Abstract
Three new terpenoids of mixed biosynthetic origin were isolated from the culture filtrate of the endophytic fungus Pycnoporus sanguineus. Their structures were determined by extensive spectroscopic analyses. We have named these tricyclic and tetracyclic metabolites ‘coibanoles A–C’ in reference to Coiba Island and Coiba National Park, Panamá, from which the plant and endophyte were collected. The extract was inactive to the human parasites Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum at a test concentration of 10 μg/mL.
Keywords: Pycnoporus, Trypanosoma, Leishmania, Meroterpenoids, Coibanoles
Introduction
Endophytes are microorganisms including bacteria or fungi that live within apparently healthy tissues of host plants without causing discernible manifestations of disease.1,2 Endophytic microbes have been recognized as important sources of structurally novel and biologically active secondary metabolites, including terpenoids, steroids, alkaloids, isocoumarin derivatives, quinones, and lipids.3–5 As part of the research conducted by the international cooperative biodiversity group (ICBG) located in Panamá6–9 a number of endophytic fungi have been isolated and are under study to explore their capacity to produce natural products with activity against tropical diseases and cancer.
A filamentous fungal endophyte (F0305), identified by molecular phylogenetic analysis as Pycnoporus sanguineus, was isolated from an apparently healthy, mature leaf of Desmotes incomparabilis (Rutaceae), a shrub that is endemic to Coiba Island, Veraguas Province, Panamá, the largest island in the tropical Eastern Pacific, lying within the Coiba National Park which is a UNESCO World Heritage Site.10 The extract was obtained from liquid culture in malt extract broth (MEB) and was tested against Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum at a concentration of 10 lg/ mL, but no activity was observed. Nevertheless, three new mixed biogenesis terpenoids (Fig. 1), including two tricyclocoibanoles (1, 2) and one tetracyclocoibanol (3), were isolated. These compounds belong to a general class of mixed biosynthetic terpenoids characterized by a combination of two isoprene units that are biosynthesized via the classic mevalonate pathway, and a nonterpenoid moiety. These new metabolites are six-membered cyclic α,β-unsaturated ketones, having some similarity with the tricyclic arternarene series reported by Liebermann et al. and others.11–17 Their structures were determined by extensive spectroscopic experiments (especially 2D NMR, HRMS, and IR). We have named these tricyclic and tetracyclic terpenoids coibanoles A–C, in reference to Coiba Island and Coiba National Park from which the plant and endophyte were collected.
Figure 1.
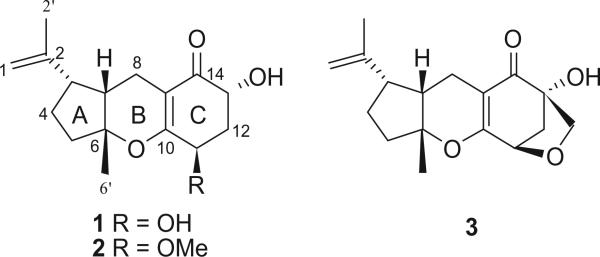
Molecular structures of coibanoles A–C.
Results and discussion
P. sanguineus was cultured in MEB, extracted with EtOAc, and fractionated by Sephadex LH-20. Selected fractions were purified by semi-preparative C18 reversed-phase HPLC, furnishing two new tricyclic mixed biosynthesis terpenoids (1, 2), together with one new tetracyclic compound (3).
Coibanol A (1) possessed a molecular formula of C16H22O4, with six degrees of unsaturation, on the basis of HREIMS (m/z 278.1515 [M+]) and NMR data. The 13C NMR and DEPT spectra contained signals for two methyl groups (one attached to an sp2 carbon, δc 19.1), five methylene units (including an olefinic carbon, δc 111.6), four methines (two oxygenated, δc 68.6 and 67.4) and five non-protonated carbons, including one α,β-unsaturated ketone carbon (δc 201.2), as well as three olefinic carbons (δc 164.7, 146.6, and 106.3). These data suggested that 1 was a tricyclic metabolite in which an oxygen atom should be integrated in one of the ring systems.
The 1H NMR spectrum of 1 showed signals corresponding to an olefinic AB spin system for gem-methylene protons at H-1a (δH 4.73), H-1b (δH 4.66) and a methyl singlet attached to H-2′(δH 1.69), suggesting the presence of an isopropenyl unit. An additional methyl singlet, H3-6′ (δH 1.34) and two oxygenated methine protons (δH 4.28 and δH 4.41) were observed. The HMBC experiments showed correlation of H2-8 with C-3, C-7, C-9, and C-10, placing C-8 between A and C. Furthermore H-13 showed cross-peaks with C-12 and C-14; whereas H-11 has with C-10 and C-12 indicating together that both hydroxyls groups and the α-β unsaturated ketone are on the same ring. This was confirmed by COSY experiments where H-11 and H-13 showed correlation with H2-12 (2.06 and 2.32). Finally H3-6′ showed correlation with C-5, C-6, and C-7.
Detailed analysis of the 2D NMR experiments (COSY, HSQC, and HMBC) led to the assignment of all signals (Table 1). Consequently, 1 was identified as a new mixed terpenoid. Further analysis of the 1H–1H coupling constants and NOESY data established the relative configuration of 1. The large trans-diaxial-type coupling constant observed between Hb-12 and H-13 (11.4 Hz) indicated that they were pseudoaxial. A small coupling constant of 3.3 Hz between H-11 and H2-12 placed H-11 in a pseudoequatorial orientation of the cyclohexanone ring. A NOESY correlation between H-7 and Me-6′ revealed that the A and B ring in 1 were cis-fused, similar to the tricycloalternarene (TCA) series. The main difference between coibanol A and the TCA metabolites was on the side chain; in place of a C8 unit in the case of the TCA series compound 1 possessed a C3 unit. Nevertheless, we have used a similar enumeration of the atom positions as used for the TCA series.11
Table 1.
NMR spectral data for compounds 1–2 (in CD3OD) and 3 (in CDCl3)
| Position | 1 |
2 |
3 |
|||
|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 1 | 111.6, CH2 | 4.73, br s | 111.6, CH2 | 4.72, br s | 111.6, CH2 | 4.72, br s |
| 4.66, br s | 4.64, br s | 4.60, br s | ||||
| 2 | 146.6, C | 147.3, C | 145.3, C | |||
| 3 | 50.1, CH | 2.34, m | 50.5, CH | 2.34, m | 48.7, CH | 2.18, m |
| 4 | 27.9, CH2 | 1.96, m | 27.9, CH2 | 1.96, m | 26.9, CH2 | 1.94, m |
| 1.59, m | 1.59, m | 1.54, m | ||||
| 5 | 38.6, CH2 | 2.14, m | 38.4, CH2 | 2.14, m | 37.5, CH2 | 2.11, m |
| 1.87, m | 1.87, m | 1.80, m | ||||
| 6 | 89.2, C | 89.4, C | 89.3, C | |||
| 7 | 44.5, CH | 2.02, m | 44.2, CH | 2.02, m | 43.3, CH2 | 2.03, m |
| 8 | 17.1, CH2 | 2.22, m | 17.4, CH2 | 2.22, m | 15.6, CH2 | 2.33, dd (1.4, 17.3) |
| 2.18, m | 2.18, m | 2.15, m | ||||
| 9 | 106.3, C | 106.4, C | 102.9, C | |||
| 10 | 164.7, C | 171.7, C | 172.9, C | |||
| 11 | 67.4, CH | 4.28, br t (3.3) | 79.4, CH | 3.84, dd (4.4, 12.1) | 78.5, CH | 4.54, d (5.9) |
| 12 | 39.3, CH2 | 2.32, m | 37.4, CH2 | 2.30, m | 44.1, CH2 | 2.45, dd (5.9, 10.7) |
| 2.06, m | 2.03, m | 2.02, d (10.7) | ||||
| 13 | 68.6, CH | 4.41, dd (5.0, 11.4) | 66.3, CH | 4.48, dd (5.1, 9.1) | 81.8, C | |
| 14 | 201.2, C | 198.7, C | 198.8, C | |||
| 2′ | 19.1, CH3 | 1.69, s | 19.0, CH3 | 1.69, s | 19.1, CH3 | 1.64, s |
| 6′ | 23.5, CH3 | 1.34, s | 23.0, CH3 | 1.34, s | 23.3, CH3 | 1.31, s |
| OCH3 | 58.4, CH3 | 3.50, s | ||||
| OCH2 | 70.7, CH2 | 3.80, d (8.3) | ||||
| 3.48, d (8.3) | ||||||
| –OH | 3.39, s | 3.39, s | 4.22, s | |||
Additionally, the NMR data for compound 1 were similar to those reported for a known analog, ACTG-toxin E, previously isolated from the cultures of Alternaria citri and Alternaria alternatα.11,18 These species of filamentous Ascomycota are taxonomically positioned in a distinct phylum relative to the endophyte examined in the present study (phylum Basidiomycota).
Coibanol B (2) gave a pseudomolecular ion peak [M+Na]+ at m/z 315.1569 by HR ESIFTMS, consistent with a molecular formula of C17H24O4, and having 14 amu more than compound 1. The 1H and 13C NMR data (Table 1) of compound 2 closely resembled those of coibanol A (1), and in addition, contained a OMe resonance (δH 3.50) and corresponding geminal proton at 3.84 ppm. The 13C NMR spectrum, in comparison with 1, showed an extra signal at 58.4 ppm, characteristic of a methoxy group. Further analysis of the 2D NMR data, including COSY, HSQC, and HMBC, placed the methoxy moiety at C-11. The relative configuration of 2 was confirmed by NOESY and shown to be identical to 1.
Coibanol C (3) had a molecular formula of C17H22O4 based on its HREIMS (m/z 290.1513 [M+]), which is 2 amu less than compound 2. The 1H NMR spectrum for 3 showed several differences relative to compounds 1 and 2. In compound 3, H-11 appeared as a very sharp doublet at 4.54 ppm (J = 5.9 Hz). This suggested that H-11 formed a dihedral angle with the pseudoequatorial proton at C-12 (δHa 2.02). H-12b appear at δH 2.45 as a double doublet (J = 5.9 and 10.3). Moreover, the 1H NMR spectrum showed an AB spin system at δH 3.80 and δH 3.48 for an oxymethylene group, and this was confirmed by 13C-NMR which showed a methylene carbon signal at δc 70.7. Finally, the presence of a tetrahydrofuran ring in 3 was established by HMBC which demonstrated that the two protons of the oxymethylene moiety were correlated with C-11, C-12, C-13, and C-14 (Fig. 2). These interactions confirmed the existence and nature of a fourth ring in compound 3.
Figure 2.
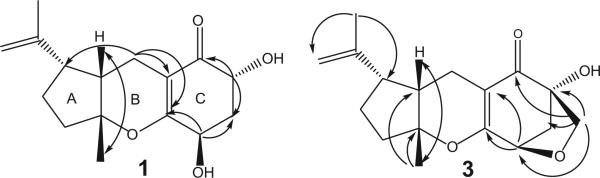
Principal NOESY (double-headed arrows) and HMBC (single-headed arrows) correlations for compounds 1 and 3.
In conclusion, bioprospecting in tropical areas still subsist today in intact regions like Coiba National Park in Panama, gives the opportunity to find new chemical entities and effective molecules to treat actual, emerging and new diseases. Examples are Coibanols A–C, a new class of meroterpenoids with an isopreinoid unit less then the TCA series. Coibanol C has an additional tetrahydrofuran ring.
Fungal material
Fungal endophyte F0305 was isolated from a fresh, apparently disease free, mature leaf of Desmotes incomparabilis (Rutaceae) which had been collected from a tropical wet forest on Coiba Island in the Coiba National Park, Veraguas, Panamá (7° 30′ 0″N/81° 51′ 0″W). A plant voucher is deposited at the University of Panamá Herbarium. As part of a larger survey of endophytic fungi, healthy leaves were rinsed in running water and cut into 1 × 2 mm pieces, which were surface sterilized following Arnold et al.19 Each piece was placed on 2% malt extract agar (MEA) and incubated on the bench top at room temperature for up to 3 weeks. The plates were examined every day for emerging hyphal tips; when observed, these were transferred to plates of 2% MEA and repeatedly streaked on fresh medium until pure cultures were obtained. Total genomic DNA was extracted from fresh mycelium following Arnold and Lutzoni.20 The nuclear ribosomal internal transcribed spacers and 5.8S gene (nrITS) and ca. 600 base pairs of the adjacent nuclear ribosomal large subunit (LSU) were amplified as a single fragment and sequenced bidirectionally at the Genomics and Technology Core at the University of Arizona using primers ITS1F and LR3.21 Forward and reverse reads were assembled and evaluated by phred and phrap22,23 with automation provided by Mesquite24 and manual editing in Sequencher v4.5 (Gene Codes Corporation, Ann Arbor, MI). The consensus sequence was submitted to a BLAST search of the NCBI GenBank database for preliminary identification at higher taxonomic levels and to establish appropriate taxon sampling for phylogenetic analysis. The top BLAST matches were to species of Trametes, with high coverage and percent identity values. However, subsequent phylogenetic analyses, which incorporated 70 sequences representing Trametes and Pycnoporus (Basidiomycota, Polyporaceae) and were conducted using a maximum likelihood approach in GARLI 0.9525 with the model GTR + I + G inferred in ModelTest26, placed F0305 within Pycnoporus with high bootstrap support. Within Pycnoporus F0305 was nested the monophyletic and well-represented P. sanguineus species, again with high statistical support (data not shown). Isolate F0305, therefore identified as P. sanguineus, was stored on slants of 2% MEA and on agar pellets suspended in sterile water. Vouchers are deposited at the ICBG Culture Collection at the Smithsonian Tropical Research Institute, Panamá, and at the Robert L. Gilbertson Mycological Herbarium at the University of Arizona (ARIZ, under TK1804/F0305). Isolate F0305 was maintained on malt extract agar at 25 °C.20 The fungal strain was cultured on 15 Petri dishes containing malt extract agar at 25 °C for 10 days. Agar plugs were used to inoculate 12 1L Erlenmeyer flasks (one dish per flask) containing 0.6 L of malt extract media (Shaarlau Chemie). Flasks were incubated at 25 °C on a rotating shaker at 170 rpm for 15 days.
Extraction and isolation
Fungal cells and broth were separated by filtration and the filtrate was extracted exhaustively with EtOAc. The organic solvent was evaporated to dryness under vacuum to afford the crude extract (213 mg). The extract was subjected to Sephadex LH-20 column chromatography (1.5 × 19 cm), and eluted with MeOH to yield 37 fractions. The resulting fractions were combined, based upon their TLC profiles, into five fractions (A1–A5). Fraction A2 (132.7 mg) was subjected to semi-preparative HPLC (Nova–Pak C18, 4 micron, 4.6 × 250 mm; MeCN–H2O (1:1); flow rate 1.2 mL/min) to furnish 1 (6.9 mg, tR 8.3 min); 2 (9.6 mg, tR 10.6 min), and 3 (5.3 mg, tR 28 min).
Coibanol A (1)
Colorless oil; [α]25D +24.0 (c 0.025, acetone); IR (film) υmax 3413, 2925, 2856, 1619, 1387, 1260 cm –1; 1H and 13C NMR data, see Table 1; HR EIMS m/z M+ 278.1515 (calcd for C16H22O4, 278.1513).
Coibanol B (2)
Colorless powder; [α]25D +95.6 (c 0.045, acetone); IR (film) υmax 3435, 2928, 1658, 1448, 1162, 1022 cm –1; 1H and 13C NMR data, see Table 1; HR ESIFTMS m/z [M+Na]+ 315.1569 (calcd for C17H24NaO4, 315.1567).
Coibanol C (3)
Colorless oil; [α]25D +65.6 (c 0.09, acetone); IR (film) υmax 3435, 2927, 1656, 1618, 1380, 1025 cm –1; 1H and 13C NMR data, see Table 1; HREIMS m/z M+ 290.1513 (calcd for C17H22O4, 290.1513).
Acknowledgments
This work was supported by US NIH Grant for the International Cooperative Biodiversity Groups program (ICBG–Panama; 2 U01 TW006634-06). We thank M. Gunatilaka and D. Mahana for assistance with DNA sequencing, and the College of Agriculture and Life Sciences at the University of Arizona for logistical and financial support. We also give our thanks to the personnel of Panamá's Autoridad Nacional del Ambiente for their assistance in conducting this research project.
References and notes
- 1.Strobel GA. Crit. Rev. Biotechnol. 2002;22:315–333. doi: 10.1080/07388550290789531. [DOI] [PubMed] [Google Scholar]
- 2.Strobel GA, Long D. ASM News. 1998;64:263–268. [Google Scholar]
- 3.De Borges W, Borges KB, Bonato PS, Said S, Pupo MT. Curr. Org. Chem. 2009;13:1137–1163. [Google Scholar]
- 4.Tan RX, Zou W. Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HW, Song YC, Tan RX. Nat. Prod. Rep. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 6.Kursar TA, Caballero-George CG, Capson TL, Cubilla-Rios L, Gerwick WH, Gupta MP, Ibanez A, Linington RG, McPhail KL, Ortega-Barria E, Romero LI, Solis PN, Coley PD. Bioscience. 2006;56:1005–1012. [Google Scholar]
- 7.Kursar TA, Caballero-George CC, Capson TL, Cubilla-Rios L, Gerwick WH, Heller MV, Ibanez A, Linington RG, McPhail KL, Ortega-Barria E, Romero LI, Coley PD. Biodivers. Conserv. 2007;16:2789–2800. [Google Scholar]
- 8.Jimenez-Romero C, Ortega-Barria E, Arnold AE, Cubilla-Rios L. Pharm. Biol. 2008;46:700–703. [Google Scholar]
- 9.Martinez-Luis S, Della-Tonga G, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios LJ. Nat. Prod. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallunki JA. Brittonia. 1992;44:107–139. [Google Scholar]
- 11.Liebermann B, Ellinger R, Günther W, Ihn W, Gallander H. Phytochemistry. 1997;46:297–303. [Google Scholar]
- 12.Sugawara F, Uzawa J, Esumi Y, Suzuki M, Yoshida S, Strobel G, Ros Steiner JL, Clardy J. Biosci., Biotechnol., Biochem. 1998;62:638–642. doi: 10.1271/bbb.62.638. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum RP, Gunther W, Heinze S, Liebermann B. Phytochemistry. 1999;52:593–599. [Google Scholar]
- 14.Bernd L, Ralph-Peter N, Wolfgang G. Phytochemistry. 2000;55:987–992. [Google Scholar]
- 15.Liebermann B, Nussbaum R-P, Günther W, Teuscher J-M. Phytochemistry. 2001;56:551–557. doi: 10.1016/s0031-9422(00)00459-3. [DOI] [PubMed] [Google Scholar]
- 16.Li-Rui Q, Lin Y, Jin-Ming G, Pei-Ji Z, Qian-Jin K, Yue-Mao SJ. Basic Microb. 2007;47:340–343. doi: 10.1002/jobm.200610306. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Pei-Ji Z, Juan M, Guo-Hong L, Yue-Mao S. Helv. Chim. Acta. 2008;91:1588–1594. [Google Scholar]
- 18.Kono Y, Gardner JM, Suzuki Y, Kondo H, Takeuchi SJ. Pestic. Sci. 1989;14:223–228. [Google Scholar]
- 19.Arnold AE, Lutzoni F. Ecology. 2007;88:541–549. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- 20.Arnold EA, Maynard Z, Gilbert GS, Coley PD, Kursar TA. Ecol. Lett. 2000;3:267–274. [Google Scholar]
- 21.Higgins KL, Coley PD, Kursar TA, Arnold AE. Mycologia. 2011;103:247. doi: 10.3852/09-158. [DOI] [PubMed] [Google Scholar]
- 22.Ewing B, Green P. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 23.Ewing B, Hillier L, Wendl M, Green P. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 24.Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2007 Available at http://mesquiteproject.org.
- 25.Zwickl DJ. Ph.D. dissertation. The University of Texas at Austin; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. [Google Scholar]
- 26.Posada D, Crandall KA. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]


