Abstract
Sonic hedgehog (Shh) is expressed and secreted from the embryonic lung epithelium and acts on the adjacent mesenchymal cells via its receptor Patched (Ptch)/Smoothened (Smo) and transcriptional effectors Gli proteins. Genetic studies showed that the Shh pathway plays critical roles in mouse lung development. However, little is known about microRNAs (miRNAs) downstream of Shh in embryonic lungs. Here we profiled miRNAs in embryonic lung cultures treated with cyclopamine, a specific Smo antagonist or with Smo agonist by next-generation of sequencing. We then performed functional screening to examine whether some of these miRNAs can modulate the induction of Gli-responsive luciferase by Shh treatment. These analyses revealed that expression of miR-326 and its host gene, Arrestin β1, is selectively enriched in embryonic lung mesenchymal cells and is specifically influenced by Shh activity. Furthermore, functional analyses showed that miR-326 acts as a negative modulator for Shh signaling by directly targeting Smo and Gli2. Together, these findings suggest a novel miR-326–negative feedback loop in regulating the activity of Shh signaling.
Keywords: miR-326, Arrestin β1, Sonic hedgehog, Gli2, the embryonic lung mesenchyme
Clinical Relevance
Sonic hedgehog (Shh) pathway plays critical roles in development and diseases. Here we identified that miR-326 is downstream of Shh and negatively regulates Shh activities by targeting Gli2 and Smoothened. These Results significantly extend our understanding the regulation of Shh pathway by microRNAs.
MicroRNAs (miRNAs) are endogenous, small, noncoding RNAs that regulate target gene expression post-transcriptionally and play important roles in diverse biological processes, including development, metabolism, cell differentiation, and proliferation (1–3). Studies have revealed a large number of miRNAs in the mammalian genome, which may target over 80% of protein-coding mRNAs (4). According to their genomic location, miRNAs can be classified into intergenic and intragenic miRNAs, and the latter include intronic and exonic miRNAs (5). Intronic miRNAs reside in the introns of protein-coding genes, called “host genes,” and account for approximately 37% of miRNAs in mammals (6). Recent studies showed that the transcription of the majority of intronic miRNAs is co-regulated with their host genes (7, 8).
Sonic hedgehog (Shh) is a secreted signal molecule and is a key regulator of patterning of multiple organs, including limb, neuronal tube, and lungs, during embryonic development (9–13). Binding of the Shh ligand with its receptor Patched (Ptch) leads to the dissociation of Ptch from Smoothened (Smo). This is followed by β-Arrestin–mediated translocation of Smo to the primary cilia, where Gli2/3 are activated by post-translational modifications (14, 15). In embryonic lungs, Shh is expressed in the distal epithelial cells. Shh signaling is activated in a paracrine fashion via its receptor Ptch, which is preferentially expressed in adjacent lung mesenchyme (16–18). Knockout of Shh results in dramatic impairment of lung branching, together with defects in the development of smooth muscle and vasculature (16, 19, 20). Inactivation of Gli1 or Gli3 led only to mild defects in the lung, whereas the Gli2 knockout mouse exhibited a severe phenotype with branching defects, similar to those of Shh-null lungs. This suggests that Gli2 is the primary mediator of Shh signaling during lung development (13, 21, 22). The temporal and spatial activities of Shh signaling is partially achieved by the complex positive and negative feedback mechanisms (23). For example, expression of Ptch and Hedgehog-interacting protein 1 is up-regulated by Shh activity; however, both act as negative feedback regulators through association with Smo and Shh, respectively (16, 24). Although expression of Gli1 is induced by Shh signaling, Gli1 functions as a positive feedback regulator (17).
The importance of the crosstalk between miRNAs and the Shh signaling pathway during embryonic development is increasingly recognized (25, 26). We reported that brain-derived neurotrophic factor is a target of miR-206. Shh induces the expression of brain-derived neurotrophic factor by suppression of miR-206 in the formation and innervation of airway smooth muscle cells (26). miR-214 is expressed in somites of Zebrafish during early stages of segmentation and plays a role in muscle cell fate specification by down-regulating the expression of Sufu, a negative regulator of Hedgehog signaling (25). miRNAs are also known to regulate Shh signaling, which is implicated in the pathogenesis of medulloblastoma and lung cancer by targeting Smo, Ptch1, and Cxcr4 (27–29).
In this study, we hypothesized that miRNAs are part of the feedback loop in balancing the activity of Shh signaling during lung development. Using an embryonic lung explant culture system and genome-wide expression profiling, we characterized the miRNAs and mRNAs downstream of Shh signaling. We found that expression of miR-326 and its host gene Arrestin β1 (Arrb1) was positively regulated by Shh signaling. Moreover, we demonstrated that miR-326 is a negative modulator of Shh signaling by targeting Gli2 and Smo. Together, our data suggest that miR-326 generates additional negative feedback loop by suppressing the expression of Gl2 and Smo.
Materials and Methods
Detailed methods are described in the online supplement.
Embryonic Lung Cultures
Embryonic lung cultures were performed as described previously (26, 30). For the various purposes, BGjb medium was supplemented with 5 μM cyclopamine (Calbiochem, Darmstadt, Germany), 1 μg/ml Smo agonist (SAG) (Calbiochem), or the TGF-β receptor 1 antagonist SB431542 (10 μM) (Sigma-Aldrich, St. Louis, MO). DMSO or tomatidine (5 μM) (Sigma-Aldrich) were selected as a negative control.
Exon Array and Real-time Quantitative RT-PCR
The Mouse Genome 430 2.0 Exon Array (Affymetrix, Santa Clara, CA) was used for mRNA expression profiling according to the manufacturer’s instructions. Array data were analyzed as described previously (31, 32). TaqMan primers and probes from Applied Biosystems (Grand Island, NY) and StepOnePlus Real-Time PCR system were selected for quantitative RT-PCR (qRT-PCR).
Next-Generation Sequencing of Small RNAs and Data Analysis
SOLiD system (Applied Biosystems) was chosen for sequencing because of its high capacity for small RNAs. CLC Genomics Workbench (CLC Bio) was selected for data analysis.
In Situ Hybridization of mRNA and miRNA
In situ hybridization (ISH) of freshly isolated embryonic lungs, cultured lungs, or frozen sections of embryonic lungs were performed as described previously (26, 31, 33, 34) by using DIG-labeled sense and antisense RNA probes or 5′-DIG–labeled LNA miRNA probes.
Generation of Lentiviral Vectors for miRNA Expression
The overexpression of miRNAs was achieved using a lentiviral vector pLVTHM (a generous gift of Dr. Darrell N. Kotton, Boston University). The lentivirus was cloned and packaged in HEK293T cells as described previously and was used to infect target cells with polybrene (5 μg/ml) (35, 36).
Functional Screening of miRNAs that Modulate the Induction of Gli-Responsive Firefly Luciferase by Shh
Shh-LIGHT2 cell line with Gli-responsive firefly luciferase and constitutive renilla luciferase was selected for the screening (37). Shh-conditioned medium was prepared by collecting the DMEM medium of HEK293 cells that were transfected with Shh-N plasmid as described (38).
3′-UTR Luciferase Reporter Assay
psiCHECK-2 dual luciferase reporter plasmid (Promega) was used for cloning and analysis of 3′-UTRs of Smo and Gli2 as described (31).
Antibodies and Western Blotting
Western blots were performed following the standard procedure. Antibodies (Abcam, Cambridge, MA) including anti-Gli2 antibody (1:200), anti-Smo antibody (1:200), and anti-GADPH antibody (1:10,000) were used.
Statistical Analysis
For quantitative analyses including qRT-PCR, mRNA array, 3′-UTR luciferase, and MTT assay, results from samples of three independent experiments were subjected to statistical analysis. RNA samples from two independent experiments were used for NGS of small RNAs. A Student's t test was performed to calculate P value. Data are presented as mean ± SD, and P < 0.05 was considered significant.
Results
Characterization of miRNAs Downstream of Shh Signaling in Embryonic Lungs by NGS
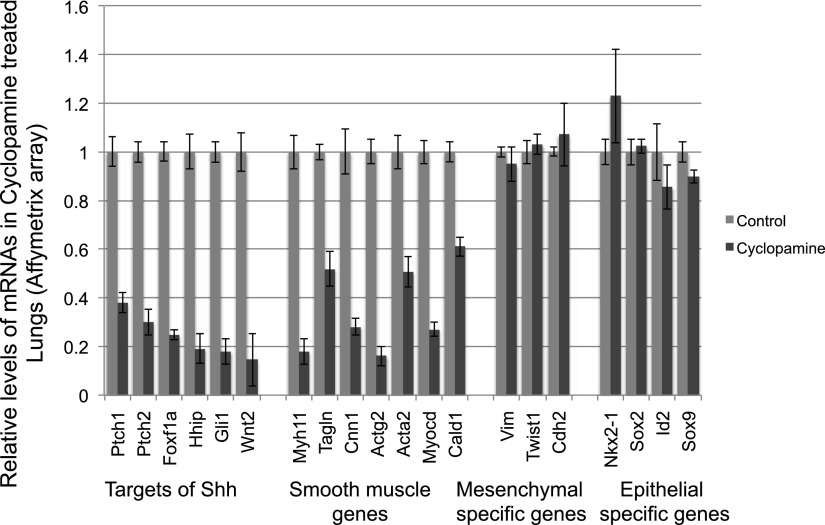
To identify mRNAs and miRNAs whose expression is influenced by Shh signaling, we first profiled mRNAs and miRNAs in embryonic lung cultures in which Shh signaling activities were modulated by the treatment of cyclopamine (the Smoothened antagonist) or SAG (the Smoothened agonist) (40, 41). Briefly, E12 lungs dissected from CD1 mouse embryos were cultured with cyclopamine (5 μM) or SAG (1 μg/ml) in serum-free medium for 48 hours. As reported, the cyclopamine-treated lung explants showed expanded distal epithelium and abnormal branching pattern and loosening of the lung mesenchyme (see Figures E1B and E1E in the online supplement) as compared with control lung explants treated with DMSO or tomatidine, which is structurally similar to cyclopamine but does not inhibit the Shh pathway (Figures E1A, E1D, and E1F) (41). SAG treatment leads to reduction of epithelial growth and expansion of the mesenchymal compartment (Figure E1C). To identify mRNAs downstream of Shh, we first compared exon array data (Affymetrix) of lung explants treated with cyclopamine with those of DMSO control. This analysis revealed that expression levels of 177 genes are significantly altered (FC > 2.0; P < 0.05) in cyclopamine-treated lungs. Gene Sets Enrichment Analysis (Broad Institute) revealed that genes involved in Shh pathway and smooth muscle differentiation are significantly enriched among genes down-regulated by cyclopamine treatment (see Table E2). These include known downstream genes of the Shh pathway (e.g., Ptch1, Gli1, Wnt2, and Hhip) and genes associated with smooth muscle cell differentiation (Figure 1). Reduced expression of these genes is not likely to be the result of perturbation of the ratio of the mesenchymal/epithelial cells because levels of some well-known mesenchymal specific genes (e.g., Vimentin and Twist 1) or epithelial cell–specific genes (e.g., Nkx2–1, Sox2, Sox9, and Id2) are not altered in cyclopamine-treated lungs (Figure 1). In addition, expression of these known Shh target genes is not altered in the exon array data of E12 lung explants treated with SB4, the TGF-β receptor 1 antagonist (10 μM), suggesting a specific connection to Shh activity (Figure E2A). To further show the specificity of cyclopamine, embryonic lung explants were treated with DMSO, cyclopamine, or tomatidine. We found that tomatidine-treated lungs are morphologically indistinguishable from DMSO lungs (Figures E1D and E1F). Moreover, we showed that levels of Gli1 and Arrb1 are not reduced by tomaditine (Figure E2B). Together, these data suggest that our experimental approaches can identify the genes that are specifically influenced by Shh signaling in developing lungs.
Figure 1.
Cyclopamine treatment leads to the significant reduction of known Sonic hedgehog (Shh) targets and genes associated with smooth muscle cells in embryonic lung explants (P < 0.05). RNA samples from three independent experiments were used for the array profiling. Levels of known mesenchymal or epithelial specific genes were not altered by cyclopamine treatment. Error bars, SD.
Next, we sequenced small RNAs (15–25 nt) isolated from embryonic lung explants treated with cyclopamine, SAG, SB4, or DMSO using Next-Generation of Sequencing (NGS) technology (SOLiD system; Applied Biosystems). CLC Genomics Workbench was used for the analysis of sequencing data. After filtering for rRNA, tRNA, and repeated sequences, a range of 4.2 to 6.7 million reads remained in each library and were used for the subsequent analysis. By aligning these sequences to miRNAs in miRbase (42), the number of reads for each known miRNA are calculated (reads/per million) and normalized to the total number of reads that are aligned with known miRNAs. This allows us to report the relative abundance of each miRNA. We identified 29 miRNAs whose levels are reduced by more than 50% in cyclopamine-treated samples, as compared with control, and are up-regulated by more than 2-fold in SAG-treated lung cultures (Table 1). The expression levels of the majority of these miRNAs are not altered by SB4 treatment (Table 1), indicating that they are specifically influenced by Shh activity.
Table 1.
MicroRNAs Whose Levels Are Influenced by Inhibition and Activation of the Sonic Hedgehog Pathway in Cultured Embryonic Lungs
|
P Values |
|||||||
|---|---|---|---|---|---|---|---|
| miRNAs | Average Reads in Control Samples | Cyclop/CTRL | SAG/CTRL | SB4/CTRL | Cyclop versus CTRL | SAG versus CTRL | SB4 versus CTRL |
| mir-374c | 5.00 | 0.00 | 5.90 | 1.10 | 0.42 | 0.50 | 0.95 |
| mir-137 | 12.50 | 0.44 | 2.36 | 0.80 | 0.19 | 0.62 | 0.56 |
| mir-501 | 22.50 | 0.38 | 2.38 | 1.22 | 0.25 | 0.45 | 0.71 |
| mir-496 | 51.00 | 0.31 | 2.55 | 1.65 | 0.21 | 0.37 | 0.49 |
| mir-374 | 99.50 | 0.49 | 13.36 | 2.23 | 0.44 | 0.45 | 0.55 |
| mir-365-1 | 182.50 | 0.32 | 3.78 | 1.00 | 0.02 | 0.38 | 1.00 |
| mir-186 | 219.00 | 0.36 | 3.81 | 2.68 | 0.58 | 0.28 | 0.43 |
| mir-1-1 | 357.00 | 0.29 | 4.02 | 0.66 | 0.04 | 0.38 | 0.25 |
| mir-30d | 594.50 | 0.36 | 4.51 | 1.49 | 0.08 | 0.41 | 0.49 |
| mir-181b-1 | 675.00 | 0.34 | 3.91 | 0.55 | 0.36 | 0.51 | 0.54 |
| mir-361 | 760.50 | 0.27 | 4.64 | 1.65 | 0.09 | 0.25 | 0.56 |
| mir-449a | 949.00 | 0.41 | 6.43 | 0.56 | 0.52 | 0.46 | 0.62 |
| mir-181d | 987.50 | 0.23 | 2.64 | 1.32 | 0.05 | 0.44 | 0.72 |
| mir-193b | 1,797.50 | 0.34 | 2.17 | 0.62 | 0.16 | 0.28 | 0.42 |
| mir-98 | 1,999.00 | 0.49 | 2.18 | 1.34 | 0.40 | 0.63 | 0.75 |
| mir-30c-1 | 2,047.50 | 0.48 | 2.41 | 1.35 | 0.09 | 0.39 | 0.72 |
| mir-181c | 2,343.00 | 0.42 | 2.52 | 1.11 | 0.30 | 0.35 | 0.93 |
| mir-495 | 3,048.00 | 0.33 | 2.20 | 1.06 | 0.18 | 0.52 | 0.95 |
| mir-296 | 3,406.50 | 0.22 | 4.17 | 0.62 | 0.02 | 0.32 | 0.07 |
| mir-151 | 3,608.50 | 0.45 | 3.37 | 1.57 | 0.35 | 0.26 | 0.58 |
| mir-326 | 4,727.50 | 0.32 | 3.38 | 0.97 | 0.07 | 0.10 | 0.96 |
| mir-24-1 | 5,414.50 | 0.05 | 2.77 | 1.47 | 0.08 | 0.37 | 0.69 |
| mir-143 | 5,955.50 | 0.36 | 3.19 | 0.79 | 0.31 | 0.56 | 0.76 |
| mir-26a-1 | 8,173.00 | 0.39 | 3.32 | 1.99 | 0.33 | 0.42 | 0.49 |
| mir-181a-1 | 13,085.00 | 0.46 | 4.30 | 0.11 | 0.45 | 0.34 | 0.16 |
| let-7f-1 | 15,378.50 | 0.37 | 2.91 | 1.37 | 0.12 | 0.29 | 0.71 |
| mir-484 | 15,429.50 | 0.38 | 2.09 | 0.82 | 0.10 | 0.30 | 0.75 |
| mir-145 | 98,439.50 | 0.33 | 3.36 | 0.48 | 0.06 | 0.26 | 0.24 |
Definition of abbreviations: CTRL, control; Cyclop, cyclopamine; miRNA, microRNA; SAG, Smoothened agonist; SB4, the TGF-β receptor 1 antagonist.
To validate the NGS data, we performed qRT-PCR analysis for three miRNAs (miR-1, miR-143, and miR-449) and found that the results of qRT-PCR are consistent with the NGS data (Figure E3A and Table 1). The Shh and Tgfb pathways are known to play an important role for the smooth muscle cell differentiation in developing lung. We showed that mRNAs associated with smooth muscle cells are down-regulated in both cyclopamine- and SB4-treated lung explants (Figure 1). We then examined the expression of smooth muscle–specific miRNAs in these cultured lung explants. In the course of this study, miR-143/145 was identified as a smooth muscle cell–specific miRNA in multiple organs, including the lung (43–45). Consistent with lacZ reporter data of miR-145/143 in airway smooth muscle cells (42), our ISH analysis showed that miR-143 is specifically expressed in the subepithelial domain where smooth muscle cells reside (Figures E3B and E3C). Moreover, levels of miR-143 are significantly reduced in cyclopamine-treated lung explants and are up-regulated in SAG-treated lungs (Figure E3A). Together, these analyses allowed us to identify and confirm miRNAs whose expression is under the influence of Shh signaling in the embryonic lungs.
Identification of miRNAs that Modulate the Induction of Gli-Luciferase Reporter by Shh Treatment
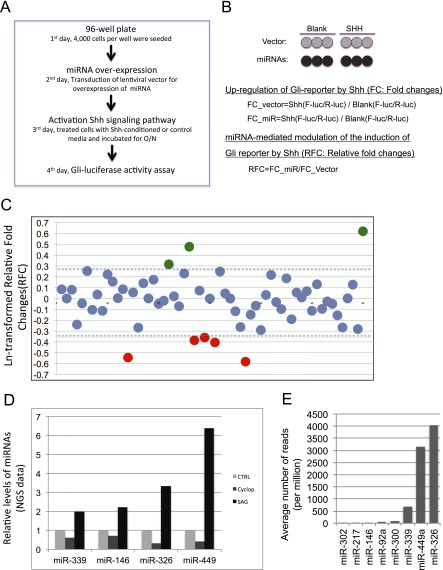
To examine whether some of these miRNAs might have feedback regulatory activities, we performed functional analysis as described in Materials and Methods for 58 individual miRNAs (Figure 2 and Table E3). Among them are miRNAs whose expression levels are influenced by Shh activities in cultured embryonic lungs (Table 1) or miRNAs whose expression levels are abundant in developing or adult lungs. To carry out the screening, 4,000 Shh-LIGHT2 cells were seeded in each well of 96-well plates and cultured overnight. Six wells were transduced with either individual miRNA lentivirus or control lentivirus. To determine the induction of Gli-responsive firefly luciferase by Shh, three wells were treated with blank control medium or Shh-conditioned medium 24 hours after transduction (Figures 2A and 2B). Firefly luciferase activities were measured 24 hours after Shh treatment by using GloMax-Multi Detection System (Promega). The constitutively expressed renilla luciferase activity was measured as an internal control. FC of Shh(F-luc/R-luc)/Control(F-luc/R-luc) was calculated to show the induction of Gli-luciferase reporter by Shh (Figures 2A and 2B). By comparing with those of cells transduced with control lenti-vector, Ln-transformed RFC was calculated (Figure 2B) to show the effect of miRNA overexpression on the induction of Gli-luciferase reporter by Shh.
Figure 2.
Screening for microRNAs (miRNAs) with modulatory activity on the induction of Gli-luciferase reporter by Shh treatment. (A) Experimental procedure. (B) Luciferase reporter data analysis. (C) miRNAs with positive or negative regulatory activities on the induction of Gli-reporter by Shh in Shh-LIGHT2 cells. Numbers on the Y axis are Ln-transformed relative fold changes. (D) Expression of miRNAs that are influenced by Shh signaling activity. (E) The relative levels of miRNAs in control lung explants. A > 30% up- or down-regulation of the induction of Gli-reporter activities by Shh was chosen to select miRNAs with modulatory activities (dashed line in C). NGS, next-generation of sequencing.
It is known that cell density of Shh-LIGHT2 influences the Shh-induced up-regulation of Gli-responsive reporter (37, 38). In the process of screening, we found that overexpression of some miRNAs results in alteration of the constitutively expressed Renilla luciferase activities, which has been used to estimate the number of viable cells in a similar functional screening using the same cell line (39) and in another unrelated study (45). By MTT assay, we further showed that reduction of renilla luciferase in wells transduced with miRNA lentivirus, such as miR-135a, is consistent with a decreased number of viable cells likely due to reduced proliferation and/or increased apoptosis (Figures E4A and E4B). This leads us to evaluate cell density by using Renilla luciferase activity as described (39, 45). To normalize the contribution of cell density, we set up wells with seven different starting densities (2,000, 3,000, 4,000, 5,000, 6,000, 7,000 and 8,000 cells per well) for the transduction of control lentivirus. Then, the cell density of these wells was estimated by the activities of Renilla luciferase (range, 3,365–9,087). Renilla luciferase activities for individual miRNA overexpressing cells range from 2,890 to 8,790. The differences (percentile) in cell densities between experimental versus each individual control was calculated based on the value of Renilla luciferase activity. A specific control was chosen for experimental wells that give smaller differences (percentile), as compared with other cell density controls (data were analyzed and sorted by using Excel table). This allows us to choose a specific control for several experimental wells with very similar cell density.
Among these miRNAs in our functional screening, miR-326 is reported to significantly suppress the Gli-responsive reporter activity in Ptch−/− MEF cells (∼ 50% reduction) (27). Similarly, we found in our screening that overexpression of miR-326 leads to 30% down-regulation of the induction of Gli-reporter activities by Shh treatment in LIGHT 2 cells. This was further confirmed in LIGHT 2 cells with different cell densities (Figure 5A). This led us to choose 30% as cut-off for selecting miRNAs that potentially modulate Shh activation in our screening. We found that overexpression of five individual miRNAs (miR-146, miR-302, miR-326, miR-339, and miR-449) leads to more than 30% down-regulation of the induction of Gli-reporter activities by Shh. Interestingly, expression of miR-326, miR-146, miR-339, or miR-449 was reduced by cyclopamine and up-regulated by SAG in our NGS data (Table 1 and Figure 2D). We also found that overexpression of miR-217, miR-300, or miR-92a enhances the induction of Gli-luciferase reporter by Shh (Figure 2C). In addition, the mean RFC values of triplicate wells were calculated and converted to Z-scores using the mean and standard deviations of all miRNA overexpressing cells, as described in a similar study (39). This analysis showed that Z-scores for several of these miRNAs, including miR-300, miR-92a, and miR-449, are greater than ± 1.96, indicating that the chance for a miRNA having more modulatory activity is less than 2.5%.
Figure 5.
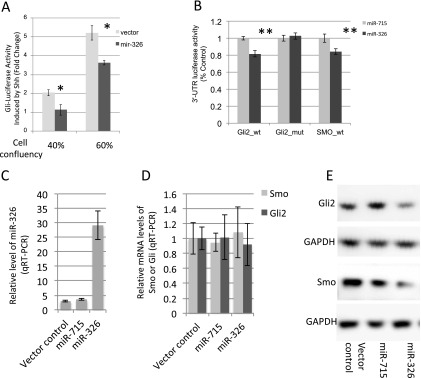
miR-326 suppresses Shh-induced Gli-reporter activities by targeting Smoothened (Smo) and Gli2. (A) miR-326 suppresses the Shh-induced Gli-reporter activities at low and medium cell densities. (B) Luciferase activities of Smo–3′-UTR or Gli2–3′-UTR reporters were decreased by miR-326 but not by miR-715, an unrelated miRNA. Suppression of luciferase activity of Gli2–3′-UTR reporter depends on the miR-326 binding site being intact. (C) Levels of miR-326 in HCT116 cells transduced with miR-326 lentivirus. (D) Overexpression of miR-326 does not alter the levels of Gli2 and Smo miRNAs. (E) Gli2 and Smo protein levels are significantly reduced in miR-326 overexpression cells, as compared with cells transduced with control or miR-715 expressing lentivirus. Results are representative of three independent experiments. Error bars = SD. *P < 0.001; **P < 0.05. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Expression of miR-326 and Its Host Gene Arrb1 Are Regulated by Shh Signaling
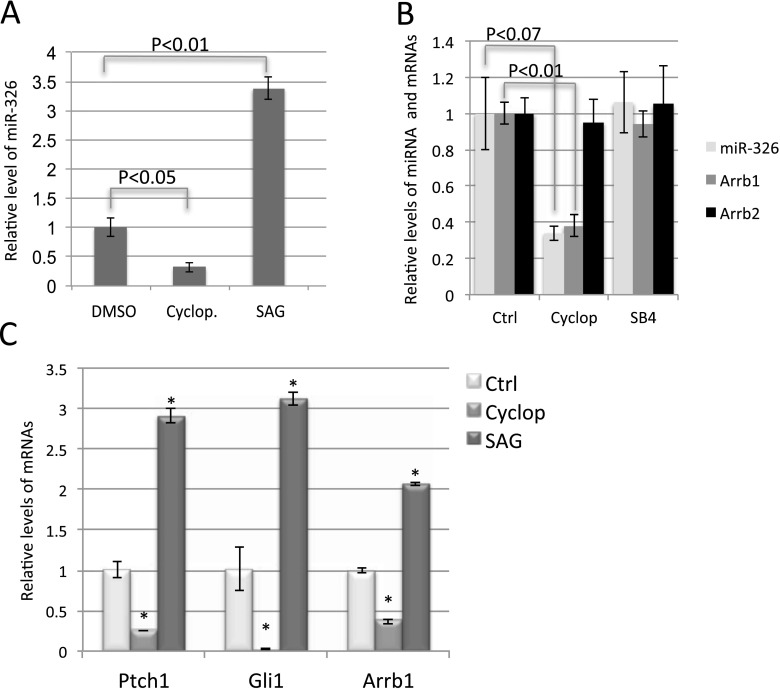
miR-326 is the most abundant among the five miRNAs that significantly suppress the induction of Gli-luciferase reporter by Shh (Figure 2E). In addition, among the 285 miRNAs detected by NGS, miR-326 ranks at 42 with 4,727 reads per million, putting miR-326 in the top 15% of most abundant miRNAs in these lung explants. In our NGS analysis, levels of miR-326 were down-regulated by cyclopamine and up-regulated by SAG (Table 1). This observation was confirmed by qRT-PCR (Figure 3A). miR-326 is localized in the intron 1 of Arrb1, a gene involved in the translocation of Smo into the primary cilia that is important for the transduction of Shh signaling (14, 46). Because the majority of intronic miRNAs and their host genes are coordinately expressed (7, 8), we examined the levels of Arrb1 in cyclopamine- or SAG-treated embryonic lungs. Our exon array data revealed that levels of Arrb1 mRNA were decreased by approximately 63% in cyclopamine-treated lungs, which correlated well with the reduction of miR-326 expression (Figure 3B and Table 1). The expression of Arrb2, another family member of Arrestin-β, was not altered by cyclopamine treatment (Figure 3B). Moreover, levels of Arrb1, miR-326, and Arrb2 were not altered in SB4-treated lungs (Figure 3B and Table 1). Finally, similar to Gli1 and Ptch1, the known downstream targets of Shh signaling, expression of Arrb1 is increased by SAG treatment (Figure 3C). Together, our data suggest that expression of miR-326 and Arrb1 is specifically co-regulated by Shh signaling in developing lung.
Figure 3.
The expression of Arrestin β1 (Arrb1) and miR-326 is regulated by Shh signaling in cultured embryonic lungs. (A) Quantitative RT-PCR (qRT-PCR) analysis of miR-326 in cultured lungs treated with cyclopamine or Smoothened agonist (SAG). (B) Relative levels of Arrb1, Arrb2, and miR-326 in cultured lung explants with treatments as indicated. (C) qRT-PCR analysis for levels of Gli1, Ptch1, and Arrb1 in cultured lungs with treatment as indicated. Error bars = SD. *P < 0.01. Ctrl, control; Cyclop, cyclopamine.
Downstream genes of Shh signaling, such as Ptch1, are known to be selectively expressed in mesenchymal cells of developing lungs. We next examined the expression pattern of Arrb1 and miR-326 in developing lung. Our ISH data revealed that expression of Arrb1 was selectively enriched in the mesenchymal cells of E13.5 embryonic lung or cultured lung explants (Figures 4A and 4C). Levels of Arrb1 were dramatically reduced by cyclopamine treatment (Figures 4C and 4D). In contrast, expression of Arrb2 was detected in the epithelium and mesenchyme of E13.5 lungs (Figure 4B). In addition, we performed ISH using the Dig-labeled Arrb1 sense probe, which did not detect specific signal in embryonic lung mesenchyme (Figure E6B). By whole mount ISH or ISH on sections using miR-326 LNA probe, expression of miR-326 was mainly detected in the mesenchyme surrounding the distal epithelial buds (Figures 4E–4G). Similar to Arrb1, ISH signal of miR-326 is consistently lower in lung explants treated with cyclopamine (Figures 4G and 4H). Scrambled LNA probes were used as negative control and did not detect robust and specific signal in control lung explants (Figure E5D). Our analysis revealed a mesenchymal-enriched expression pattern of miR-326 and Arrb1 in developing lung, similar to those of Ptch1, further supporting the idea that they are downstream genes of the Shh pathway.
Figure 4.
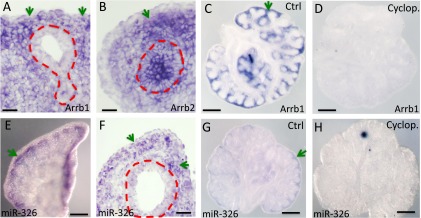
The expression of Arrb1 and miR-326 is selectively enriched in mesenchyme of embryonic lungs and reduced in cyclopamine-treated lung explants. In situ hybridization (ISH) signal of Arrb1 on frozen section of E13.5 lungs (A) or in lung explant (C). In contrast, Arrb2 is expressed in both epithelium and mesenchyme (B). Expression pattern of miR-326 in the middle lobe of E14 lung (E), in embryonic lung explant (G), and on frozen section of E13.5 lungs (F). Expression levels of Arrb1 and miR-326 are significantly reduced in embryonic lungs treated with cyclopamine (C, D, G, and H). Images are representative of three independent experiments. Green arrows point to the ISH signal of Arrb1 and miR-326. The epithelium is highlighted by red dashed line A, B, and F. Scale bar in A, B, and F = 20 μM; scale bar in C, D, E, G, and H = 300 μM.
miR-326 Modulates Shh Signaling by Targeting Smo and Gli2
We first confirmed the effect of miR-326 on the induction of Gli-luciferase reporter by Shh in Shh-LIGHT2. Considering the cell density–dependent manner of the induction of Gli-luciferase reporter by Shh, we assessed the effect of miR-326 overexpression on Shh signaling in wells with different cell densities. Our data confirmed that overexpression of miR-326 reduced the induction of Gli-luciferase reporter by Shh treatment (Figure 5A). We next investigated whether this suppression is achieved by miR-326–mediated repression of components of the Shh pathway. It has been reported that miR-326 directly repressed Smo expression in Daoy medulloblastoma cells (27). Moreover, miR-326 is predicted to target Arrb1, Sufu, Gli2, and Gli3. We first cloned fragments of the 3′-UTRs of Smo, Arrb1, Sufu, Gli2, and Gli3 that contain the predicted miR-326 binding sites into the psiCHECK2 dual luciferase reporter plasmid. Plasmid of luciferase reporter was cotransfected with miRNA expression vector of miR-326 or miR-715, an unrelated miRNA with no binding site in these 3′-UTRs. Whereas overexpression of miR-326 has no significant effect on the 3′-UTR reporter of Arrb1, Sufu, and Gli3, activities of luciferase reporters containing the wild-type 3′-UTR of Gli2 or Smo were significantly suppressed by miR-326 but not by miR-715 (Figure 5B). A close examination of the 3′-UTRs of Smo and Gli2 (TargetScan) revealed that the miR-326 binding site is conserved among several organisms, including human (Figure E7). We then cloned 3′-UTR of Gli2, in which miR-326 binding site is mutated, and found that the suppression of Gli2 3′-UTR reporter by miR-326 depends on the intact miR-326 binding site (Figure 5B). This suggests that Gli2 is a direct target of miR-326.
To further examine whether miR-326 regulates the level of Gli2 protein, we first searched for cell lines with relatively high levels of Gli2 or Smo mRNA by examining the mRNA array database (Gene Sets Enrichment Analysis; Broad Institute) of NCI-60 cell lines. We found that the human colon carcinoma cell line HCT116 expresses relatively high levels of Smo, Gli2, and other components of the Shh pathway. Human Gli2 3′-UTR contains two miR-326 binding sites, including the one that is conserved in murine Gli2 3′-UTR and confirmed in our luciferase assay (Figure E6). Therefore, we tested whether miR-326 can suppress Gli2 protein levels in HCT116 cells. HCT116 cells were transduced with control lentivirus that do not express miRNA or with lentivirus expressing miR-326 or miR-715. Transduction of miR-326 lentivirus leads to an approximately 10-fold increase of miR-326 levels as compared with cells infected with control or miR-715 lentivirus (Figure 5C). By Western blot, we showed that overexpression of miR-326 consistently reduced the protein levels of Gli2 and Smo without altering their mRNA levels, as compared with cells transduced with control or miR-715 overexpression lentivirus (Figures 5D and 5E). These results strongly suggest that miR-326 regulates the expression of Smo and Gli2 post-transcriptionally.
Discussion
In this study we used NGS and exon array approaches to identify miRNAs and mRNAs downstream of Shh pathway in developing lungs. We report for the first time that expression of miR-326 and its host gene Arrb1 is under the influence of Shh signaling. Using a Gli-responsive luciferase reporter cell line, we screened the activities of 58 miRNAs in modulating Shh signaling and identified miR-326 as a negative feedback regulator of Shh pathway by targeting Gli2 and Smo.
Lung explant culture system and pharmacological approaches have been successfully applied to study signaling pathways in developing lung (26, 41). The expression level of Ptch1 and Gli1 is up-regulated by SAG and reduced by cyclopamine, which reflects the activity of Shh signaling in developing lung (Figure 2C) (26, 41). Moreover, the expression level of Shh target genes and Arrb1 is not altered in embryonic lung explants treated with tomatidine, a compound structurally similar to cyclopamine, but does not inhibit the hedgehog (Shh) pathway (Figure E2B). This indicates the specific activities of cyclopamine and SAG in modulating Shh signaling in embryonic lung explants. The Shh signaling is important for regulating the proliferation and differentiation of epithelial and mesenchymal cells in developing lung. However, by examining the levels of mesenchyme or epithelial-specific genes in response to Shh signaling manipulations, we found that there are no significant changes in cell populations in embryonic lung explant 48 hours after cyclopamine treatment.
It has been shown that levels of miR-326 were negatively correlated with Gli activity, and miR-326 directly regulated the expression of Smo in Daoy medulloblastoma cells (27). In contrast to these observations in medulloblastoma, we found that Shh signaling induces the expression of miR-326 in developing lungs. Here, we provide several lines of evidence suggesting that expression of miR-326 and its host gene Arrb1 is co-regulated by Shh signal in developing lung. (1) Inhibition of Shh signaling led to down-regulation of levels of miR-326 and Arrb1. Conversely, activation of Shh signaling led to up-regulation of miR-326 and Arrb1 expression. (2) Expression of miR-326 and Arrb1 is selectively enriched in the mesenchymal cells of embryonic lungs, which is in agreement with the expression pattern of downstream target of Shh signaling. (3) Our findings of coexpression of miR-326 and Arrb1 are in agreement with reported data from human glioblastoma, where down-regulation of miR-326 was strikingly correlated with reduced Arrb1 expression (47). However, in contrast to our findings, the level of miR-326 is lower in medulloblastoma with high Gli activity, indicating a negative correlation of miR-326 expression with Shh signaling activity (27). Whether expression of Arrb1 is correlated with mir-326 was not examined in this study (27). It is unclear whether expression of Arrb1/miR-326 is directly under the control of Gli. Further study is needed to determine the mechanisms underlying the regulation of Arrb1 and miR-326 by Shh signaling.
We further showed that overexpression of miR-326 significantly reduce the induction of Gli-responsive reporter by Shh, at least partially by repressing the expression of Gli2 and Smo.
miRNAs are known to repress the expression of mRNA targets by promoting translational repression and mRNA degradation (48, 49). Although our initial findings suggest that miRNA regulates target gene expression mainly by translational repression in mammalian cells, recent evidence indicates that target degradation provides a major contribution (49). However, for a subset of targets and reporters, mRNA-mediated target gene repression is independent of mRNA degradation (50–52). Our finding that overexpression of miR-326 resulted in the reduction of protein levels of Smo and Gli2 without changing their mRNA levels might fall into this category.
Our genome-wide profilings of miRNA expression and cell-based functional screening identified four other miRNAs with negative modulatory activity, including miR-146, miR-302, miR-339, and miR-449. miR-449 and miR-339 are hosted in the intron of cell division cycle 20B (cdc20b) and transcript 3110082I17Rik, respectively. miR-302 is transcribed from the antisense of La ribonucleoprotein domain family, member 7 (Larp7), a chaperon protein for noncoding RNA. miR-146a is an intergenic miRNA. miR-302 has been reported to negatively affect the SHH-GLI-NANOG pathway in glioma-initiating cells by targeting CXCR4 (28). miR-146a is known for its role in inflammation (53). However, a recent publication showed that miR-146 enhances Shh signaling activity by suppressing the expression of numb (54). In addition, we identified three miRNAs with positive regulatory activities. Among them, miR-217 is hosted in the intron of transcript ENSMUST00000138164. miR-92a is part of miR-17/92 cluster, and miR-300 is transcribed from a large miRNA cluster in chromosome 12. It has been shown that Shh treatment of primary cerebellar granule neuron precursors resulted in increased miR-17/92 expression (55). In addition, overexpression of miR-92 in engineered tumors resulted in loss of Ptch1 and up-regulation of Gli1 (56). These suggest a potential connection between these miRNAs and the Shh pathway. Little is known about these miRNAs and their host genes in relation to the Shh pathway, and these will be the subject of future studies.
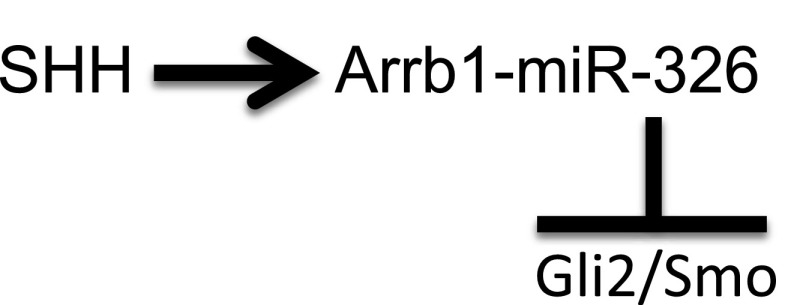
In conclusion, we report that expression of miR-326 and its host gene Arrb1 was regulated by Shh signaling in mouse developing lung. Moreover, cell-based functional screening identified that miR-326 negatively modulates Shh signaling by directly targeting Gli2 and Smo. These findings suggest that there is an additional negative feedback mechanism of Shh signaling in developing lung that is mediated by miRNA and acts on the post-transcriptional level (Figure 6). Our data suggest that regulation of Shh activity by the miR-326–Gli2/Smo feedback loop may be a relevant for proper regulation of Shh activity in lung development and disease.
Figure 6.
A negative feedback role of miR-326 in the Shh signaling pathway.
Acknowledgments
Acknowledgments
The authors thank Wellington Cardoso, Alan Fine, and Lindsey Madden for critical comments and thoughtful discussions; Feng Chen and Thanh Tran for technical support; and Matthew Jones for the support in NGS data analysis.
Footnotes
This work was supported by National Heart, Lung and Blood Institute grants P01 HL47049 and R01 HL081800.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0127OC on March 11, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 2.Asli NS, Pitulescu ME, Kessel M. Micrornas in organogenesis and disease. Curr Mol Med. 2008;8:698–710. doi: 10.2174/156652408786733739. [DOI] [PubMed] [Google Scholar]
- 3.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 4.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 6.Lutter D, Marr C, Krumsiek J, Lang EW, Theis FJ. Intronic microRNAs support their host genes by mediating synergistic and antagonistic regulatory effects. BMC Genomics. 2010;11:224. doi: 10.1186/1471-2164-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microrna host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RL, Riddle RD, Tabin CJ. Mechanisms of limb patterning. Curr Opin Genet Dev. 1994;4:535–542. doi: 10.1016/0959-437x(94)90069-f. [DOI] [PubMed] [Google Scholar]
- 10.Ho KS, Scott MP. Sonic hedgehog in the nervous system: functions, modifications and mechanisms. Curr Opin Neurobiol. 2002;12:57–63. doi: 10.1016/s0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 11.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 12.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 16.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol. 1997;188:337–348. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Kugler MC, Loomis CA, Samdani R, Zhao Z, Chen GJ, Brandt JP, Brownell I, Joyner AL, Rom WN, et al. Hedgehog signaling in neonatal and adult lung. Am J Respir Cell Mol Biol. 2013;48:703–710. doi: 10.1165/rcmb.2012-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 20.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 21.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 22.Rutter M, Wang J, Huang Z, Kuliszewski M, Post M. Gli2 influences proliferation in the developing lung through regulation of cyclin expression. Am J Respir Cell Mol Biol. 2010;42:615–625. doi: 10.1165/rcmb.2008-0390OC. [DOI] [PubMed] [Google Scholar]
- 23.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 24.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynt AS, Li N, Thatcher EJ, Solnica-Krezel L, Patton JG. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat Genet. 2007;39:259–263. doi: 10.1038/ng1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A shh/mir-206/bdnf cascade coordinates innervation and formation of airway smooth muscle. J Neurosci. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I, et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008;27:2616–2627. doi: 10.1038/emboj.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fareh M, Turchi L, Virolle V, Debruyne D, Almairac F. de-la-Forest Divonne S, Paquis P, Preynat-Seauve O, Krause KH, Chneiweiss H, et al. The mir 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through cxcr4 repression and consequent disruption of the shh-gli-nanog network. Cell Death Differ. 2012;19:232–244. doi: 10.1038/cdd.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhang D, Chen C, Ruan Z, Li Y, Huang Y. MicroRNA-212 displays tumor-promoting properties in non-small cell lung cancer cells and targets the hedgehog pathway receptor PTCH1. Mol Biol Cell. 2012;23:1423–1434. doi: 10.1091/mbc.E11-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lü J, Qian J, Izvolsky KI, Cardoso WV. Global analysis of genes differentially expressed in branching and non-branching regions of the mouse embryonic lung. Dev Biol. 2004;273:418–435. doi: 10.1016/j.ydbio.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 33.Lü J, Qian J, Keppler D, Cardoso WV. Cathespin H is an Fgf10 target involved in Bmp4 degradation during lung branching morphogenesis. J Biol Chem. 2007;282:22176–22184. doi: 10.1074/jbc.M700063200. [DOI] [PubMed] [Google Scholar]
- 34.Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci USA. 2012;109:12592–12597. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Qian J, Li C, Kwok L, Cheng F, Liu P, Perdomo C, Kotton D, Vaziri C, Anderlind C, et al. miR-129 regulates cell proliferation by downregulating Cdk6 expression. Cell Cycle. 2010;9:1809–1818. doi: 10.4161/cc.9.9.11535. [DOI] [PubMed] [Google Scholar]
- 36.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 38.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci USA. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillman RT, Feng BY, Ni J, Woo WM, Milenkovic L, Hayden Gephart MG, Teruel MN, Oro AE, Chen JK, Scott MP. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333–2346. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyer LA, Makadia FA, Scott A, Pegram K, Hutson MR, Kirby ML. BMP signaling modulates hedgehog-induced secondary heart field proliferation. Dev Biol. 2010;348:167–176. doi: 10.1016/j.ydbio.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–231. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Wellcome Trust Sanger Institute. Version 18.0, November 2011. Available from: http://miRNA.sanger.ac.uk/sequences/index.shtml [Google Scholar]
- 43.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir 143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiba-Mizutani T, Miura H, Matsuda M, Matsuda Z, Yokomaku Y, Miyauchi K, Nishizawa M, Yamamoto N, Sugiura W. Use of new T-cell-based cell lines expressing two luciferase reporters for accurately evaluating susceptibility to anti-human immunodeficiency virus type 1 drugs. J Clin Microbiol. 2007;45:477–487. doi: 10.1128/JCM.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 48.Kefas B, Comeau L, Floyd DH, Seleverstov O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca EA, et al. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci. 2009;29:15161–15168. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 50.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 51.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 53.Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat Struct Mol Biol. 2006;13:1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 55.Ghorpade DS, Sinha AY, Holla S, Singh V, Balaji KN. NOD2-nitric oxide-responsive microRNA-146a activates Sonic hedgehog signaling to orchestrate inflammatory responses in murine model of inflammatory bowel disease. J Biol Chem. 2013;288:33037–33048. doi: 10.1074/jbc.M113.492496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Northcott PA, Fernandez-L A, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uziel T, Karginov FV, Xie S, Parker JS, Wang YD, Gajjar A, He L, Ellison D, Gilbertson RJ, Hannon G, et al. The miR-17∼92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci USA. 2009;106:2812–2817. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]