Abstract
Glucocorticoids induce COX-2 expression in rat cardiomyocytes. While investigating whether phosphatidylinositol 3 kinase (PI3K) plays a role in corticosterone (CT) induced COX-2, we found that LY294002 (LY29) but not wortmannin (WM) attenuates CT from inducing COX-2 gene expression. Expression of a dominant-negative mutant of p85 subunit of PI3K failed to inhibit CT from inducing COX-2 expression. CT did not activate PI3K/AKT signaling pathway whereas LY29 and WM decreased the activity of PI3K. LY303511 (LY30), a structural analogue and a negative control for PI3K inhibitory activity of LY29, also suppressed COX-2 induction. These data suggest PI3K independent mechanisms in regulating CT induced COX-2 expression. LY29 and LY30 do not inhibit glucocorticoid receptor transactivity. Both compounds have been reported to inhibit Casein Kinase 2 activity and modulate potassium and calcium levels independent of PI3K, while LY29 has been reported to inhibit mammalian Target of Rapamycin (mTOR), DNA-dependent Protein Kinase (DNA-PK). Inhibitor of Casein Kinase 2 (CK2), mTOR or DNA-PK failed to prevent CT from inducing COX-2 expression. Tetraethylammonium (TEA), a potassium channel blocker, and nimodipine, a calcium channel blocker, both attenuated CT from inducing COX-2 gene expression. CT was found to increase intracellular Ca2+ concentration, which can be inhibited by LY29, TEA or nimodipine. These data suggest a possible role of calcium instead of PI3K in CT induced COX-2 expression in cardiomyocytes.
Introduction
LY294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, abbreviated as LY29] is a flavonoid-based synthetic compound that inhibits phosphatidylinositol 3-kinase (PI3K). LY29 competes for the occupancy of the ATP pocket of the catalytic subunit of PI3K (Vlahos et al., 1994). The average inhibitory concentration (IC50) is 1 µM for most tissues. Although LY29 is widely used as an experimental tool to inhibit PI3K, recent studies have suggested that this compound exhibits inhibitory activities on targets other than PI3K, such as mammalian Target of Rapamycin (mTOR), DNA-dependent Protein Kinase (DNA-PK) and Casein Kinase-2 (CK2) (Vanhaesebroeck et al., 2001). LY303511 (2-Piperazinyl-8-phenyl-4H-1-benzopyran-4-one, abbreviated as LY30), a compound with a single substitution of the oxygen with an amine in the morpholine ring of LY29, does not inhibit PI3K and therefore serves as a negative control for PI3K inhibitory activity of LY29 (Vlahos et al., 1994).
Unlike LY29, wortmannin (WM) is a cell-permeable fungal metabolite and a potent irreversible PI3K inhibitor that has a distinct structure than LY29. The average IC50 for PI3K inhibition is in 1–10 nM range. WM binds deeper than ATP in the ATP pocket of PI3K catalytic subunit, resulting in conformation change and covalent binding with a lysine residue (K833) (Vanhaesebroeck et al., 2001). Like LY29, WM also inhibits mTOR and DNA-PK. The majority use of WM in the literature is its PI3K inhibitory activity (Vanhaesebroeck et al., 2001).
PI3K represents a key signaling molecule that transduces extracellular signals to gene expression in a variety of cell types. In human keratinocytes, PI3K regulates UV-B light induced cyclooxygenase-2 (COX-2) expression through activation of AKT and consequential inhibition of glycogen synthase kinase 3β (GSK3β) (Tang et al, 2001; Bachelor et al., 2005; Sheu et al., 2005; Wu et al., 2005). COX-2 gene responds to a diverse range of stimuli from growth factors or cytokines to physical or chemical stress by increasing expression (Smith, 2000; Hinz and Brune, 2002; Turini and DuBois, 2002; Warner and Mitchell, 2004). Several signaling transduction pathways have been shown to regulate COX-2 gene expression, including the Mitogen-Activated Protein Kinases (Smith, 2000), Protein Kinase A (Choudhary et al., 2004; Pino et al., 2005) or Protein Kinase C (Xuan et al., 2005). Although PI3K has been reported to mediate UV-B induced COX-2 expression in keratinocytes, it is not known whether PI3K is essential for COX-2 gene induction by a variety of stimuli or in different cell types.
Recent studies from our laboratory have found that glucocorticoids induce COX-2 gene expression by transcriptional activation in cardiomyocytes (Chen et al., 2005)(Sun and Chen, manuscript submitted). Physical or psychological stresses increase the level of glucocorticoids in the circulating system due to stimulation of glucocorticoid synthesis from the adrenal glands. Synthetic glucocorticoids are often prescribed as immune suppressants. In cardiomyocytes, glucocorticoids have been found to protect cells from apoptosis (Chen et al., 2005). Since prostaglandins have been shown to elicit a cardiac protective effect against oxidant, doxorubicin, or ischemia-reperfusion induced injury (Adderley and Fitzgerald, 1999; Camitta et al., 2001; Bolli et al., 2002; Calabresi et al., 2003; Xiao et al., 2004; Ma et al., 2005; Shinmura et al., 2005), induction of COX-2 may serve as an important mechanism of glucocorticoid induced cytoprotection. The goal of this study is to address whether PI3K plays a role in CT induced COX-2 expression. However we found that LY29 abolishes COX-2 induction via a mechanism independent of PI3K.
Materials and Methods
Chemicals
Most chemicals including steroids, nimodipine and tetraethylammonium (TEA) were purchased from Sigma. LY29, LY30, WM, and 4,5,6,7-Tetrabromo-2-azabenzimidazole (TBB) were obtained from Calbiochem.
Cell Culture and Drug Treatment
Cardiomyocytes were prepared from 1-to 2-day old neonatal Sprague-Dawley rats (Harlan, Indianapolis, IN) as described previously (Coronella-Wood et al., 2004; Purdom and Chen, 2005). Cells were seeded at a density of 0.5 × 106 per well in 6-well plates with low glucose DMEM containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA). After 24-hrs serum starvation, cells were treated with pharmacological inhibitors 30 – 60 minutes prior to addition of steroids.
Western Blot Analysis
Cells were dissolved in a SDS lysis buffer [120 mM Tris-HCl, pH 6.8, 2.4% (w/v) SDS, and 50% (v/v) glycerol]. An equal volume of sample buffer [60 mM Tris-HCl, pH 6.8, 2% (w/v) SDS, 0.05% (v/v) beta-mercaptoethanol, and 10% (v/v) glycerol] was added to the cell lysates for 10-minutes boiling. Protein concentration was measured by the Warburg-Christian method (Layne, 1957; Chen et al., 1998). Western blots were performed using antibodies against COX-2 (1:1600 dilution, #160106, Cayman Chemical, Ann Arbor, MI), total GSK (1:6000, #44–610, Invitrogen, Carlsbad, CA), Ser21/Ser9 phospho GSK-3α/β (1:1000), total AKT (1:1500), Thr308 phospho AKT (1:1000), or Ser473 phospho AKT (1:1000, #9331, #4058, #9275, or #9272, respectively, Cell Signaling Technology, Inc. Danvers, MA). Equal protein loading between samples was verified by Western blot to detect vinculin (1:8000, V9131, Sigma-Aldrich, St. Louis, MO).
RNA Isolation and Semiquantitative RT-PCR
Total RNA was isolated by dissolving cells in Trizol following manufacturer’s instruction (Invitrogen, Carlsbad, CA). After converting RNA to cDNA via reverse transcription (RT) using hexanucleotide random primers, COX-2 cDNA was amplified by PCR using forward primer of 5'-TACAAGCAGTGGCAAAGGCC and reverse primer of 5'-CAGTATTGAGGAGAACAGATGGG. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified in parallel as an internal loading control using primers of forward 5'-AGACAGCCGCATCTTCTTGT and reverse 5'-CCACAGTCTTCTGAGTGGCA.
Promoter Luciferase Construct, Transient Transfection and Luciferase Assay
To generate rat COX-2 promoter-luciferase construct, a fragment of the rat COX-2 promoter DNA sequence (−449 to +24) was amplified by PCR using rat liver genomic DNA as a template. The forward primer (5'-GGG GTA CCA GAG CAG CAA GCA CGT CAG ACT) contains a Kpn I restriction site, while the reverse primer (5'-CCT AGC TAG CAG CTC TCC GCT CAG TTT GAC AA) has a Nhe I restriction site, allowing restriction digestion and subcloning of the PCR product into a pGL3 Basic vector at 5’ upstream of the firefly luciferase gene (Promega, Madison, WI). The reporter construct (0.2 µg) was transfected into rat cardiomyocytes using Fugene 6 liposomes (Roche). For cotransfection, 0.2 µg DNA of dominant-negative p85, wild type p85 or corresponding empty vector pSRα (generous gifts from Dr. Wataru Ogawa) was included in the transfection. A mouse mammary tumor virus promoter luciferase (MMTV-luc) in pGL3 basic vector) was obtained from Dr. Roger Miesfeld. MMTV-luc (0.2 µg) was cotransfected with a TK-Renilla luciferase vector (0.05 µg, Promega) using Fugene 6 liposomes (Roche). After 5-hours incubation with plasmids and tranfecting reagents, cells were replenished with fresh DMEM containing 10% FBS for overnight recovery. After 24 hours serum starvation and experimental treatments, cells were lysed for luciferase assays (Promega, Madison, WI) using a luminometer (Turner Designs). The activity of luciferase was normalized to protein content of cells or Renilla luciferase.
PI3K Activity Assay
Cardiomyocytes were harvested for PI3K activity assay as described (Tu et al., 2002). Samples containing 500 µg protein were incubated with 5 µl anti-p85 antibody (#06–195, Upstate Biotechnology, Lake Placid, NY) for 2 h on ice and for additional 1 h following addition of 40 µl protein A-Sepharose. Kinase reaction was carried at 30°C for 20 min in a 50-µl reaction mixture containing p85 immunoprecipitates, 0.2 mg/ml phosphatidylinositol (Avantis Polar-Lipid, Alabaster, AL) and 10 µCi [γ-32P]ATP in a reaction buffer (20 mM Tris-HCL, pH 7.4, 50 mM NaCl, 10 mM MgCl2, 0.5 mM EGTA, 120 µM adenosine and 50 µM ATP). The resulting 32P-labeled phospholipids were extracted using 200 µl of a chloroform and methanol mixture (1:1 volume ratio) for thin layer chromatography usng Silica Gel 60 plates and a solvent containing chloroform, methanol, 25% ammonia hydroxide and water (43:38:5:7). The product of PI3K reaction, i.e. [γ-32P] labeled phosphatidylinositol phosphate, was detected by autoradiography.
Intracellular Ca2+ Measurement
Intracellular Ca2+ concentration ([Ca2+]i) was measured using a fluorescence microplate reader (BioTek ELX800). Cardiomyocytes were seeded in 12-wells plates (5×104 cells/cm2 or 2.5×105 cells/well). After washing with HEPES buffer (130 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM HEPES, 11 mM glucose, and 0.2 mM CaCl2, pH 7.4), the cells were incubated with 4 µM fluo-4 AM (Molecular Probes) for 45 min at 37 °C in HEPES buffer. LY29 (10 µM), TEA (30 mM) or nimodipine (20 nM) were added to cells during dye incubation. After removal of unincorporated dye by 3 washes, the cells were treated with 1 µM CT in HEPES buffer in the presence or absence of inhibitors. The fluorescence generated from intracellular Ca2+ bound fluo-4 was recorded at excitation wavelength of 485 nm and emission wavelength of 530 nm. The concentration of [Ca2+]i was calculated as Kd[(F − Fmin)/(Fmax − F)], where Kd is the dissociation constant of 345 nM for fluo-4, F is the fluorescence of samples, Fmax is the fluorescence of saturated Ca2+, which was obtained by addition of 0.1% triton X-100, and Fmin is the fluorescence of the samples in the presence of 10 mM EGTA.
Results
LY294002 and LY303511 but not Wortmannin or Dominant Negative p85 Inhibit CT-Induced COX-2 Expression
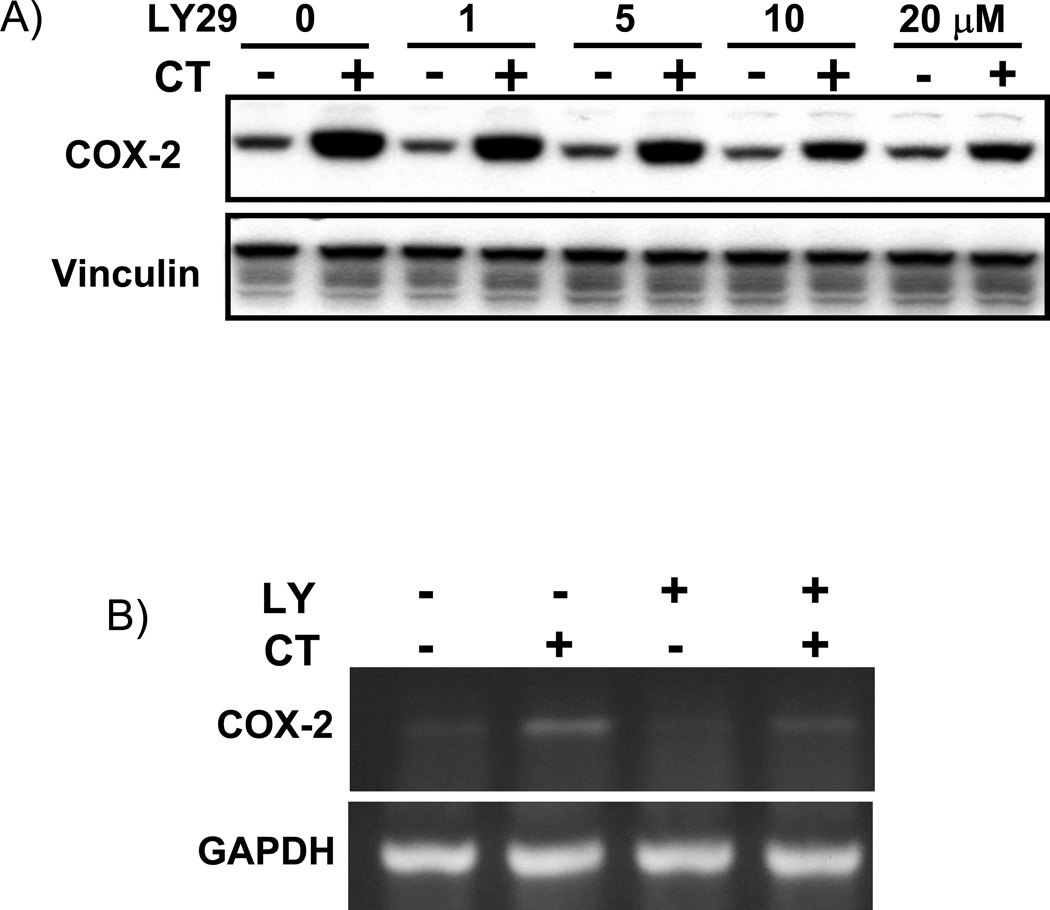
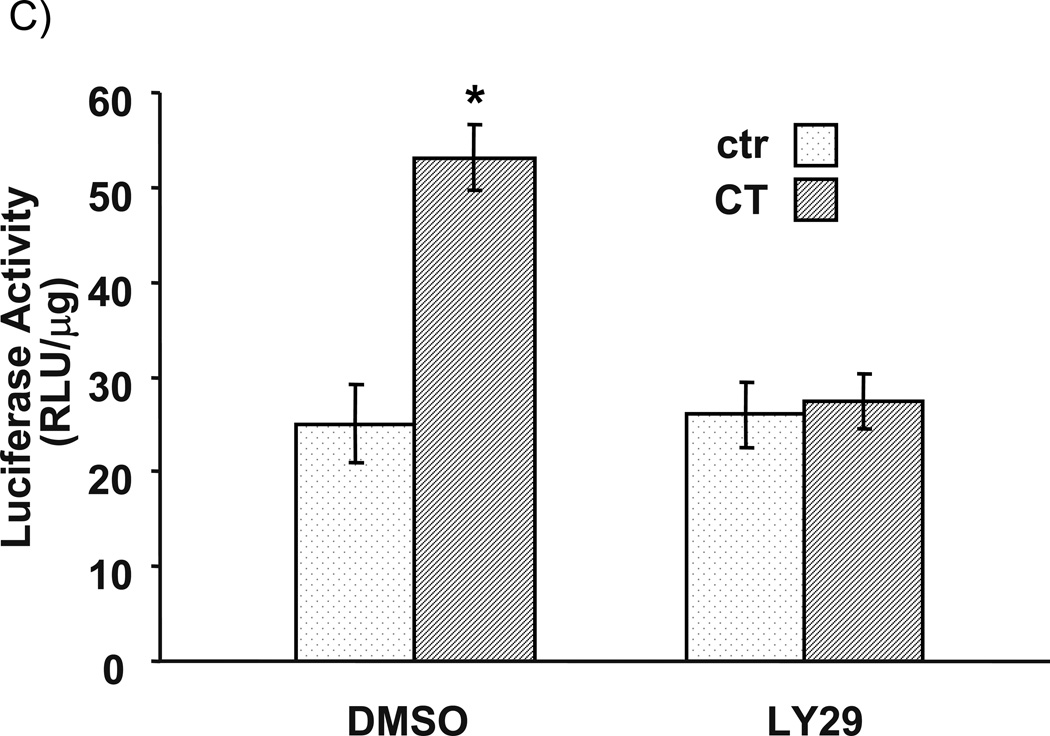
LY29 was first used as an inhibitor of PI3K to examine the effect on CT-induced COX-2 expression in cardiomyocytes. This inhibitor has been shown to inhibit PI3K pathway in our experimental system (Purdom-Dickinson et al., 2007). As shown in Fig. 1A, CT treatment elevates COX-2 protein level, while LY 29 inhibited the effect of CT in a dose-dependent manner. Although 10 µM has been the most commonly used dose of LY29 in the literature, about 40% reduction in the intensity of CT induced COX-2 protein band has been observed (Fig. 1A). COX-2 induction is regulated at the transcriptional level (Sun and Chen, manuscript submitted). We measured the level of COX-2 mRNA and COX-2 promoter to address whether LY29 inhibits CT from inducing transcriptional activation of COX-2 gene. These assays show an inhibition of LY29 on CT induced COX-2 mRNA elevation and promoter activation (Fig. 1B&C).
Figure 1. LY294002 dose dependent inhibition of CT-induced COX-2 elevation.
Cardiomyocytes were pretreated with LY29 at the dose indicated (A) or 10 µM (B) 60 mins before treatment of 1 µM CT for 4 hours (A) or 2 hours (B). COX-2 was measured by Western blot (A) or RT-PCR (B) with vinculin or GAPDH as a loading control. Alternatively, cardiomyocytes were transfected with rat COX-2 promoter luciferase vector (C). Transfected cells with or without 60-mins pretreatment of 10 µM LY29 were treated with 1 µM CT for 12 hours before luciferase assay (C). The activity of luciferase is expressed as the Relative Light Unit (RLU) per µg protein (C). The data represent one of three independent experiments. An asterisk (*) indicates significant difference (p<0.05) as judged by the Student's t test when the means of CT treated groups were compared to that of the control in three experiments (C).
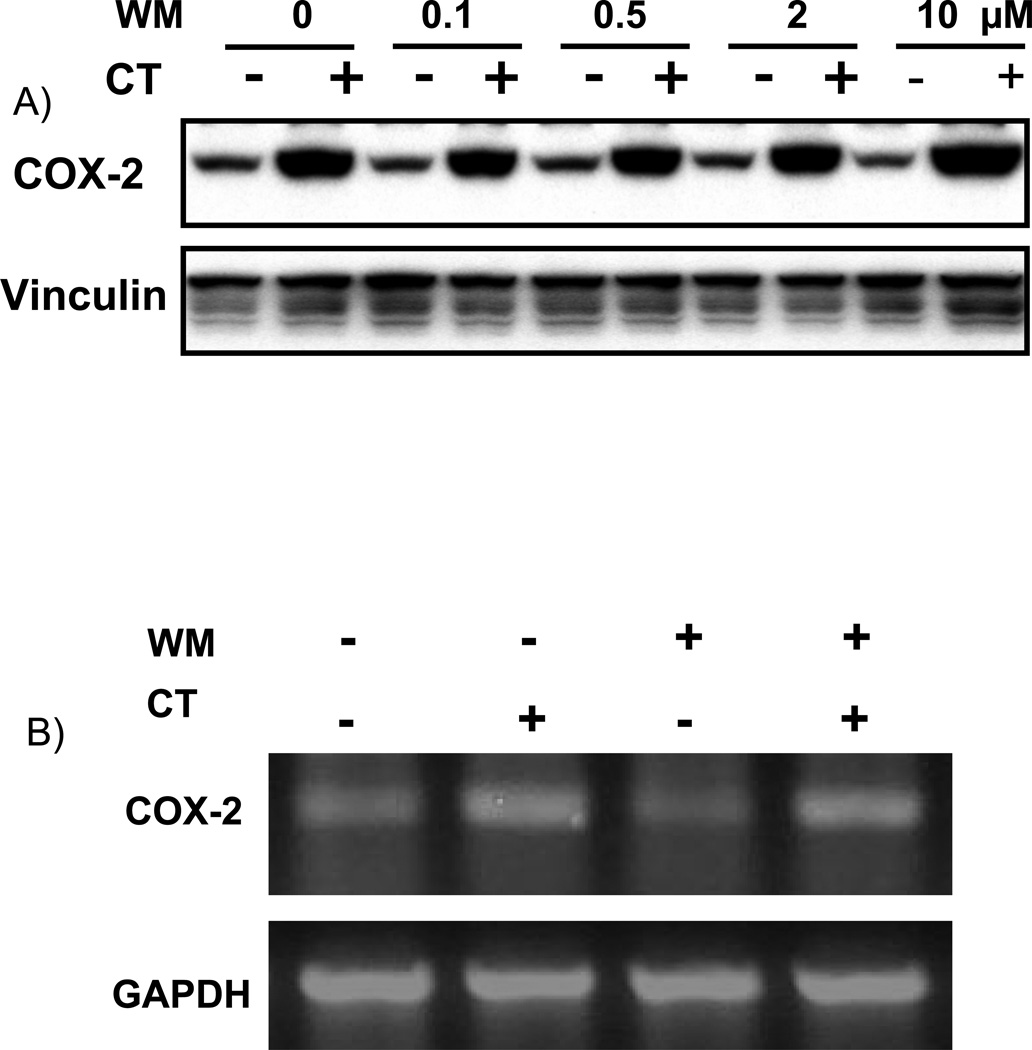
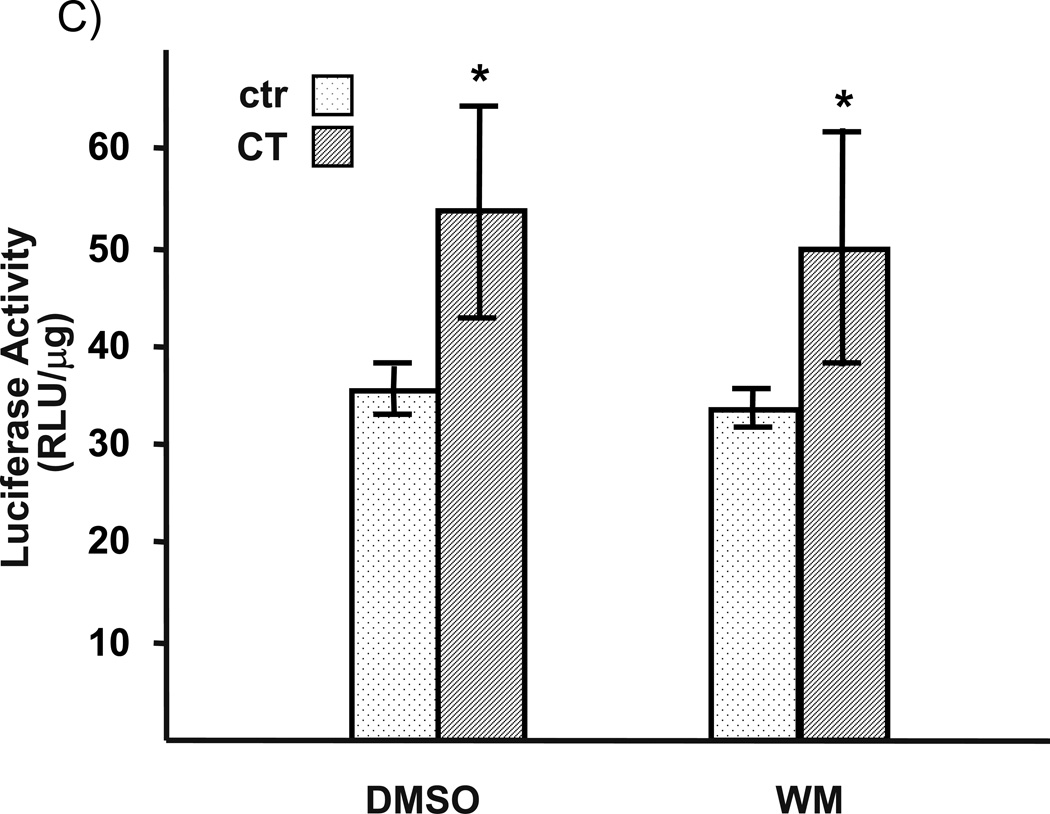
To address whether the observed effect of LY29 is indeed related to PI3K inhibition, we test the effect of WM. WM at the dose used is capable of inhibiting PI3K in cardiomyocytes as shown in our previous work (Tu et al., 2002). Despite of numerous experiments with several different batches of WM, we found that WM could not prevent CT from inducing COX-2 expression, either at protein or mRNA level (Fig. 2A&B). Consistent with the negative data on COX-2 protein and mRNA, WM failed to inhibit COX-2 promoter activation by CT (Fig. 2C).
Figure 2. Wortmannin failed to inhibit CT-induced COX-2 elevation.
Cardiomyocytes were pretreated with WM at the dose indicated (A) or 2 µM (B) 60 mins before treatment of 1 µM CT for 4 (A) or 2 hours (B). COX-2 was measured by Western blot (A) or RT-PCR (B) with vinculin or GAPDH as a loading control. Transfected cardiomyocytes with or without 60-mins pretreatment of 2 µM WM were treated with 1 µM CT for 12 hours before harvesting for measurements of luciferase activity, which is expressed as the RLU per µg protein (C). The data represent one of three independent experiments. An asterisk (*) indicates significant difference (p<0.05) as judged by the Student's t test when the means of CT treated groups were compared to that of the control from three independent experiments (C).
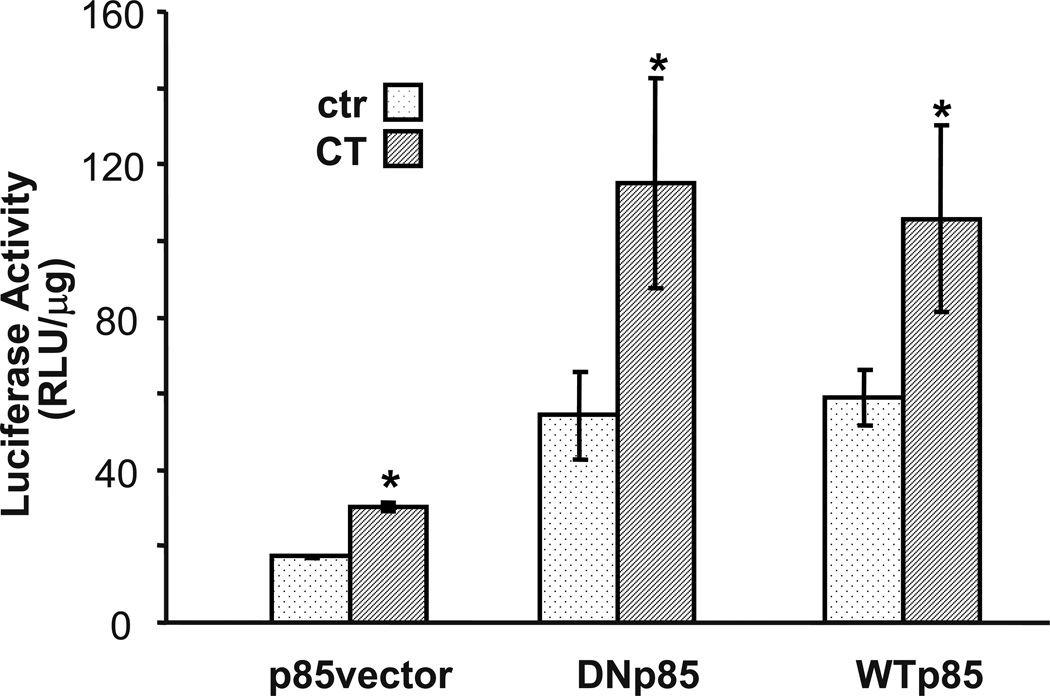
The discrepancy between LY29 and WM data led to the question whether or not PI3K plays a role in CT induced COX-2 expression. We addressed this question by expressing a wild type or dominant-negative mutant of p85 expression vector (Hara et a.l., 1994). PI3K contains a regulatory and a catalytic subunit. Activation of PI3K requires the recruitment of its p85 regulatory subunit to p110 catalytic subunits to form a complex. Transfection of p85 dominant negative subunit into cardiomyocytes is capable of inhibiting PI3K mediated event (Purdom-Dickinson et al., 2007). For a reason unknown, either dominant negative or wild type p85 increased the basal level of COX-2 promoter luciferase. However the ratios of luciferase between control and CT treatment indicate that p85 dominant negative or wild type p85 does not affect CT induced COX-2 promoter activation (Fig. 3). This result argues against a role of PI3K in CT induced COX-2 gene expression.
Figure 3. Dominant negative mutant of PI3K failed to prevent COX-2 induction.
Cardiomyocytes were transfected with rat COX-2 promoter reporter vector along with empty vector, wild type or dominant-negative p85 expression vector. Transfected cells were incubated with or without 1 µM CT for 12 hours before luciferase activity assay. The activity of luciferase is expressed as the RLU per µg protein. The data show one experiment representative of three. An asterisk (*) indicates significant difference (p<0.05) as judged by the Student's t test when the means of CT treated groups were compared to that of the control from three independent experiments.
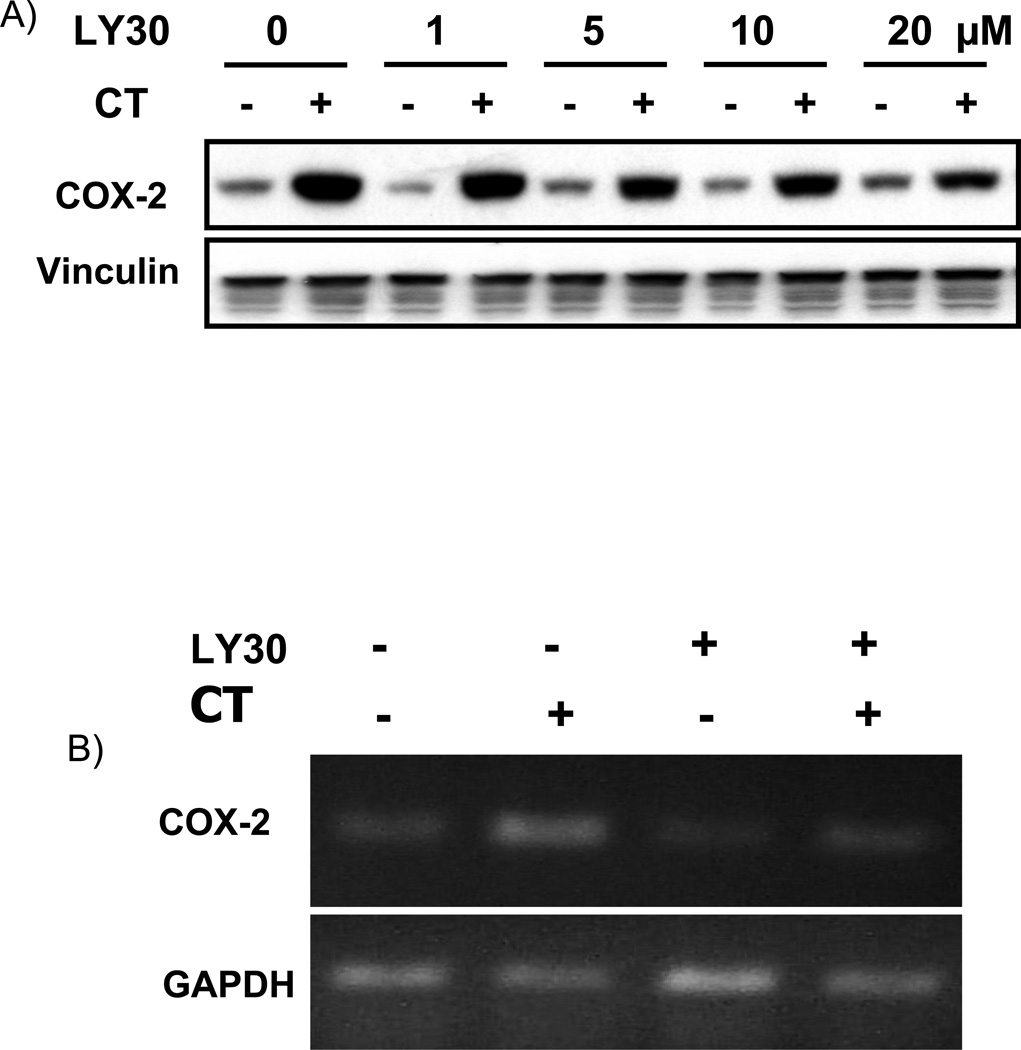
The lack of inhibitory effect of WM or dominant negative p85 suggested that LY29 inhibited CT from inducing COX-2 expression via a PI3K independent mechanism and the inhibition could be reproduced with a LY29 releted structural analogue. LY30 has been used as a negative control of LY29 and does not inhibit PI3K at a dose below 100 µM (Vlahos et al., 1994). When cells were treated with LY30 at the same dose range as LY29, LY30 prevented CT from inducing COX-2 protein expression (Fig 4A). Measurements of RNA level also confirmed the inhibition (Fig. 4B).
Figure 4. LY303511 attenuates CT-induced COX-2 elevation.
Cardiomyocytes were pretreated with LY30 at the dose indicated (A) or 10 µM (B) 60 mins before treatment of 1 µM CT for 4 hours (A) or 2 hours (B). COX-2 was measured by Western blot (A) or RT-PCR (B) with vinculin (A) or GAPDH as a loading control (B).
Lack of PI3K/AKT Signaling Pathway Activation by CT Treatment
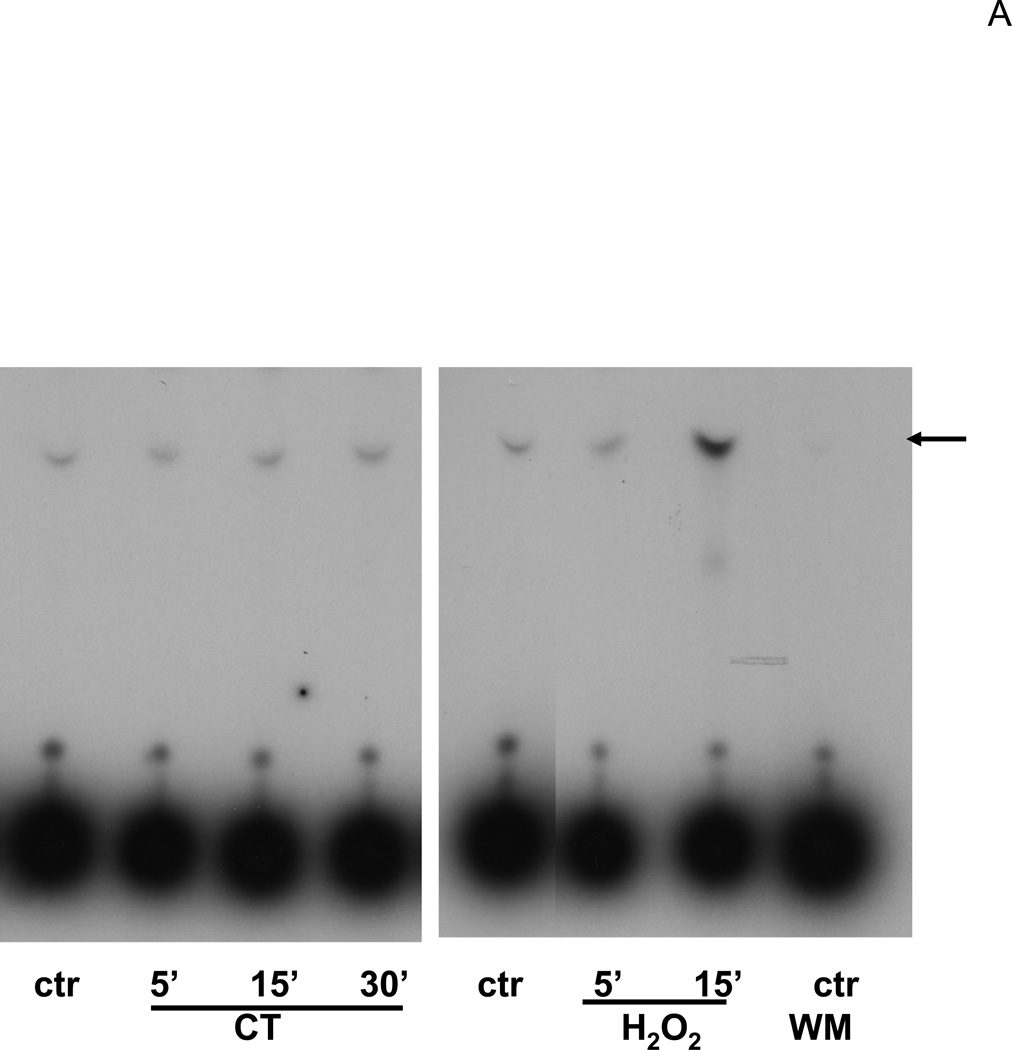
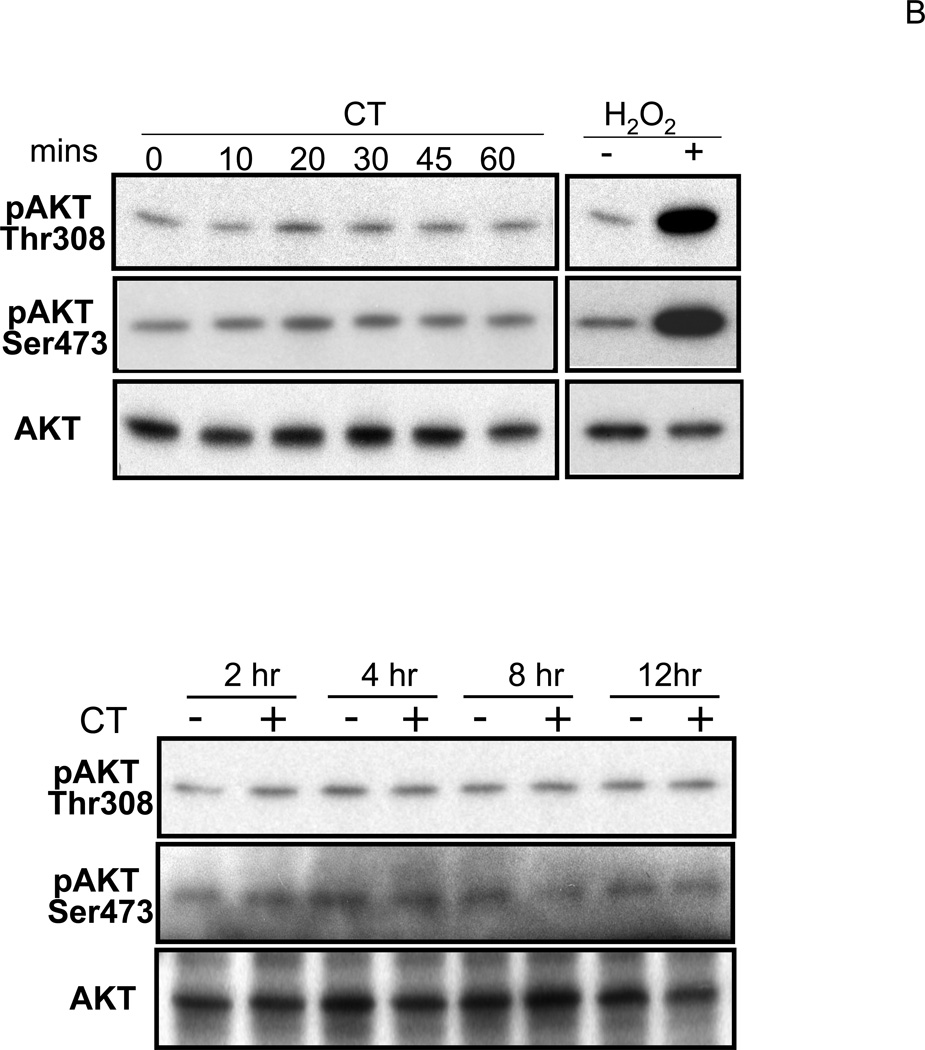
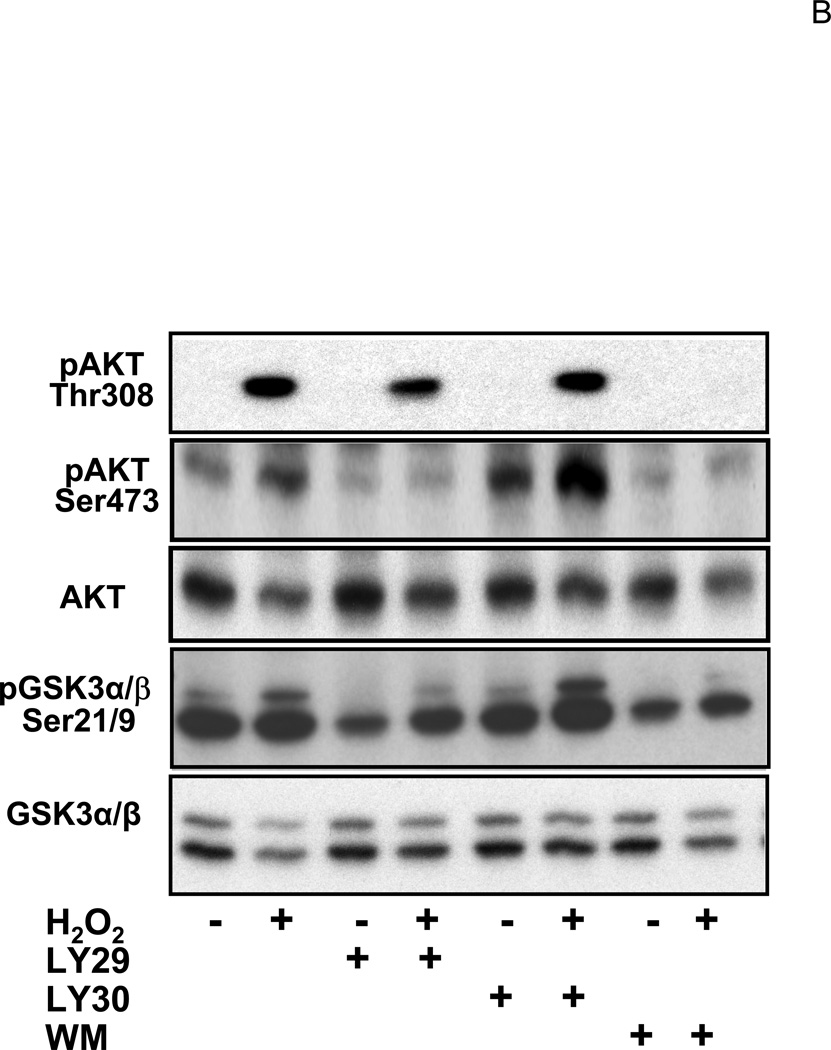
Glucocorticoids have been shown to activate PI3K in endothelial cells (Hafezi-Moghadam et al., 2002). The activity of PI3K was measured to address whether CT treatment resulted in activation of PI3K/AKT signaling pathway in cardiomyocytes. Figure 5A shows a representative experiment indicating that CT treatment did not increase PI3K activity, whereas H2O2, a positive control, activated PI3K (Tu et al., 2002). AKT is a well-known downstream target of PI3K and its activation can be measured by phosphorylation at a signature site Ser473 or Thr308. PI3K adds phosphate to the 3-position of inositol ring of phosphatidylinositol, leading to activation of phosphoinositide-dependent kinase 1 (PDK1) and PDK2, which phosphorylate Thr308 in the activation loop and Ser473 in the C-terminal region of Akt respectively (Alessi et al., 1996; Jacinto et al., 2006). Akt is activated by phosphoinositide binding and phosphorylation at these two residues. Compared to the positive control, i.e. H2O2 exposure, CT treatment did not cause significant AKT activation as measured by Thr308 or Ser473 phosphorylation within 60 mins or 12 hrs (Fig. 5B).
Figure 5. CT failed to activate PI3K/AKT signaling pathway.
Cardiomyocytes were treated with 1 μM CT for indicated time before harvesting for measurements of PI3K activity (A), Thr308 or Ser473 phospho AKT, or total AKT (B). H2O2 treatment (100 μM, 15 mins for A or 30 mins for B) was included as a positive control. In the last lane, wortmannin (WM) was added to the reaction mixture to inhibit PI3K in vitro and indicate the specific product of PI3K activity assay (A). The data represent one of three independent experiments.
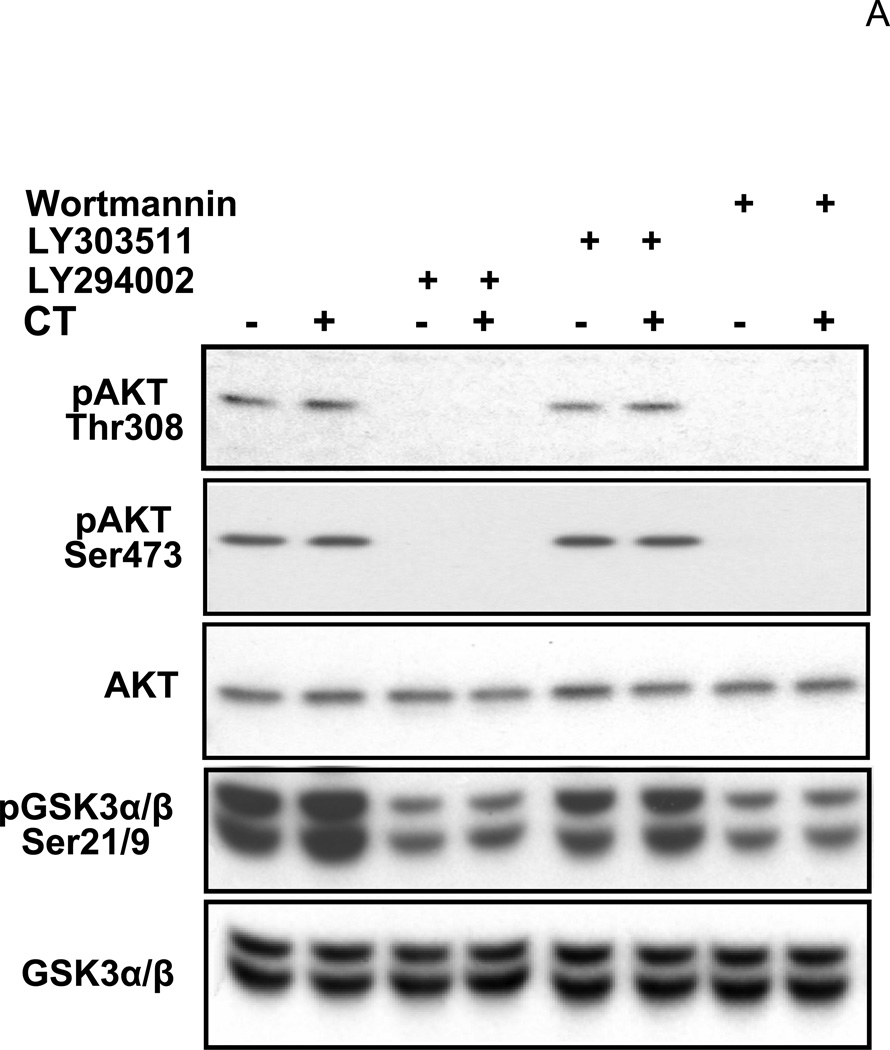
While measuring the activity of PI3K/AKT, we verified that LY29 and WM indeed inhibited PI3K while LY 30 did not. Phosphorylations of AKT and GSK3, a downstream target of AKT, were measured with cells treated with these drugs. AKT phosphorylates GSK3 α or β at Ser21 or Ser9 residue respectively. LY29 and WM but not LY30 decreased the basal level of AKT phosphorylation at Thr308 or Ser473, and GSK3α/β phosphorylation (Fig. 6). These data suggest that LY29 and WM indeed inactivate PI3K while LY30 serves well as a negative control.
Figure 6. Effects of LY294002, LY303511 and Wortmannin on PI3K/AKT pathway.
Cardiomyocytes were treated with 1µM CT for 4hr before harvesting for measurements of Thr308 or Ser473 phospho AKT, total AKT, Ser21/9 phospho GSK3 α/β or total GSK3 α/β. LY29 (20 µM), LY30 (20 µM) or WM (2 µM) was added to cells 60 mins prior to CT. The data represent one of three independent experiments.
LY29 and LY30 Do Not Affect GR Activation
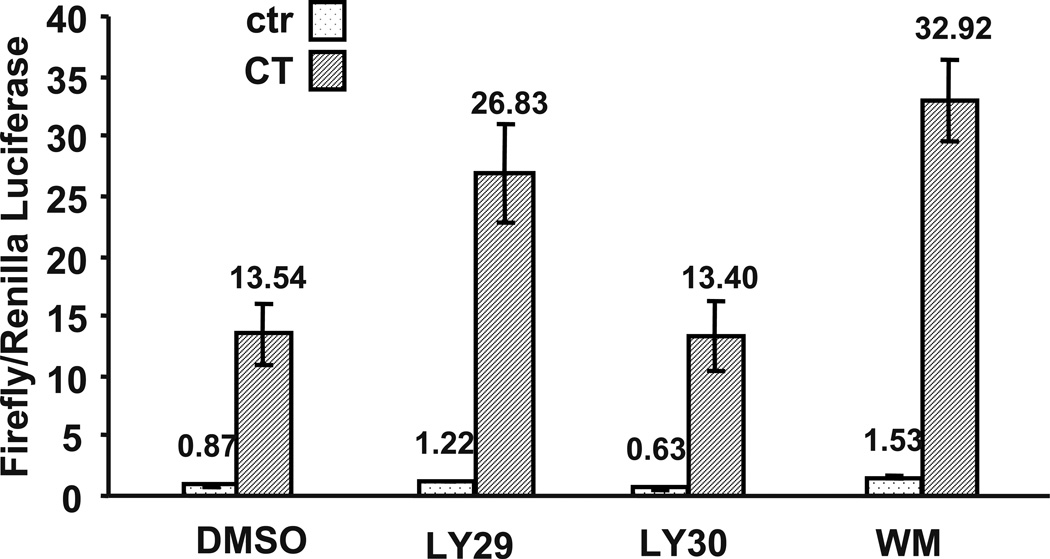
Previously work in our laboratory has found that activation of the glucorticoid receptor (GR) mediates CT-induced COX-2 expression (Sun and Chen, manuscript submitted). LY29 has been reported to bind to the estrogen receptor and inhibit estrogen from activating gene expression (Pasapera Limon et al., 2003). We examined whether LY29 and LY30 inhibited COX-2 expression by blocking GR activation. Mouse mammary tumor virus (MMTV) promoter contains a well-characterized Glucocorticoid Response Element (GRE), a cis-element of GR transcription factor. MMTV luciferase reporter construct was transfected into cardiomyocytes for measurement of GR activation. CT treatment increased MMTV-luciferase signal about 15.6 fold, which was not inhibited by WM, LY29 or LY30 (Fig. 7). This result suggests that LY29 and LY30 prevent COX-2 induction via a mechanism independent or downstream of GR activation.
Figure 7. Effects of LY294002, LY303511, and Wortmannin on glucocorticoid receptor activation.
Cardiomyocytes were transfected with MMTV-luc and TK-Renilla luciferase vector. Cells were treated with LY29 (10 µM), LY30 (10 µM) or WM (2 µM) for 60 mins before 24 hr treatment of 1 µM CT. Cells were harvested for duel luciferase assay and MMTV firefly luciferase activity was normalized to TK-Renilla luciferase activity. The bars represent the ratio of MMTV firefly luciferase versus TK-Renilla luciferase. The fold of induction by CT treatment is indicated above the bars. An asterisk (*) indicates significant difference (p<0.05) as judged by the Student's t test when the means of CT treated groups were compared to that of the control from three independent experiments.
TEA and Nomidipine Inhibit COX-2 Induction
LY29 and LY30 have been reported to inhibit Casein Kinase 2 (CK2) while WM does not do so (Davies et al., 2000; Kristof et al., 2005). To test whether inhibition of CK2 composes a mechanism for the observed inhibitory effect of LY29 and LY30, the CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) was applied to cardiomyocytes. We found that TBB at 1–10 µM had no inhibitory effect on CT induced COX-2 expression (data not shown). LY29 and WM have been shown to inhibit mTOR and DNA-PK (Vanhaesebroeck et al., 2001). Inhibitors of mTOR (rapamycin) or DNA-PK (Nu7026) failed to attenuate CT induced COX-2 expression (data not shown).
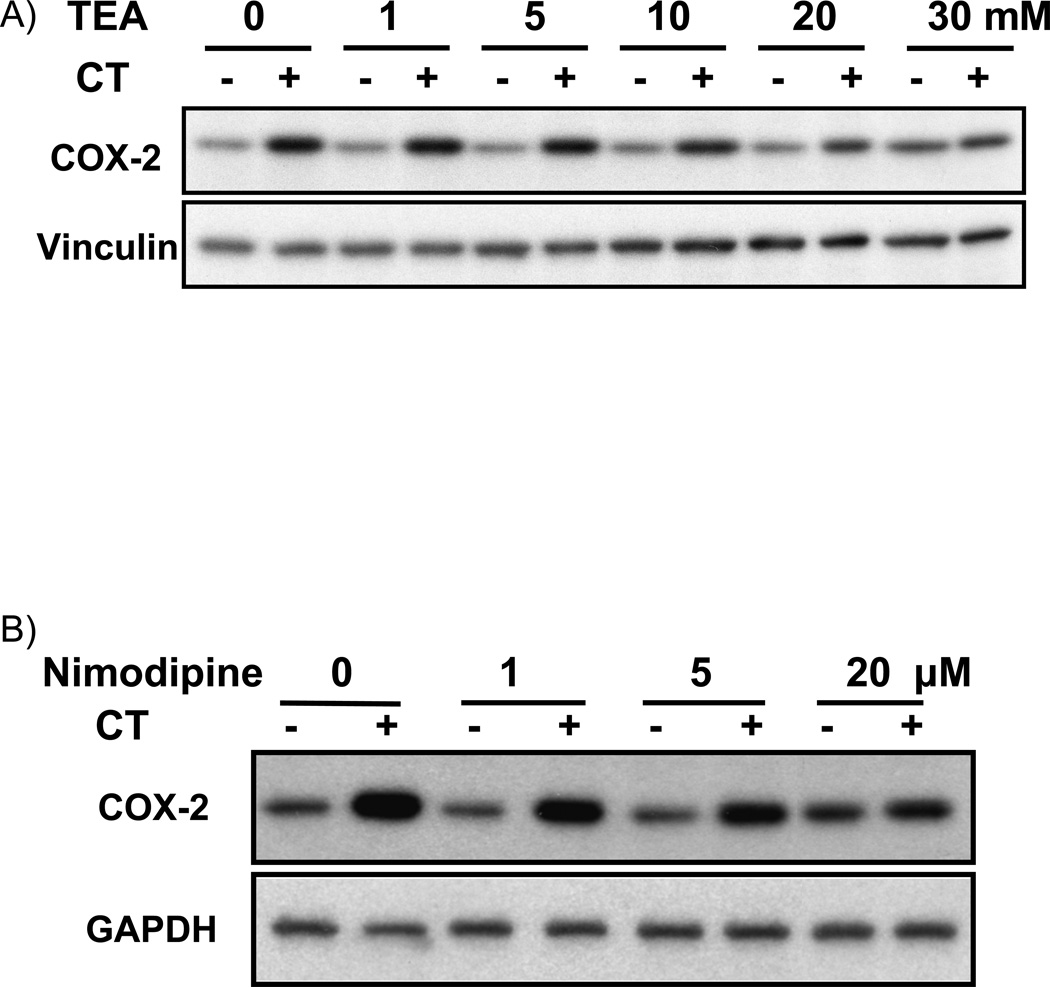
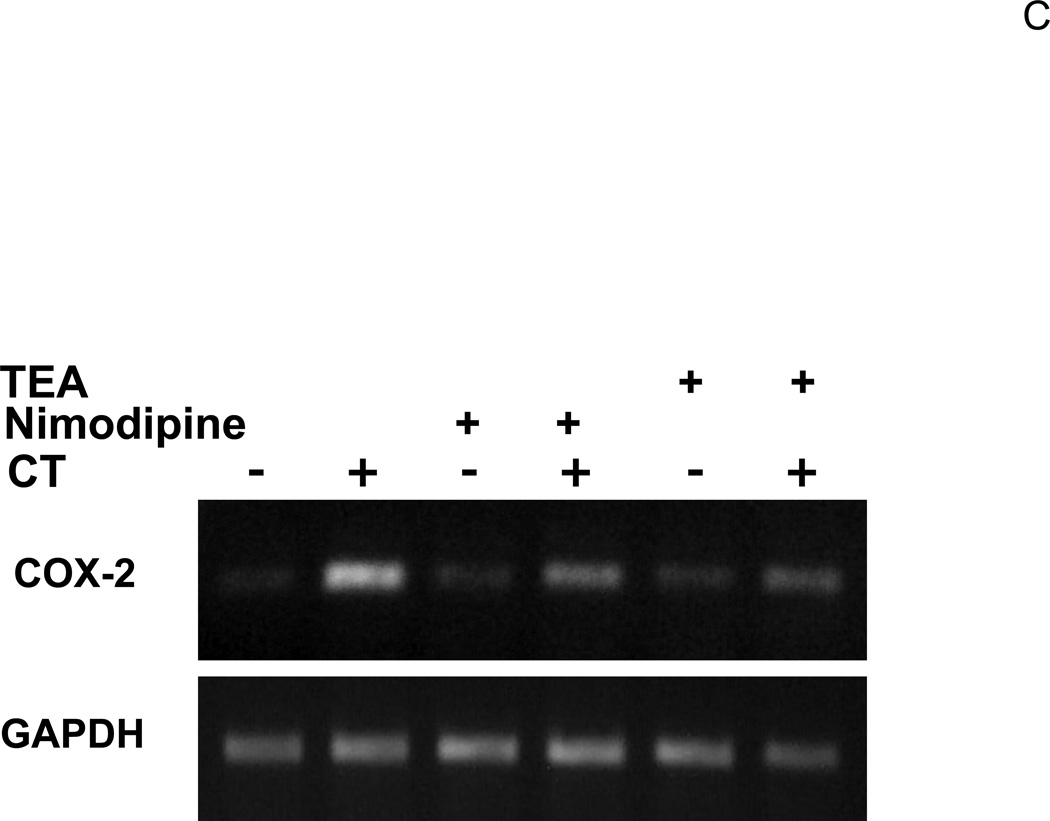
LY29 and LY30 but not WM can bind to potassium channel to block potassium current (El-Kholy et al, 2003). Tetraethylammonium (TEA) is a general potassium channel blocker with potency slightly lower than that of LY29 in inhibiting the voltage-gated potassium channel (El-Kholy et al., 2003; Sun et al., 2004). TEA at mM concentration has been commonly used to inhibit potassium channel in various experimental models. In addition to blocking potassium channel, LY29 has also been shown to alter intracellular calcium levels by regulating calcium channel (Straube and Parekh, 2001; Ethier and Madison, 2002; Welling et al., 2005). The effect of nimodipine, a voltage-gated calcium channel blocker, was examined on CT-induced COX-2 induction. When cardiomyocytes were treated with TEA or nimodipine, we found TEA at 10 mM or above and nimodipine at 1 µM or above were capable of inhibiting CT induced COX-2 expression (Fig. 8A&B). Measurements of COX-2 mRNA level confirmed the inhibitory effect of TEA and nimodipine (Fig. 8C).
Figure 8. Effect of TEA or nimodipine on CT induced COX-2 expression.
Cardiomyocytes were pretreated 60 mins with TEA (A) or nimodipine (nimo, B) at the dose indicated, or with TEA (30 mM) or nimodipine (20 µM) (C), before treatment of 1 µM CT for 4 hours. COX-2 was measured by Western blot (A,B) or RT-PCR (C) with with vinculin (A, B) or GAPDH (C) as a loading control. The data represent one of three independent experiments.
CT Causes an Increase in Intracellular Calcium
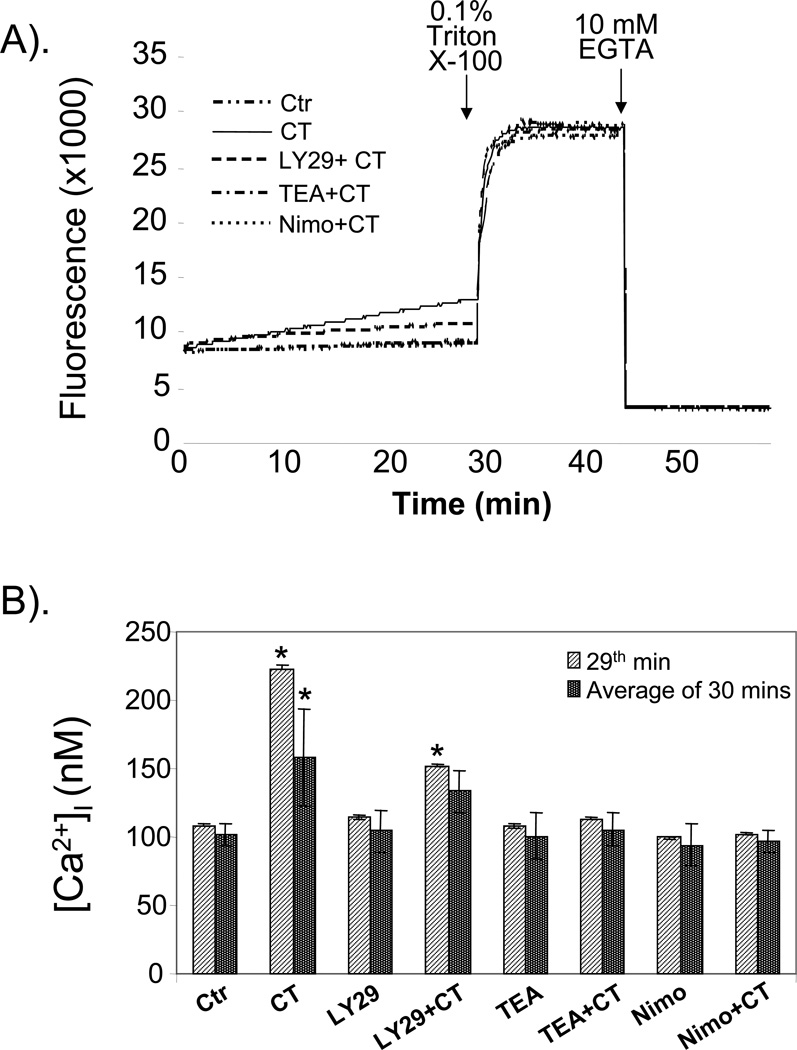
Our data with nimodipine and the fact that LY29 has been shown to regulate calcium channel suggest that CT may increase intracellular calcium. Inhibition of potassium channels often lead to changes in intracellular calcium (Tokuno et al., 1999; Doi et al., 2000; Tahara et al., 2001; Sah et al., 2002; Wang et al., 2006), further suggesting the importance to determine intracellular calcium concentration ([Ca2+]i) following CT treatment. A protocol has been developed to measure the overall [Ca2+]i in cardiomyocytes treated with CT in the presence or absence of inhibitors. The results show that CT treatment caused a gradual increase in [Ca2+]i (Fig. 9A). The average [Ca2+]i during 30 mins of measurement time was 101. 8 ° 7.9 or 158.1 ° 35.9 nM for control or CT treated cells (Fig. 9B). At 29th minute after CT addition, [Ca2+]i was doubled in CT treated cells compared to the control (Fig. 9B). While the presence of TEA or nimodipine abolished [Ca2+]i increases, LY29 attenuated CT induced [Ca2+]i increase (Fig. 9A&B).
Figure 9. Efffect of LY29, TEA and nimodipine on CT induced [Ca2+]i increase.
Cardiomyocytes seeded in a 12-well plate were placed in HEPES buffer for fluo-4 AM dye loading and fluorescence measurement using a microplate reader (BioTek ELX800) as described in the Method. The instrument measures fluorescence intensity every 7 seconds. The fluorescence reflecting [Ca2+]i changes for control, CT or CT plus inhibitors are shown (A). The [Ca2+]i for each sample was calculated as average ± standard deviation based on 258 measurements during 30 mins interval or 9 measurements during the last minute (29th min) before Triton X-100 addition (B). The data are from one experiment representative of three independent experiments. An asterisk (*) indicates significant difference (p<0.05) as judged by the Student's t test when the means of CT treated groups were compared to that of the control.
Discussion
In the present study, we aimed to address whether PI3K pathway plays a role in CT induced COX-2 gene expression. While LY29 and WM indeed inhibited PI3K/Akt pathway, LY29 but not WM attenuated COX-2 induction by CT. LY30 does not inhibit PI3K but inhibited CT-induced COX-2 expression in cardiomyocytes. The dominant negative mutant of p85 regulatory subunit of PI3K failed to alter COX-2 expression. These data suggest a PI3K/AKT independent mechanism mediating CT induced COX-2 gene expression. We have found that CT causes an increase in [Ca2+]i. The facts that LY29 attenuates CT induced [Ca2+]i increase and that nimodipine blocks COX-2 induction by CT suggest a role of [Ca2+]i in CT induced COX-2.
As an ATP binding site inhibitor, LY29 was developed based on the structure of quercetin by enhancing the specificity for PI3K inhibition (Vlahos et al., 1994). The oxygen atom in the morpholino ring of LY29 forms a hydrogen bond with Val 882 of the catalytic subunit of PI3K, displacing ATP from the catalytic site (Walker et al., 2000). Kinases or ATP binding proteins with the steric structure of ATP binding pocket similar to PI3K will likely be a candidate for LY29 inhibition. In fact, CK2, DNA-PK and mTOR have been shown as a target of LY 29 (Vanhaesebroeck et al., 2001). While WM also inhibits DNA-PK and mTOR, CK2 is inhibited by LY30 but not WM (Davies et al., 2000; Vanhaesebroeck et al., 2001; Kristof et al., 2005). The overlaps versus differential activities of these three inhibitors led to the testing of mTOR, DNA-PK and CK2 inhibitors. The negative the data from these inhibitors drove our attension to other targets.
LY29 and LY30 share many common features in perturbing cellular metabolism. Both compounds block potassium current in rat pancreatic β cells (El-Kholy et al., 2003), and inhibit monocyte chemoattractant protein-1 expression in endothelial cells (Choi et al., 2004). LY29 and LY30 induce H2O2 production in tumor cells (Poh and Pervaiz, 2005) but inhibit LPS induced NO production in macrophages (Kim et al., 2005). LY29 and LY30 have been shown to inhibit the nuclear factor-κB (NF-κB) in human umbilical vein endothelial cells (Choi et al., 2004). Although COX-2 promoter contains a NF-kB cis-element (Smith, 2000), using COX-2 promoter deletion and mutation approaches, we have not found a role of NF-κB in CT induced COX-2 expression in cardiomyocytes (Sun and Chen, manuscript submitted). In fact, CT has been reported to inhibit NF-κB transcription factor (Adcock et al., 2004). While our study has demonstrated GR dependent induction of COX-2 gene expression (Sun and Chen, manuscript submitted), neither LY29 nor LY30 suppressed GR activity in this study.
Glucocorticoids induce multitude of changes at the cellular level. The genomic effect of glucocorticoids involves ligand binding and GR translocation. The non-genomic effect comprises activation of kinases and membrane-associated molecules. Glucocorticoids have been shown to cause a sustained elevation of intracellular potassium in cardiomyocytes (Shimoni, 2005). Levitan, et al (Levitan et al., 1996) reported that in vivo adminstration of dexamethasone, a potent synthetic form of glucocorticoid, caused rapid induction of the gene encoding Kv1.5 potassium channel in cardiomyocytes. LY29 and LY30 can bind to the voltage-gated potassium channel and block outward potassium current in pancreatic beta-cells (El-Kholy et al., 2003). In rat ventricular myocytes, LY29 and LY30 inhibit the Kv1.5/2.1 potassium channels (Oudit et al., 2004). Inhibition of potassium channels often results in changes in intracellular calcium levels (Tokuno et al., 1999; Doi et al., 2000; Tahara et al., 2001; Sah et al., 2002; Wang et al., 2006). Despite of the complexity of potassium channels and the coupling of intracellular potassium versus calcium concentration changes in cardiomyocytes, we have found that a general potassium channel blocker TEA inhibited CT from increasing [Ca2+]i and inducing COX-2 expression.
Our data show that CT causes an increase in [Ca2+]i and LY inhibits CT induced [Ca2+]i increases. Although measurements of PI3K, Akt phosphorylation and GSK phosphorylation indicate no significant activation of PI3K/Akt/GSK pathway by CT at the time points chosen (Figs. 5 & 6A), we cannot exclude the possibility that CT causes a minor or transient spike of PI3K activity outside these time points that is sufficient to trigger [Ca2+]i increase. However the negative data of WM and dominant negative p85 on CT induced COX-2 do not support this possibility. LY29 has been shown to act as a direct blocker of L-type Ca2+ channel with IC50 of 20 µM (Welling et al., 2005). LY has also been shown to prevent [Ca2+]i increases triggered by thapsigargin, carbachol, caffeine and histamine in a PI3K independent manner (Ethier and Madison, 2002). Although how CT causes an elevation of [Ca2+]i in cardiomyocytes remains to be determined, an earlier study has reported a stimulatory effect of glucocorticoids on voltage-gated calcium channels (Fomina et al., 1993). A reduction of calcium efflux can also contribute to an increase in [Ca2+]i (Elliott and Sapolsky, 1993). Nevertheless calcium dependent increase in COX-2 gene transcription has been reported (Puga et al., 1997; Pham et al., 2006; Jerde et al., 2008), suggesting that CT may increase COX-2 expression by elevating [Ca2+]i in cardiomyocytes.
The finding that LY29 may abolish CT-induced COX-2 expression through a PI3K independent mechanism is potentially important for studies involving the usage of LY29. Although LY29 has been widely used as a pharmacological inhibitor of PI3K, additional approaches such as dominant negative inhibition or pharmacological inhibitors with different structures or mechanisms of action are required to confirm the involvement of PI3K in a particular biological process. With cardiomyocytes and other excitatory cells, potassium and calcium channels play an important role in contraction-relaxation cycle and cellular signaling for various biochemical reactions. The fact that LY29 can block potassium and calcium channels in a PI3K independent manner warrants the caution of using this inhibitor among these excitatory cells.
Acknowledgements
Work from our laboratory has been supported by NIH R01 ES10826, R01 HL 076530, T32 ES007091, Arizona Disease Control Research Commission, and the American Heart Association. We thank Ms. Yan Lin for technical assistance. MMTV-Luciferase construct was generous gift from Dr. Roger Miesfeld. PI3K p85 subunit dominant-negative mutant and wild type constructs were generous gifts from Dr. Masato Kasuga.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM, Ito K, Barnes PJ. Glucocorticoids: Effects on Gene Transcription. Proc. Am. Thorac. Soc. 2004;1:247–254. doi: 10.1513/pats.200402-001MS. [DOI] [PubMed] [Google Scholar]
- Adderley SR, Fitzgerald DJ. Oxidative Damage of Cardiomyocytes Is Limited by Extracellular Regulated Kinases 1/2-mediated Induction of Cyclooxygenase-2. J. Biol. Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Bachelor MA, Cooper SJ, Sikorski ET, Bowden GT. Inhibition of p38 Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-Kinase Decreases UVB-Induced Activator Protein-1 and Cyclooxygenase-2 in a SKH-1 Hairless Mouse Model. Mol. Cancer Res. 2005;3:90–99. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc. Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ. Res. 2003;92:330–337. doi: 10.1161/01.res.0000054201.60308.1a. [DOI] [PubMed] [Google Scholar]
- Camitta MGW, Gabel SA, Chulada P, Bradbury JA, Langenbach R, Zeldin DC, Murphy E. Cyclooxygenase-1 and −2 Knockout Mice Demonstrate Increased Cardiac Ischemia/Reperfusion Injury but Are Protected by Acute Preconditioning. Circulation. 2001;104:2453–2458. doi: 10.1161/hc4401.098429. [DOI] [PubMed] [Google Scholar]
- Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. Corticosteroids Inhibit Cell Death Induced by Doxorubicin in Cardiomyocytes: Induction of Antiapoptosis, Antioxidant, and Detoxification Genes. Mol. Pharm. 2005;67:1861–1873. doi: 10.1124/mol.104.003814. [DOI] [PubMed] [Google Scholar]
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E-K, Park H-J, Ma J-S, Lee H-C, Kang H-C, Kim B-G, Kang I-C. LY294002 inhibits monocyte chemoattractant protein-1 expression through a phosphatidylinositol 3-kinase-independent mechanism. FEBS Lett. 2004;559:141–144. doi: 10.1016/S0014-5793(04)00058-4. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Kumar A, Kale RK, Raisz LG, Pilbeam CC. Extracellular calcium induces COX-2 in osteoblasts via a PKA pathway. Biochem. Biophys. Res. Comm. 2004;322:395–402. doi: 10.1016/j.bbrc.2004.07.129. [DOI] [PubMed] [Google Scholar]
- Coronella-Wood J, Terrand J, Sun H, Chen QM. c-Fos Phosphorylation Induced by H2O2 Prevents Proteasomal Degradation of c-Fos in Cardiomyocytes. J. Biol. Chem. 2004;279:33567–33574. doi: 10.1074/jbc.M404013200. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S, Damron DS, Ogawa K, Tanaka S, Horibe M, Murray PA. K(+) channel inhibition, calcium signaling, and vasomotor tone in canine pulmonary artery smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L242–L251. doi: 10.1152/ajplung.2000.279.2.L242. [DOI] [PubMed] [Google Scholar]
- El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003;17:720–722. doi: 10.1096/fj.02-0802fje. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Sapolsky RM. Corticosterone impairs hippocampal neuronal calcium regulation--possible mediating mechanisms. Brain Res. 1993;602:84–90. doi: 10.1016/0006-8993(93)90245-i. [DOI] [PubMed] [Google Scholar]
- Ethier MF, Madison JM. LY294002, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium. 2002;32:31–38. doi: 10.1016/s0143-4160(02)00111-2. [DOI] [PubMed] [Google Scholar]
- Fomina AF, Kostyuk PG, Sedova MB. Glucocorticoid modulation of calcium currents in growth hormone 3 cells. Neuroscience. 1993;55:721–725. doi: 10.1016/0306-4522(93)90437-k. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson TR, et al. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Brune K. Cyclooxygenase-2---10 Years Later. J. Pharm. Exp. Therap. 2002;300:367–375. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jerde TJ, Mellon WS, Bjorling DE, Checura CM, Owusu-Ofori K, Parrish JJ, Nakada SY. Stretch induction of cyclooxygenase-2 expression in human urothelial cells is calcium- and protein kinase C zeta-dependent. Mol. Pharm. 2008;73:18–26. doi: 10.1124/mol.108.035519. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi K-H, Park J-W, Kwon TK. LY294002 inhibits LPS-induced NO production through a inhibition of NF-[kappa]B activation: independent mechanism of phosphatidylinositol 3-kinase. Immun. Lett. 2005;99:45–50. doi: 10.1016/j.imlet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kristof AS, Pacheco-Rodriguez G, Schremmer B, Moss J. LY303511 (2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one) acts via phosphatidylinositol 3-kinase-independent pathways to inhibit cell proliferation via mammalian target of rapamycin (mTOR)- and non-mTOR-dependent mechanisms. J. Pharm. Exp. Therap. 2005;314:1134–1143. doi: 10.1124/jpet.105.083550. [DOI] [PubMed] [Google Scholar]
- Layne E. Spectrophotometric and turbidimetric methods for measuring proteins. Meth. Enzymol. 1957;3:447–454. [Google Scholar]
- Levitan ES, Hershman KM, Sherman TG, Takimoto K. Dexamethasone and Stress Upregulate Kv1.5 K+ Channel Gene Expression in Rat Ventricular Myocytes. Neuropharmacology. 1996;35:1001–1006. [PubMed] [Google Scholar]
- Ma XQ, Fu RF, Feng GQ, Wang ZJ, Ma SG, Weng SA. Hypoxia-reoxygenation-induced apoptosis in cultured neonatal rat cardiomyocyets and the protective effect of prostaglandin E. Clin. Exp. Pharm. Physiol. 2005;32:1124–1130. doi: 10.1111/j.1440-1681.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J. Mol. Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Pasapera Limon AM, Herrera-Munoz J, Gutierrez-Sagal R, Ulloa-Aguirre A. The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17[beta]-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol. Cell. Endocrinol. 2003;200:199–202. doi: 10.1016/s0303-7207(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Pham H, Shafer LM, Slice LW. CREB-dependent cyclooxygenase-2 and microsomal prostaglandin E synthase-1 expression is mediated by protein kinase C and calcium. J. Cell. Biochem. 2006;98:1653–1666. doi: 10.1002/jcb.20899. [DOI] [PubMed] [Google Scholar]
- Pino M, Nawrocki S, Cognetti F, Abruzzese J, Xiong H, McConkey D. Prostaglandin E(2) Drives Cyclooxygenase-2 Expression via Cyclic AMP Response Element Activation in Human Pancreatic Cancer Cells. Cancer Biol. Therapy. 2005;4:1263–1269. doi: 10.4161/cbt.4.11.2138. [DOI] [PubMed] [Google Scholar]
- Poh TW, Pervaiz S. LY294002 and LY303511 Sensitize Tumor Cells to Drug-Induced Apoptosis via Intracellular Hydrogen Peroxide Production Independent of the Phosphoinositide 3-Kinase-Akt Pathway. Cancer Res. 2005;65:6264–6274. doi: 10.1158/0008-5472.CAN-05-0152. [DOI] [PubMed] [Google Scholar]
- Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharm. 1997;54:1287–1296. doi: 10.1016/s0006-2952(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Purdom-Dickinson S, Sheveleva E, HP S, Chen Q. Translational Control of Nrf2 Protein in Activation of Antioxidant Response Element by Oxidants. Mol. Pharm. 2007;72:1074–1081. doi: 10.1124/mol.107.035360. [DOI] [PubMed] [Google Scholar]
- Purdom S, Chen QM. Epidermal growth factor receptor-dependent and -independent pathways in hydrogen peroxide-induced mitogen-activated protein kinase activation in cardiomyocytes and heart fibroblasts. J. Pharm. Exp. Therap. 2005;312:1179–1186. doi: 10.1124/jpet.104.077057. [DOI] [PubMed] [Google Scholar]
- Sah R, Oudit GY, Nguyen TT, Lim HW, Wickenden AD, Wilson GJ, Molkentin JD, Backx PH. Inhibition of calcineurin and sarcolemmal Ca2+ influx protects cardiac morphology and ventricular function in K(v)4.2N transgenic mice. Circulation. 2002;105:1850–1856. doi: 10.1161/01.cir.0000014211.47830.4d. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, Liu S-H. High Glucose Induces Human Endothelial Cell Apoptosis Through a Phosphoinositide 3-Kinase-Regulated Cyclooxygenase-2 Pathway. Arterioscler Thromb. Vasc. Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- Shimoni Y. Dexamethasone and cardiac potassium currents in the diabetic rat. Brit. J. Pharm. 2005;146:280–287. doi: 10.1038/sj.bjp.0706314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2093–H2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt David L, Garavito R. Michael. Cyclooxygenases: structural, cellular, and molecular biology. Ann. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Straube S, Parekh AB. Effects of phosphatidylinositol kinase inhibitors on the activation of the store-operated calcium current ICRAC in RBL-1 cells. Pflugers Arch. 2001;442:391–395. doi: 10.1007/s004240100546. [DOI] [PubMed] [Google Scholar]
- Sun H, Oudit GY, Ramirez RJ, Costantini D, Backx PH. The phosphoinositide 3-kinase inhibitor LY294002 enhances cardiac myocyte contractility via a direct inhibition of Ik, slow currents. Cardiovasc. Res. 2004;62:509–520. doi: 10.1016/j.cardiores.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S. Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J. Am. Coll. Cardiol. 2001;38:1554–1563. doi: 10.1016/s0735-1097(01)01558-3. [DOI] [PubMed] [Google Scholar]
- Tang Q, Gonzales M, Inoue H, Bowden GT. Roles of Akt and Glycogen Synthase Kinase 3{beta} in the Ultraviolet B Induction of Cyclooxygenase-2 Transcription in Human Keratinocytes. Cancer Res. 2001;61:4329–4332. [PubMed] [Google Scholar]
- Tokuno T, Muraki K, Watanabe M, Imaizumi Y. Effects of K+ channel modulators on the relationship between action potential duration and Ca2+ transients in single ventricular myocytes of the guinea pig. Jpn. J. Pharm. 1999;80:243–253. doi: 10.1254/jjp.80.243. [DOI] [PubMed] [Google Scholar]
- Tu VC, Bahl JJ, Chen QM. Signals of Oxidant-Induced Cardiomyocyte Hypertrophy: Key Activation of p70 S6 Kinase-1 and Phosphoinositide 3-Kinase. J. Pharm. Exp. Therap. 2002;300:1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
- Turini M, DuBois R. CYCLOOXYGENASE-2: A Therapeutic Target. Ann. Rev. Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. SYNTHESIS AND FUNCTION OF 3-PHOSPHORYLATED INOSITOL LIPIDS. Ann. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4- morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng J, Tandan S, Jiang M, McCloskey DT, Hill JA. Transient-outward K+ channel inhibition facilitates L-type Ca2+ current in heart. J. Cardiovasc. Electrophysiol. 2006;17:298–304. doi: 10.1111/j.1540-8167.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Warner T, Mitchell J. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- Welling A, Hofmann F, Wegener JW. Inhibition of L-Type Cav1.2 Ca2+ Channels by 2,(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) and 2-[1-(3-Dimethyl-aminopropyl)-5-methoxyindol-3-yl]-3-(1H-indol-3-yl) Maleimide (Go6983) Mol. Pharm. 2005;67:541–544. doi: 10.1124/mol.104.006049. [DOI] [PubMed] [Google Scholar]
- Wu W, Silbajoris RA, Whang YE, Graves LM, Bromberg PA, Samet JM. p38 and EGF receptor kinase-mediated activation of the phosphatidylinositol 3-kinase/Akt pathway is required for Zn2+-induced cyclooxygenase-2 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L883–L889. doi: 10.1152/ajplung.00197.2005. [DOI] [PubMed] [Google Scholar]
- Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- Xuan Y-T, Guo Y, Zhu Y, Wang O-L, Rokosh G, Messing RO, Bolli R. Role of the Protein Kinase C-{epsilon}-Raf-1-MEK-1/2-p44/42 MAPK Signaling Cascade in the Activation of Signal Transducers and Activators of Transcription 1 and 3 and Induction of Cyclooxygenase-2 After Ischemic Preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]