Abstract
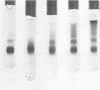
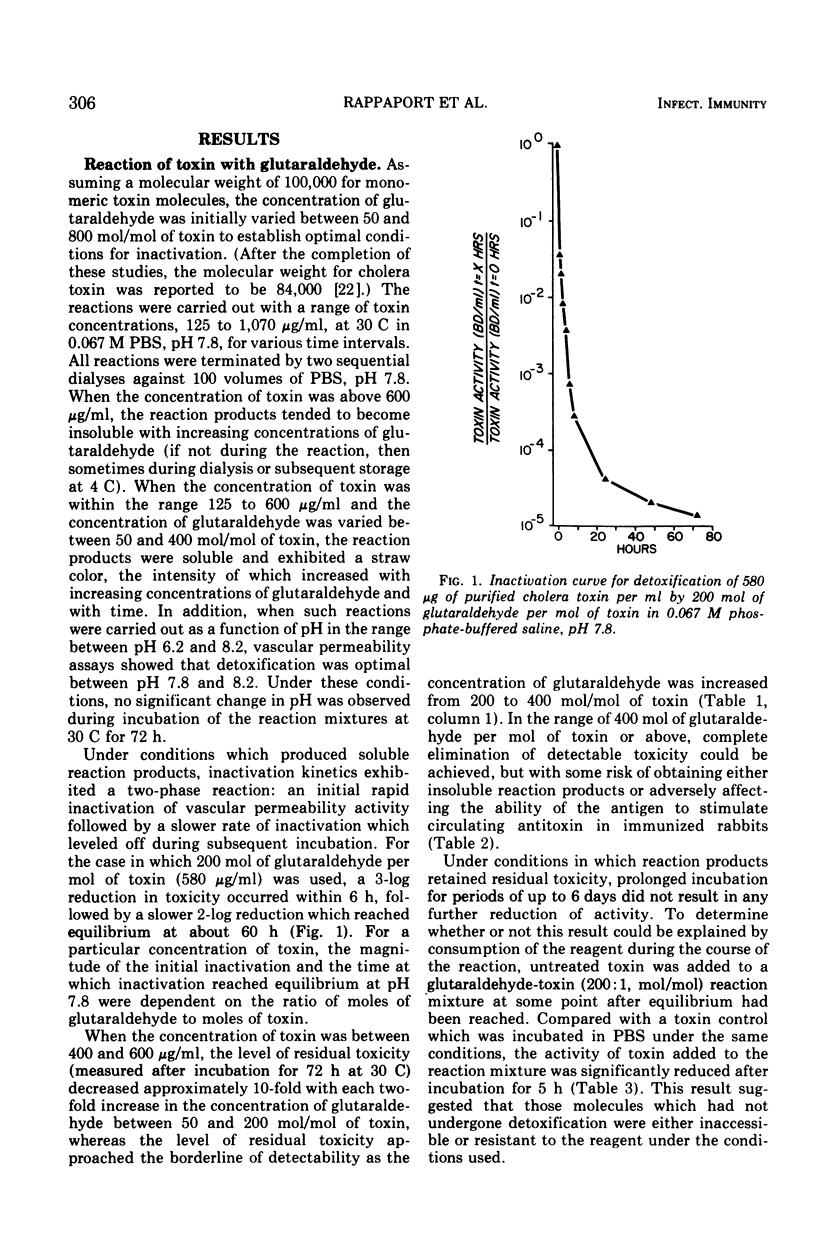
Evidence is presented which confirms that cholera toxoids obtained by reaction of purified toxin with Formalin possess the ability to partially reactivate both in vivo and in vitro. At the same time, conditions are presented for the preparation of stable, antigenic cholera toxoids by reaction of purified toxin with glutaraldehyde. Treatment of purified cholera toxin with approximately 200 mol of glutaraldehyde per mol of toxin at pH 7.8 reproducibly resulted in the preparation of toxoids which: (i) possessed less than 20 bluing doses per 100 μg; (ii) did not reactivate in vivo or in vitro; (iii) precipitated with, and neutralized antitoxin; (iv) elevated prolonged serum antitoxin in immunized rabbits; (v) protected immunized guinea pigs against toxin skin challenge; and (vi) lent themselves to enhanced antigenicity by means of an in situ adjuvant system which may be suitable for man. Acrylamide gel electrophoresis and molecular sieve chromatography of a series of glutaraldehyde-derived toxoids suggested that the reaction products consisted of monomeric and polymeric species and that the proportion of higher-molecular-weight species was determined by the relative concentrations of toxin and glutaraldehyde. The results suggested a relationship between complete and irreversible elimination of toxicity and the formation of higher-molecular-weight toxoids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akama K., Ito A., Yamamoto A., Sadahiro S. Reversion of toxicity of tetanus toxoid. Jpn J Med Sci Biol. 1971 Jun;24(3):181–183. doi: 10.7883/yoken1952.24.181. [DOI] [PubMed] [Google Scholar]
- Akama K., Kameyama S., Otani S., Sadahiro S., Murata R. Reversion of toxicity of diphtheria toxoid. Jpn J Med Sci Biol. 1971 Jun;24(3):183–187. doi: 10.7883/yoken1952.24.183. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Benyajati C. Experimental cholera in humans. Br Med J. 1966 Jan 15;1(5480):140–142. doi: 10.1136/bmj.1.5480.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W., Ishizuka M. Cyclic AMP and immune responses. II. Phosphodiesterase inhibitors as potentiators of polynucleotide effects on antibody formation. J Immunol. 1971 Oct;107(4):1036–1042. [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Eichner E. R., Hornick R. B. Cutaneous responses to cholera skin toxin in man. I. Responses in unimmunized American males. J Infect Dis. 1972 Mar;125(3):203–215. doi: 10.1093/infdis/125.3.203. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Jehl J. J., Goth A. Pathogenesis of experimental cholera: choleragen-induced rat foot edema; a method of screening anticholera drugs. Proc Soc Exp Biol Med. 1969 Dec;132(3):835–840. doi: 10.3181/00379727-132-34318. [DOI] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Preparation of enzymically active, water-insoluble derivatives of trypsin. Arch Biochem Biophys. 1967 Mar;119(1):264–268. doi: 10.1016/0003-9861(67)90453-5. [DOI] [PubMed] [Google Scholar]
- Habeeb A. J., Hiramoto R. Reaction of proteins with glutaraldehyde. Arch Biochem Biophys. 1968 Jul;126(1):16–26. doi: 10.1016/0003-9861(68)90554-7. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Andersson A., Wallerstrom G., Ouchterlony O. Experimental studies on cholera immunization. II. Evidence for protective antitoxic immunity mediated by serum antibodies as well as local antibodies. Infect Immun. 1972 May;5(5):662–667. doi: 10.1128/iai.5.5.662-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E. F., Olson A. C. Properties and enzymatic activities of papain insolubilized with glutaraldehyde. Arch Biochem Biophys. 1969 Jan;129(1):221–227. doi: 10.1016/0003-9861(69)90169-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Northrup R. S., Chisari F. V. Response of monkeys to immunization with cholera toxoid, toxin, and vaccine: reversion of cholera toxoid. J Infect Dis. 1972 May;125(5):471–479. doi: 10.1093/infdis/125.5.471. [DOI] [PubMed] [Google Scholar]
- Northrup R. S., Fauci A. S. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis. 1972 Jun;125(6):672–673. doi: 10.1093/infdis/125.6.672. [DOI] [PubMed] [Google Scholar]
- Ogata K., Ottesen M., Svendsen I. Preparation of water-insoluble, enzymatically active derivatives of subtilisin type Novo by cross-linking with glutaraldehyde. Biochim Biophys Acta. 1968 Jun 4;159(2):403–405. doi: 10.1016/0005-2744(68)90090-9. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Graybill J. R., Kaplan M. M., Bouwman D. L. Systemic effects of parenteral cholera enterotoxin in dogs. J Lab Clin Med. 1972 Jan;79(1):145–156. [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- QUIOCHO F. A., RICHARDS F. M. INTERMOLECULAR CROSS LINKING OF A PROTEIN IN THE CRYSTALLINE STATE: CARBOXYPEPTIDASE-A. Proc Natl Acad Sci U S A. 1964 Sep;52:833–839. doi: 10.1073/pnas.52.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Rubin B. A., Tint H. Development of a purified cholera toxoid. I. Purification of toxin. Infect Immun. 1974 Feb;9(2):294–303. doi: 10.1128/iai.9.2.294-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyveld E. H. Studies on the detoxification of purified diphtheria toxin. Prog Immunobiol Stand. 1967;3:258–263. [PubMed] [Google Scholar]
- Richards F. M., Knowles J. R. Glutaraldehyde as a protein cross-linkage reagent. J Mol Biol. 1968 Oct 14;37(1):231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. II. Production by cell-free culture filtrates of Vibrio cholerae. J Infect Dis. 1969 Feb;119(2):150–157. doi: 10.1093/infdis/119.2.150. [DOI] [PubMed] [Google Scholar]
- Stainer D. W. Preparation and properties of diphtheria toxoids in submerged culture. II. Purification, detoxification, antigenicity, and stability. Can J Microbiol. 1968 Apr;14(4):327–330. doi: 10.1139/m68-053. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]
- Zieve P. D., Pierce N. F., Greenough W. B., 3rd Stimulation of glycogenolysis by purified cholera exotoxin in disrupted cells. Johns Hopkins Med J. 1971 Dec;129(6):299–303. [PubMed] [Google Scholar]