Abstract
We previously identified the intracellular nicotinamide phosphoribosyltransferase (iNAMPT, aka pre–B-cell colony enhancing factor) as a candidate gene promoting acute respiratory distress syndrome (ARDS) and ventilator-induced lung injury (VILI) with circulating nicotinamide phosphoribosyltransferase potently inducing NF-κB signaling in lung endothelium. iNAMPT also synthesizes intracellular nicotinamide adenine dinucleotide (iNAD) in response to extracellular oxidative stress, contributing to the inhibition of apoptosis via ill-defined mechanisms. We now further define the role of iNAMPT activity in the pathogenesis of ARDS/VILI using the selective iNAMPT inhibitor FK-866. C57/B6 mice were exposed to VILI (40 ml/kg, 4 h) or LPS (1.5 mg/kg, 18 h) after osmotic pump delivery of FK-866 (100 mg/kg/d, intraperitoneally). Assessment of total bronchoalveolar lavage (BAL) protein, polymorphonuclear neutrophil (PMN) levels, cytokine levels (TNF-α, IL-6, IL-1α), lung iNAD levels, and injury scores revealed that FK-866–mediated iNAMPT inhibition successfully reduced lung tissue iNAD levels, BAL injury indices, inflammatory cell infiltration, and lung injury scores in LPS- and VILI-exposed mice. FK-866 further increased lung PMN apoptosis, as reflected by caspase-3 activation in BAL PMNs. These findings support iNAMPT inhibition via FK-866 as a novel therapeutic agent for ARDS via enhanced apoptosis in inflammatory PMNs.
Keywords: apoptosis, FK-866, nicotinamide phosphoribosyltransferase, polymorphonuclear neutrophil, vascular endothelium
Clinical Relevance
We previously identified NAMPT as a candidate gene promoting both acute respiratory distress syndrome (ARDS) and ventilator–induced lung injury (VILI). We now further define the role of intracellular nicotinamide phosphoribosyltransferase (NAMPT) activity in the pathogenesis of ARDS/VILI using the selective intracellular NAMPT inhibitor FK-866. These findings support intracellular NAMPT inhibition via FK-866 as a novel therapeutic agent for ARDS via enhanced apoptosis in inflammatory polymorphonuclear neutrophils.
Acute respiratory distress syndrome (ARDS) is a devastating inflammatory lung syndrome characterized by diffuse alveolar infiltration, hypoxemia, and respiratory failure that develops in response to a variety of local and systemic insults (e.g., sepsis, pneumonia, and trauma). Hallmarks of ARDS include profound inflammation, deranged alveolar capillary permeability, leukocyte extravasation, spatial heterogeneity, and lung edema, which contribute to multiorgan dysfunction and increased mortality. The exposure to mechanical stress via mechanical ventilation, a supportive intervention strategy for severe respiratory failure, also contributes to multiorgan dysfunction and ARDS mortality.
We and others have previously demonstrated that the circulating cytozyme pre–B-cell colony enhancing factor (NAMPT) is a biomarker in sepsis and sepsis-induced ARDS with genetic variants conferring ARDS susceptibility (1–3). Gene and protein expression of NAMPT/pre–B-cell colony enhancing factor (PBEF) are consistently elevated in bronchoalveolar lavage (BAL) fluid and in serum samples from critically ill intensive care unit patients with ARDS and sepsis versus control subjects. Elevated levels of circulating NAMPT are associated with diabetes (4), atherosclerosis (5), cardiac hypertrophy (6), and sepsis-induced ARDS (3). NAMPT is a mediator of innate immunity (7) and, as we have detailed, is a potent extracellular proinflammatory inducer of the NF-κB pathway and an attractive target for therapeutic neutralization (8). NAMPT is an intracellular nicotinamide phosphoribosyltransferase (iNAMPT) that is the rate-limiting enzymatic step in the salvage pathway, which synthesizes intracellular nicotinamide adenine dinucleotide (iNAD) from nicotinamide, an intracellular function that reduces oxidant stress and inhibits apoptosis (7).
FK-866 is a noncompetitive intracellular iNAMPT inhibitor, currently in phase II clinical trials as an anticancer agent for solid and advanced tumors (9), presumably via reduced intracellular nicotinamide adenine dinucleotide (NAD) production and increased apoptosis of malignant cells (10, 11). FK-866 has been tested in spinal cord injury and experimental hepatitis in in vitro and in vivo models. FK-866 administration results in iNAD depletion and reduced proinflammatory cytokine expression (12, 13). Although the extracellular proinflammatory effects of NAMPT/PBEF are well studied, little is known regarding how intracellular iNAMPT enzymatic activity contributes to ARDS pathogenesis.
This study further explores the role of iNAMPT in preclinical models of ARDS and addresses the hypothesis that iNAMPT activity extends polymorphonuclear neutrophil (PMN) survival, thereby exaggerating lung injury. Our results, using a variety of complementary molecular, pharmacologic, inhibitory, and genetic approaches in two murine models of ARDS (LPS and high tidal volume ventilation-induced lung injury [VILI]), indicate substantial FK-866–mediated protection against ARDS, likely via augmented PMN apoptosis, warranting consideration as a novel ARDS therapeutic agent.
Materials and Methods
Drug and Reagent Sources
FK-866 was purchased from Cayman Chemical (Ann Arbor, MI), NAMPT/PBEF ELISA kits were purchased from MBL International (Woburn, MA), Bio-Plex cytokine assays were purchased from Bio-Rad (Hercules, CA), and NAD assay kits were obtained from BioVision (Milpitas, CA).
Animal Housing and Procedures
All animal protocols were approved by the University of Illinois Chicago Animal Care Committee and met the Institutional guidelines for the use of animals for research. These mice were housed in an environmentally controlled animal facility (BRL Barrier Animal Facility) at the University of Chicago with free access to food and water.
Preclinical Models of ARDS
C57/B6 mice were exposed to LPS and VILI models of lung injury as previously described (8, 14, 15). C57/B6 mice received FK-866 (100 mg/kg/24 h) or saline via osmotic pumps (Alzet, Cupertino, CA) (intraperitoneally) for 4 hours beginning at the initiation of mechanical ventilation in VILI-challenged mice (high tidal volume mechanical ventilation, 40 ml/kg) or for 18 hours beginning at the time of LPS instillation (1.5 mg/kg intratracheally).
BAL Assessment
Lungs were lavaged with 1 ml cold Hanks’ balanced salt solution, and supernatants were used for protein levels as previously described (16). Cell pellets were examined for total number of white blood cells, counted with a hemacytometer and cell differential analyses using cytocentrifugation, and double stained with caspase 3 cleavage and Diff-Quik staining. The BAL fluid was used to measure total protein according to the manufacturer’s manual (BCA Protein Assay Kit; Bio-Rad).
BAL Cytokine Assays
Bio-Plex cytokine assays were used to measure levels of TNF-α, IL-6, and IL-1β in mouse lung BAL fluids, assayed according to the manufacturer’s recommended protocol (Bio-Rad) and as previously described (15). Quantification of NAMPT/PBEF levels was performed on mouse plasma samples using an ELISA kit (AdipoGen Corp., San Diego, CA) following the manufacturer’s instructions as previously described (1).
NAD Assessment
Quantification of iNAD contents in murine lung tissues (extracted with 0.5 M perchloric acid using a quantification kit [BioVision, Milpitas CA]) was assayed according to the method described previously (17).
Lung Histology and Immunochemistry
Lungs were harvested and fixed in formalin for histological evaluation by hematoxylin and eosin using Genie TM machine learning recognition software (Aperio, Vista, CA), quantifying (1) percent of lung tissue area damaged, (2) cell density in the damaged area, or (3) combined metric HScore, represented as injured area × cell density. In separate studies, lungs sections were stained for active caspase 3 with anticleaved caspase-3 antibody (1:200) (Cell Signaling, Danvers, MA). Quantification was performed using Genie and the Nuclear Algorithm (Aperio) software to score nuclear density in the alveolar compartment as described previously, and data were expressed as number of caspase 3–active positive cells/mm2 (18, 19).
Statistical Analysis
Except where noted, results were analyzed using standard one-way ANOVA. Groups were compared using the Newman-Keuls test. Lung injury scores group comparison was done with Tukey post hoc test analysis. Differences between groups were considered statistically significant at P < 0.05. Results are expressed as mean ± SEM.
Results
Inhibition of Intracellular NAMPT Ameliorates LPS- and Ventilator-Induced Murine Lung Injury
We assessed the effects of the iNAMPT inhibitor FK-866 in mice exposed to high tidal volume ventilation (VILI, 40 ml/kg, 4 h) or to endotoxin (LPS, 1.5 mg/kg, 18 h). When compared with VILI-exposed control mice, VILI mice receiving FK-866 demonstrated significantly decreased BAL protein concentrations, total BAL cell counts, and percent BAL PMNs and lower BAL levels of IL-6, TNF-α, and IL-1β (Figure 1).
Figure 1.
FK-866 (FK) reduces ventilator-induced murine lung injury. FK-866 administration attenuated increases in bronchoalveolar lavage (BAL) protein concentration (A), the number of total BAL cells (B), and total BAL polymorphonuclear neutrophils (PMNs) (C) in addition to concentrations of IL-6 (D), TNF-α (E), and IL-1β (F) in ventilator-induced lung injury (VILI)-challenged mice. *P < 0.05 compared to VILI only group. CTRL, control.
Similarly, FK-866 attenuated LPS-induced increases in BAL protein, total BAL cell counts, BAL PMN percentage, and BAL IL-6, TNF-α, and IL-1β (Figure 2) when compared with LPS-challenged mice. FK-866 alone did not increase BAL protein levels or cell counts but appeared to slightly increase cytokine levels. KC and IL-10 cytokines were measured in BAL but were not significantly altered (data not shown).
Figure 2.
FK-866 reduces LPS-induced murine lung injury. FK-866 administration attenuated increases in BAL protein concentration (A), BAL cellularity (B), PMN percentage (C), and concentrations of IL-6 (D), TNF-α (E), and IL-1β (F) in LPS-challenged mice. *P < 0.05 compared to VILI only group.
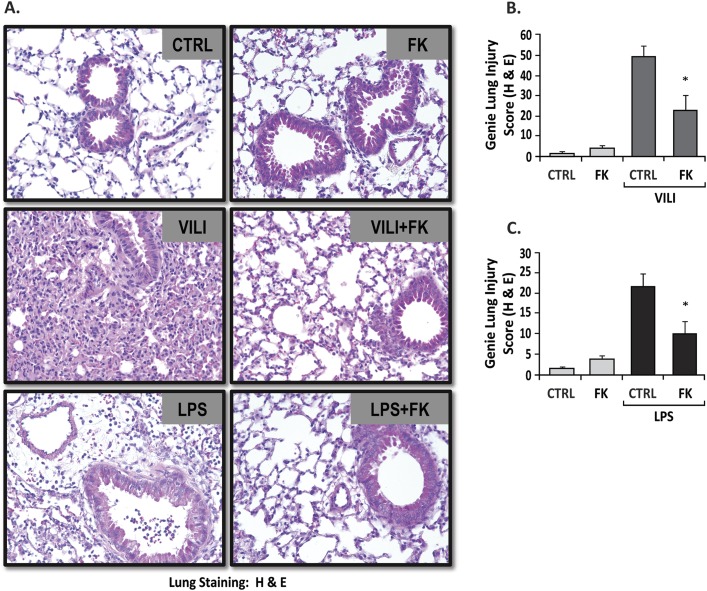
The degree of parenchymal lung injury was assessed in hematoxylin and eosin–stained lung sections (Figure 3A). VILI or LPS challenges induced significant increases in inflammatory cell infiltration compared with control groups (Figure 3A, upper panel). FK-866 significantly decreased inflammatory cell infiltration and lung injury scores in the VILI (Figure 3B) and LPS models (Figure 3C).
Figure 3.
FK-866 decreases inflammatory cell infiltration and lung injury scores in VILI- and LPS-challenged mice. Representative hematoxylin and eosin images (A) and bar graph quantifications (B and C) demonstrate VILI- or LPS-induced significant increases in lung injury compared with spontaneous breathing or vehicle-treated control mice (P < 0.001). When compared with VILI (B) or LPS (C) alone, treatment with FK-866 reduces parenchymal inflammatory cell infiltration and lung injury scores in VILI-challenged (B) and LPS-challenged (C) mice (P < 0.05). Infusion of FK-866 did not produce increased inflammation in the lungs. Genie lung injury score was defined as Percent Alveoli Damage × Cell Density. *P < 0.05 compared to VILI only group.
Inhibition of Intracellular NAMPT Induces Neutrophil Apoptosis
To explore the potential mechanisms for the beneficial effects of FK-866 in LPS and VILI preclinical models of lung injury, we assessed the apoptotic activity of cells infiltrating the lung parenchyma (Figure 4A). The number of cleaved caspase-3–positive PMNs (expressed as fold change) was considerably increased in VILI-exposed mice and in VILI mice treated with FK-866 (Figure 4B). In LPS-challenged mice, there was evidence of increased caspase-3 staining when compared with control mice, with this effect being more pronounced in LPS/FK-866–treated mice compared with LPS-challenged mice (Figure 4C).
Figure 4.
Inhibition of intracellular NAMPT with FK-866 induces neutrophil apoptosis. Representative lung sections from control or FK-866 groups, VILI- and VILI/FK-866–challenged mice, and LPS- and LPS/FK-866–challenged mice (A), all stained with a caspase-3 antibody, demonstrate that FK-866 induces PMN apoptosis. Bar graphs represent caspase-3 staining quantification in VILI-challenged (B) and LPS-challenged (C) mice using Genie software (P < 0.05; n = 3–4). s = data were expressed as number of apoptotic cells/mm2. *P < 0.05 between two selected groups.
Intracellular NAMPT Enzymatic Activity Increases NAD Levels in Cells
NAD content was quantified in lung extracts to examine the role of iNAD in ARDS. iNAD levels significantly decreased in FK-866–treated mice compared with vehicle-treated control mice (0.46 ± 0.05 versus 1.0 ± 0.18; P = 0.04) (Figures 5A and 5B) and compared with control hepatic tissues, confirming iNAMPT inhibition by FK-866. Intracellular NAD levels were modestly reduced in VILI-challenged mice but remained significantly higher than the VILI/FK-866 group (Figure 5A). LPS-challenged mice exhibited higher iNAD levels than VILI mice (Figures 5A and 5B), with iNAD content elevations abrogated by FK-866 (Figure 5B).
Figure 5.
Effect of FK-866 on intracellular nicotinamide adenine dinucleotide (iNAD) and intracellular nicotinamide phosphoribosyltransferase (iNAMPT) levels in LPS- and VILI-challenged mice. FK-866 decreases iNAD levels with or without VILI or LPS challenge (A and B). NAMPT/pre–B-cell colony enhancing factor (PBEF) levels were also assessed in blood, with FK-886 treatment decreasing plasma NAMPT/PBEF levels only in control mice (noninjury control group) (C). Values denote mean ± SEM for three to five mice in each group. *P < 0.05 between two selected groups.
Assessment of plasma NAMPT/PBEF levels in VILI and LPS samples (Figure 5C) revealed consistent elevations in NAMPT/PBEF protein content when compared with control mice. Considerable reductions in NAMPT/PBEF levels were observed in FK-866–treated samples and in LPS/FK-866–treated samples compared with control mice. In VILI/FK-866–treated mice, no changes in extracellular NAMPT/PBEF levels were observed when compared with VILI control mice.
Discussion
NAMPT is a candidate gene in human sepsis and ALI and encodes NAMPT/PBEF, which functions as an ALI biomarker in BAL and blood (1–3). Extracellular NAMPT/PBEF is a mediator of innate immunity (7) via potent activation of the NF-κB pathway and is an attractive target for therapeutic neutralization (8). iNAMPT is the rate-limiting enzymatic step in the salvage pathway that synthesizes iNAD from nicotinamide and serves to reduce oxidant stress and inhibit apoptosis (7). The roles of NAMPT/PBEF and iNAMPT, as well as iNAMPT-generated intracellular and extracellular NAD, in inflammatory lung injury are complex. Our prior studies demonstrated that extracellular NAMPT/PBEF is a PMN chemotactic factor and increases the release of PMN chemoattractants, producing a robust PMN alveolitis with lung vascular and alveolar permeability when administered in vivo via an intratracheal route. Consistent with the role of extracellular NAMPT/PBEF as an inflammatory mediator, heterozygous NAMPT/PBEF+/− mice were significantly protected from exposure to a model of severe VILI (reduced BAL protein and IL-6 levels, peak inspiratory pressures), and strategies to reduce extracellular NAMPT/PBEF availability (neutralizing antibody) resulted in significantly reduced VILI-mediated lung injury. In the current report, we have addressed the complex role of iNAMPT enzymatic activity on lung iNAD levels and lung injury in two well-established preclinical models of murine ARDS.
This study demonstrated that systemic inhibition of iNAMPT enzymatic activity (FK-866) attenuates LPS- and VILI-induced pulmonary inflammatory cell influx, vascular barrier dysfunction, inflammatory cytokine expression, and parenchymal injury. Measurements of intracellular lung NAD levels in LPS- or VILI-challenged mice confirmed reductions in NAD levels by FK-866, a noncompetitive iNAMPT inhibitor, currently in phase II clinical trials as an anticancer therapy (9). The protective effects of FK-866 in ARDS and VILI are related, at least in part, to reductions in iNAD production and induction of neutrophil apoptosis, as evidenced by increases in caspase-3–positive BAL neutrophils in mice treated with FK-866 (Figure 4). These findings are consistent with apoptosis inhibition by iNAMPT enzymatic activity and increased iNAD, leading to prolonged inflammatory cell survival. iNAMPT is overexpressed in circulating neutrophils from critically ill patients with sepsis, resulting in profoundly delayed rates of PMN apoptosis (20). Silencing of iNAMPT expression completely abrogates the inhibitory effects of LPS, IL-1, GM-CSF, IL-8, and TNF-α on PMN apoptosis (20). iNAMPT inhibition and reductions in iNAD also attenuate inflammatory responses in murine models of myocardial infarction (21), inflammatory arthritis (22), and sepsis (23). In contrast to the effects of VILI and LPS on PBEF/NAMPT expression activation, VILI or LPS can induce iNAD reduction (Figure 5), which potentially mediates apoptosis alone or with VILI/LPS challenge (Figure 4). The differential regulation of PBEF/NAMPT and iNAD is not fully understood, possibly due to other mechanisms besides iNAMPT. Although neutrophil apoptosis occurs with these inflammatory challenges, it cannot overcome the drastic infiltration into the bronchoalveolar area from blood stream with accumulation of PMN in the lung. However, with bronchoalveolar neutrophilia induced by these stimuli, FK-866 accelerates apoptosis to potently reduce neutrophilia.
Although iNAMPT and iNAMPT-generated iNAD are associated with reduced caspase-8 and caspase-3 activities (20), the mechanism by which iNAMPT and iNAD function as inhibitors of apoptosis induced by inflammatory stimuli is poorly understood but may involve the MAPK-PI3–kinase pathway (24, 25) as well as increased SIRT-1 activity leading to PARP overexpression and increased PARP activities (26). These findings indicate that iNAMPT promotes endogenous prosurvival factors.
Taken together, these data suggest that iNAMPT inhibition serves as a novel, effective therapy for ARDS. We speculate that in response to acute inflammatory stimuli, iNAMPT-derived iNAD exerts a predominantly proinflammatory effect by promoting neutrophil survival. These data suggest that, in addition to strategies to neutralize extracellular NAMPT/PBEF, tissue-specific inhibition of iNAMPT enzymatic activity represents potential ARDS and VILI therapeutic strategies.
Acknowledgments
Acknowledgments
The authors thank Lakshmi Natarajan for her expert technical assistance with the endothelial cell studies.
Footnotes
This study was supported by National Heart, Lung and Blood Institute grant HL94394 (J.G.N.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0519OC on March 3, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T, et al. Pre-b-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 2.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-b-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med. 2007;35:1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 3.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL, Verin AD, Natarajan V, Garcia JG. Pre-b-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res. 2005;70:142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, Rappl G, Abken H, Hahn M, Schulz O, et al. Visfatin/pbef/nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm Metab Res. 2010;42:268–273. doi: 10.1055/s-0029-1243638. [DOI] [PubMed] [Google Scholar]
- 5.Filippatos TD, Randeva HS, Derdemezis CS, Elisaf MS, Mikhailidis DP. Visfatin/pbef and atherosclerosis-related diseases. Curr Vasc Pharmacol. 2010;8:12–28. doi: 10.2174/157016110790226679. [DOI] [PubMed] [Google Scholar]
- 6.Pillai VB, Sundaresan NR, Kim GH, Samant S, Moreno-Vinasco L, Garcia JG, Gupta M. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304:H415–H426. doi: 10.1152/ajpheart.00468.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luk T, Malam Z, Marshall JC. Pre-b cell colony-enhancing factor (pbef)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 8.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma SF, Mirzapoiazova T, Evenoski C, Reeves RR, et al. Essential role of pre-b-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holen K, Saltz LB, Hollywood E, Burk K, Hanauske AR. The pharmacokinetics, toxicities, and biologic effects of fk866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest New Drugs. 2008;26:45–51. doi: 10.1007/s10637-007-9083-2. [DOI] [PubMed] [Google Scholar]
- 10.Pogrebniak A, Schemainda I, Azzam K, Pelka-Fleischer R, Nussler V, Hasmann M. Chemopotentiating effects of a novel nad biosynthesis inhibitor, fk866, in combination with antineoplastic agents. Eur J Med Res. 2006;11:313–321. [PubMed] [Google Scholar]
- 11.Hasmann M, Schemainda I. Fk866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 12.Esposito E, Impellizzeri D, Mazzon E, Fakhfouri G, Rahimian R, Travelli C, Tron GC, Genazzani AA, Cuzzocrea S. The nampt inhibitor fk866 reverts the damage in spinal cord injury. J Neuroinflammation. 2012;9:66. doi: 10.1186/1742-2094-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moschen AR, Gerner R, Schroll A, Fritz T, Kaser A, Tilg H. A key role for pre-b cell colony-enhancing factor in experimental hepatitis. Hepatology. 2011;54:675–686. doi: 10.1002/hep.24416. [DOI] [PubMed] [Google Scholar]
- 14.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 15.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer NJ, Huang Y, Singleton PA, Sammani S, Moitra J, Evenoski CL, Husain AN, Mitra S, Moreno-Vinasco L, Jacobson JR, et al. Gadd45a is a novel candidate gene in inflammatory lung injury via influences on akt signaling. FASEB J. 2009;23:1325–1337. doi: 10.1096/fj.08-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous nad blocks cardiac hypertrophic response via activation of the sirt3-lkb1-amp-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klapczynski M, Gagne GD, Morgan SJ, Larson KJ, Leroy BE, Blomme EA, Cox BF, Shek EW. Computer-assisted imaging algorithms facilitate histomorphometric quantification of kidney damage in rodent renal failure models. J Pathol Inform. 2012;3:20. doi: 10.4103/2153-3539.95456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekontso Dessap A, Voiriot G, Zhou T, Marcos E, Dudek SM, Jacobson JR, Machado R, Adnot S, Brochard L, Maitre B, et al. Conflicting physiological and genomic cardiopulmonary effects of recruitment maneuvers in murine acute lung injury. Am J Respir Cell Mol Biol. 2012;46:541–550. doi: 10.1165/rcmb.2011-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-b cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco F, Bauer I, Braunersreuther V, Bruzzone S, Akhmedov A, Luscher TF, Speer T, Poggi A, Mannino E, Pelli G, et al. Inhibition of nicotinamide phosphoribosyltransferase reduces neutrophil-mediated injury in myocardial infarction. Antioxid Redox Signal. 2013;18:630–641. doi: 10.1089/ars.2011.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, Leo O, So A, De Smedt T. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to nad. PLoS ONE. 2008;3:e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Gool F, Galli M, Gueydan C, Kruys V, Prevot PP, Bedalov A, Mostoslavsky R, Alt FW, De Smedt T, Leo O. Intracellular nad levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl TB, Haukeland JW, Yndestad A, Ranheim T, Gladhaug IP, Damas JK, Haaland T, Loberg EM, Arntsen B, Birkeland K, et al. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:3039–3047. doi: 10.1210/jc.2009-2148. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q, Dong W, Qian L, Wu J, Peng Y. Visfatin inhibits apoptosis of pancreatic beta-cell line, min6, via the mitogen-activated protein kinase/phosphoinositide 3-kinase pathway. J Mol Endocrinol. 2011;47:13–21. doi: 10.1530/JME-10-0106. [DOI] [PubMed] [Google Scholar]
- 26.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(adp-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by nad+ depletion and reduced sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]