependent upon the identification of the anatomic source or of the transcriptional phenotype of the master cardiac progenitors, both of which remain unresolved issues in the field (Hansson et al., 2009). Moreover, although others have proposed endogenous progenitor activation as a mechanism of benefit from cell therapy (Tang et al., 2010), our study represents the demonstration of this process with the powerful approach of genetic lineage mapping.
We did not detect direct cardiomyocyte transdifferentiation by exogenously administered bone marrow progenitors, as was first demonstrated by Orlic and colleagues (Orlic et al., 2001). Although our analysis cannot rule out rare transdifferentiation, we can conclude that transdifferentiation cannot account for the c-kit+ cell-stimulated cardiomyocyte repletion observed in this study. Our results suggest that transdifferentiation under these circumstances is probably very rare, as others have suggested (Balsam et al., 2004; Murry et al., 2004). We speculate, as have others, that interstudy differences have led to variations in the observed magnitudes of the direct regenerative capacity of bone marrow progenitors (Laflamme and Murry, 2005).
Genetic fate mapping is an important approach to tracking cell fate, but there are important considerations to cardiomyocyte turnover to consider in these experiments. We cannot definitively exclude the possibility that the c-kit+ cell-mediated reduction in the percentage of GFP+ cardiomyocytes is a result of either selective loss of GFP+ cardiomyocytes or selective proliferation by a subset of immature β-galactosidase+ cardiomyocytes, rather than cardiomyocyte repletion by GFP− progenitors. The cumulative evidence, however, suggests that our central finding is not an artifact of either selective cell death or proliferation. We have previously shown that GFP+ cardiomyocytes in the MerCreMer-ZEG mouse do not exhibit a heightened sensitivity to apoptosis after myocardial infarction (Hsieh et al., 2007). In the current study, in the absence of injury, we did not detect a size difference between GFP+ and β-galactosidase+ populations as might be expected if β-galactosidase+ cardiomyocytes were immature. Furthermore, the improvement in cardiac function associated with c-kit+ cell therapy coupled with a c-kit+ cell therapy-mediated increase in the number of nkx2.5- and GATA4-positive cells after MI lends further support to our conclusion that c-kit+ cell therapy stimulates proregenerative activity by endogenous cardiomyocyte progenitors rather than proliferation of pre-existing cardiomyocytes as has been demonstrated in nonmammalian organisms (Bersell et al., 2009; Kikuchi et al., 2010). Ultimately, further experiments in different experimental models and in human tissues via methods with separate limitations and capabilities will be necessary for a definitive picture of cardiomyocyte refreshment in mammalian myocardium.
The observed benefits of c-kit+ cell therapy in our study were contrasted by the absence of an effect of mesenchymal stem cell therapy on endogenous progenitors, although this study cannot exclude the possibility that mesenchymal stem cells have additional beneficial properties. We speculate that the inconsistent effects of cell therapies in both animal and human studies may in part be attributable to specific cell preparations and their capacity to activate endogenous progenitors. Elucidating factor(s) that enable certain cell populations, like bone marrow-derived c-kit+ cells, to stimulate endogenous progenitors may facilitate optimization of cell therapy for the heart, a prerequisite to widespread clinic application.
Understanding the mechanisms underpinning the proregenerative properties of c-kit+ cells could provide noncellular therapies in the future. If, as suggested by our study, exogenously delivered c-kit+ cells exert regenerative effects by releasing a paracrine signal to endogenous progenitors, it may be possible to stimulate endogenous regeneration pharmacologically. In the current study, we tested one such paracrine candidate, SDF-1α. Although a number of soluble factors have been attributed regenerative properties, SDF-1α represents a particularly attractive candidate because of its capacity to both recruit a wide variety of stem cell populations and improve cardiac function after ischemic injury (Ceradini et al., 2004; Saxena et al., 2008; Segers et al., 2007; Zaruba et al., 2009). As previously demonstrated, SDF-1α stimulated neovascularization at the MI border but did not appear to stimulate endogenous cardiomyocyte progenitors as previously hypothesized (Segers et al., 2007).
In summary, intramyocardial delivery of c-kit+ bone marrow cells after MI induces endogenous progenitor-derived cardiomyocytes and improves ventricular function, an effect not observed with MSCs. With multiple methods, these effects of c-kit+ cells could not be explained by their direct transdifferentiation into cardiomyocytes. The c-kit+ cell-mediated activation of endogenous progenitors may depend on paracrine communication between donor and recipient cells. Understanding why specific cell types have the potential to stimulate endogenous cardiac progenitors may enable the future development of pharmacoregenerative therapies as well as improving current cell therapy strategies.
EXPERIMENTAL PROCEDURES
Generation and Maintenance of MerCreMer-ZEG Colony
Double transgenic MerCreMer-ZEG mice were generated by cross-breeding transgenic B6129-Tg(Myh6-cre/Esr1)1Jmk/J (MerCreMer) mice with the B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J reporter strain (ZEG, Jackson Laboratories) as previously described. Genotyping was performed by PCR on tail DNA with the following primers: MerCreMer forward: 5′-GTCTGACTAGGTGTCCTTCT-3′; MerCreMer backward: 5′-CGTCCTCCTGCTGGTATAG-3′; ZEG forward: 5′-AAGTTCATCTGCACCACCG-3′; ZEG backward: 5′-TCCTTGAAGAAGATGGTGCG-3′; ZEG control forward: 5′-CTAGGCCACAGAATTGAAAGATCT-3′; and ZEG control backward: 5′-GTAGGTGGAAATTCTAGCATCATCC-3′. Mice used for cell transplantation experiments were derived from a transgenic colony subjected to inbreeding for >4 years and >30 generations. Maintenance of the colony and all experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals and approved by the Harvard Medical School Standing Committee on Animals.
4-OH-Tamoxifen Pulse
A previously validated protocol was used to induce Cre recombinase-mediated GFP expression and permanent deletion of β-galactosidase gene expression in the cardiomyocytes of MerCreMer-ZEG mice. 4-OH-tamoxifen (provided by a generous gift from Laboratoires Besins) was dissolved in peanut oil (Sigma) at a concentration of 5 mg/mL and injected intraperitoneally at a dose of 20 mg/kg/d for 2 weeks. The same dosing schedule was administered to wild-type mice, 6 weeks after treatment with bone marrow stem cells derived from MerCreMer-ZEG bitransgenic mice.
Experimental Myocardial Infarction and Cellular Delivery
Mice were subjected to experimental myocardial infarction as described. Surgeries were performed by a single operator with more than 20 years of experience in the performance of coronary ligation in rodents. In brief, the left coronary artery was permanently ligated approximately 2 mm below the left atrial appendage. For sham operations, the thoracic cavity was opened and the heart exposed, but no intramyocardial sutures were placed. All intramyocardial injections (cells, vehicle control, or protein therapy) were performed immediately after coronary ligation, prior to closure of the thoracic cavity. 5 μl injections with a 31 g syringe (Hamilton) were administered to the medial and lateral infarct border regions (total of two injections per mouse). BrdU (Sigma) was administered at a rate of 20 μg/hr experiments via osmotic minipump (Alzet), implanted subcutaneously at the time of experimental myocardial infarction.
Bone Marrow Stem Cell Isolation
c-kit+ bone marrow cells were isolated via immunomagnetic sorting (Miltenyi Biotec). Bone marrow was harvested from the femurs and tibias of adult male mice (aged 8–12 weeks). Marrow was suspended in PBS containing 0.5% BSA and 2 mM EDTA. The cell suspension was first depleted of lineage+ cells (expressing the following cell surface antigens: CD5, CD45R [B220], CD11b, Gr-1 [Ly-6G/C], 7–4, and Ter-119) prior to positive selection for c-kit expression. This system yielded a cellular preparation of >97% c-kit+ cells assayed by flow cytometry (Figure S1). Bone marrow mesenchymal stem cells were isolated from adult mice (aged 8–12 weeks) and grown in hypoxic conditions (1% O2) with Mesencult medium/protocol (Stem Cell Technologies). Mesenchymal stem cells used for cell therapy were taken from passages 7–9. For all experiments in which MSCs were delivered therapeutically, the mesenchymal stem cell surface marker profile was verified with flow cytometry one passage prior to cell administration. Both c-kit+ cells and MSCs were resuspended in unsupplemented DMEM (vehicle) and placed on ice prior to intramyocardial delivery. Cells were administered within 3 hr of isolation (c-kit+ cells) or harvest from the culture flask (MSCs). A total of 6 × 105 cells were delivered per mouse in two divided injections.
Immunohistochemistry and Immunofluorescent Microscopy
Mouse hearts were fixed with 4% paraformaldehyde, paraffin embedded, sectioned, and stained with standard immunohistochemistry and fluorescent microscopy methods as previously described. An antigen retrieval step was used in all experiments, by heating samples in a citrate-based buffer (Dako) to 95°C for 20 min. Primary antibodies were used as follows: rabbit GFP-specific antibody 1:400 (Abcam); rabbit β-galactosidase-specific antibody 1:200 (Invitrogen); mouse troponin T-specific antibody 1:200 (Abcam); rat BrdU-specific antibody 1:200 (Abcam); rabbit nkx2.5-specific antibody 1:50 (Abcam); goat nkx2.5-specific antibody 1:50 (Santa Cruz); rabbit GATA4-specific antibody 1:50 (Santa Cruz); rabbit Mef2c-specific antibody 1:200 (Abcam); rabbit TBX5-specific antibody 1:400 (Abcam); rabbit c-kit-specific antibody 1:50 (Santa Cruz); and Isolectin 1:200 (Invitrogen). Alexa-Fluor (Invitrogen) secondaries were used at 1:200 dilution for all immunofluorescence. A biotinylated anti-rabbit secondary followed by ABC reagent and DAB (Vector Laboratories) were used for immunohistochemistry.
In Situ Hybridization
To probe for Y chromosomes, sections were incubated in 1 M sodium thiocyanate for 10 min at 80°C, washed in PBS, and digested in 0.4% pepsin in 0.1 M HCl at 37°C. Sections were quenched in 0.2% glycine in 2× PBS, rinsed in 1× PBS, postfixed in 4% paraformaldehyde/PBS, dehydrated through graded ethanol, and air-dried. Biotinylated-labeled Y chromosome paint (Star-FISH, Cambio) was suspended in hybridization mix, applied to sections, and sealed under glass with rubber cement. Samples were heated to 60°C for 10 min. After an overnight incubation at 37°C, slides were washed three times with 50% formamide/2× standard saline citrate, three washes with 2× standard saline citrate, and two washes with 4× standard saline citrate/0.1% TWEEN, all at 37°C. Samples were blocked with PBS/10% horse serum and incubated for 2 hr in streptavidin-conjugated Alexa Fluor 488 (Invitrogen). Any immunofluorescent staining was performed prior to digestion. Sections were photographed at the completion of immunofluorescent staining and again after in situ hybridization. Images were aligned with Adobe Photoshop.
Flow Cytometry
All flow cytometry was performed on fresh, unfixed cells kept on ice during all incubation steps. Cells were blocked with PBS/0.5% BSA for 10 min prior to resuspension at a concentration of 1 × 106 cells per 250 μL. Cells were incubated for 30 min in directly conjugated primary antibodies specific for PE-c-kit (eBioscience), PE-sca-1 (eBioscience), PE-CD105/endoglin (eBioscience), PE-CD45 (Miltenyi), FITC-CD31 (eBioscience), and PE-CD166 (eBioscience), washed twice in PBS, prior to flow analysis. Conjugated isotype control antibodies were used in all experiments.
Cardiac Function Assessment
Hemodynamic parameters were acquired with the close-chest invasive method with a Millar Pressure Volume Catheter (Millar Instruments) through the right carotid artery. Hemodynamic tracings were analyzed by PowerLab Chart5 (ADInstruments). Serial echocardiography was performed by a single operator. All data were acquired and analyzed by an operator who was blinded to treatment assignment.
Study Design
Treatment assignments for cell therapy and protein studies were made by randomization. Both the operator who performed the surgeries and intra-myocardial injections and the operative assistant charged with drawing up therapies for intramyocardial injection were blinded to the identity of the administered agent.
Image Analysis
After sections were stained for GFP or β-galactosidase, images were captured by an observer blinded to all experimental information. For each animal, images were captured from a section taken from two independent levels (mid-ventricle and mid-apical) by a photographer blinded to experimental information. Four images were taken from the remote area, defined as tissue from the posterior wall of the left ventricle. Four images were also taken from the MI border zone defined as an image not farther away than one high-power field from scar tissue but not including the scar. Positive and negative cardiomyocytes were counted in each image by an independent observer, also blinded to experimental information. Only cells with clear cardiomyocyte morphology were included. The denominator was taken as the sum of negatively and positively stained cells. Isolectin-positive vessels were also photographed and analyzed by an observer blinded to experimental information. The number of isolectin-positive vessels per mm2 was quantitated in the MI border zone by selecting areas in which the majority of vessels were displayed in cross-section. Vessels were counted with automated software (ImageJ). Infarct size was determined by the midline method with trichrome-stained (Sigma) sections taken from multiple ventricular levels (Takagawa et al., 2007). Border zone cardiomyocyte cross-sectional area was determined by averaging a minimum of 50 cardiomyocytes per animal by an observer unaware of experimental group. Suitable cross-sections were defined as having circular capillary profiles and circular myocyte sections.
Statistical Analyses
Data comparing the rates of GFP+ or β-galactosidase+ cardiomyocytes (Figures 1, 2, and 5; Figure S2), the number of nkx2.5+ or GATA4+ cells (Figure 4), vessel density (Figure 5), or functional data (Figure 2; Figures S4 and S5) were subjected to one-way ANOVA and posthoc Bonferonni correction. Statistical significance was assigned for p < 0.05; results are shown with standard estimate of the mean.
Supplementary Material
Acknowledgments
R.T.L. is a founder of Provasculon, Inc. This work was supported by a grant from the NIH (R01 AG032977).
Footnotes
Supplemental Information includes five figures and can be found with this article online at doi:10.1016/j.stem.2011.02.002.
References
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson EM, Lindsay ME, Chien KR. Regeneration next: Toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AA, van der Laarse A, Ypey DL, Atsma DE. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: Comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.