Abstract
Objectives
Thoracic aneurysms are the main cardiovascular complication of Marfan syndrome (MFS) resulting in premature death. MFS has been associated with mutations of the gene encoding fibrillin-1 (FBN1), a major constituent of the elastic fibers. Matrix metalloproteinases (MMPs) are important in the pathogenesis of abdominal aortic aneurysms but their precise role in MFS is not clear. Doxycycline is a nonspecific MMP inhibitor. The objective of the study was to determine whether docycycline can attenuate matrix degradation and prolong the survival of mice with MFS.
Methods
The study employed a well-characterized animal model of MFS, namely fibrillin-1 under-expressing mice (mgR/mgR mice) that die spontaneously from rupture of the thoracic aorta between 2 to 4 months of age. Mutant and wild type mice were given doxycycline in their drinking water at a concentration designed to provide 100 mg/kg/day beginning at postnatal day (PD) 1, whereas control mice were given water. Treated mice were divided into two groups. One group of animals was followed until death or for 7 months to determine lifespan. In the second group of mice, the ascending thoracic aortas were collected for histological analysis (H&E staining, Trichrome staining) and zymography for examining MMP-2 and MMP-9 levels at six weeks.
Results
MMP-2 and MMP-9 levels were higher in the thoracic aorta of mgR/mgR mice compared to wild type littermates. Doxycycline-treated mgR/mgR mice lived 132 ± 14.6 days (n=16) or significantly longer than untreated mutant mice (79 ± 6.7 days, n=30) (p<0.01). Connective tissue staining showed that doxycycline treatment decreased elastic fiber degradation in mgR/mgR mice. Furthermore, mgR/mgR mice treated with doxycycline had lower MMP-2 and MMP-9 levels compared to untreated mgR/mgR mice.
Conclusions
This study demonstrates that doxycycline significantly delays aneurysm rupture in MFS-like mice by inhibiting expression of tissue MMP-2 and MMP-9 and thus, degradation of the elastic matrix. The results suggest that MMPs contribute to the progression of thoracic aneurysm in MFS and that doxycycline has the potential to significantly alter the course of the disease.
Keywords: aortic aneurysm; Marfan syndrome; doxycycline; matrix metalloproteinase 2, 9; mouse
INTRODUCTION
Marfan syndrome (MFS) is an inherited disorder of the connective tissue with prominent abnormalities in the ocular, skeletal and cardiovascular systems [1]. The most common cardiovascular manifestation in patients with MFS is progressive aortic root dilatation that can precipitate life-threatening complications, such as aortic regurgitation, dissection or rupture. MFS is caused by mutations in the gene encoding fibrillin-1 (FBN1), the major constituent of extracellular microfibrils [2,3]. Fibrillin-rich microfibrils are found in a wide variety of connective tissues, either associated with elastin in the elastic fibers or as elastin-free assemblies [4]. Experimental evidence and biosynthetic considerations originally predicted that FBN1 mutations in MFS would reduce tissue integrity by interfering with the normal assembly of microfibrils. The combination of a structurally impaired tissue and chronic cyclic stress was believed to be the main cause of the mechanical failure of the aorta [4]. This concept was recently revised by the studies of mouse models of MFS that have implicated TGF-β-driven secondary cellular events in the progression of aortic aneurysm [5-7]. These studies have also documented the ability of TGF-β antagonism to effectively rescue aortic aneurysm in MFS-like mice [8]. Additional work in mutant mice has indicated that proteolysis of fibrillin-rich microfibrils contributes to aneurysm progression as well by stimulating macrophage chemotaxis and the expression of matrix metalloproteinases (MMPs) [9,10].
MMPs represent a family of zinc endopeptidases that are responsible for the degradation of the extracellular matrix (ECM) in abdominal aortic aneurysms (AAAs) [11-13]. It has been demonstrated in previous studies that MMP-2 from mesenchymal cells and MMP-9 from macrophages are required for aneurysm formation [14]. Studies of aneurysm tissues from patients with MFS suggest that upregulation of MMP-2 and MMP-9 may also play a primary role in MFS [15,16]. Doxycycline is a non-specific MMP inhibitor that has been used to treat a number of conditions associated with excess MMP expression, including periodontal disease and rheumatoid arthritis [17-20]. Experimental AAAs are inhibited by doxycycline and a single small randomized trial demonstrated suppression of aortic aneurysm expansion by doxycycline [21-23]. Previous work from our laboratory has shown that doxycycline inhibits MMP-2 secretion from explanted human AAA tissue [24]. These findings provide evidence that doxycycline can effectively treat diseases in which MMPs play a pathogenic role. Based on these data, we hypothesized that doxycycline could delay spontaneous rupture of the thoracic aorta in mice with MFS by inhibiting MMP-2 and MMP-9.
The present study was designed to test the above hypothesis by examining the effects of doxycycline treatment in mice with MFS that typically develop thoracic aneurysms and die spontaneously from rupture of the proximal aorta at between 2 to 4 months of age [5]. The results showed that doxycycline treatment significantly prolonged the survival of the mutant mice while reducing MMP expression and improving histopathological signs of aortic matrix degradation compared to placebo-treated control animals. These findings strongly suggest that doxycycline has the potential to significantly alter the course of the disease in MFS patients.
METHODS
Mice
Heterozygous mutant mice (mgR/+) in a mixed C57Bl/6J;129 SvEv background were mated to generate homozygous mutant mice (mgR/mgR) and wild type littermates [5]. Genotyping of mice was performed at post-natal day 14 (P14) by PCR using DNA from tail biopsies and the following primers to amplify the wild type and mgR allele, respectively: (5’-CTCCGTGGGACCTACAAATG-3’ and 5’-CCAGGTGTGTTTCGACATTG-3’) and (5’-CTCCGTGGGACCTACAAATG-3’ and 5’-TGAATGAACTGCAGGACGAG-3’). All experiments were carried out in accordance with the guidelines of the University of Nebraska Medical Center Animal Care Committee for the use and care of laboratory animals. All mice were maintained in the pathogen free animal facility.
Doxycycline treatment and Kaplan-Meier’s survival curve
Beginning on postnatal day (PD)1, mouse mothers were given plain water or doxycycline containing drinking water. The concentration of doxycycline in the water was calculated to provide 100 mg/kg/day based on average daily water intake. We have shown in a previous study that this concentration of doxycycline achieved a mean plasma concentration of 4.14 ± 0.557[22]. Serum doxycycline levels at these doses were similar to the plasma doxycycline levels of AAA patients taking 200 mg of doxycycline per day [22]. One group of wild type littermates (n=18) and one group of homozygous MFS mice (n=17) with or without doxycycline treatment were sacrificed at 6 weeks of age. The ascending thoracic aortas were perfusion-fixed with 10% neutral buffered formalin and collected for histological studies [25]. Some of the samples were snap frozen in liquid nitrogen for protein extraction and zymographic analysis [25]. To configure the Kaplan-Meier survival curve, mice were evaluated daily and survival recorded. Mice were followed up to 7 months, at which time, all surviving mice were sacrificed.
Masson’s trichrome connective tissue staining
Mouse ascending thoracic aortas were harvested, perfusion-fixed with 10% neutral buffered formalin, embedded in paraffin, and cut into 4 μm sections. The slides were stained with hematoxylin, Crocein Scarlet, Acid Fuchsin, and Aniline Blue (Sigma, St. Louis, MO). Each staining cycle alternated between fixing and washing procedures. The slides were examined and photographed using light microscopy (Kodak) (40×).
Gelatin zymography
Mouse aortic protein was extracted as described previously [25]. The protein content of the aortic samples was determined by protein assay (Bio-Rad Laboratories, Hercules, CA) and equalized by protein content for loading. Gelatin zymography was conducted using SDS-polyacrylamide gels containing 0.8 % gelatin (Sigma, St. Louis, MO). After electrophoresis, the gels were washed five times in a Triton X-100 solution (50 mM Tris-HCl (pH 7.5) / 5 mM CaCl2/1 μM ZnCl2 / 0.02 %NaN3 / 2.5 %Triton X-100) to remove the SDS and renature the gelatinases. Gels were then developed in the same buffer excluding Triton X-100 (development buffer) overnight at 37°C. Enzymatic activity was visualized as negative staining with 0.5 % Coomassie Brilliant Blue R-250 (Sigma, St. Louis, MO). The molecular sizes of gelatinolytic activity were determined by comparison to Molecular Weight Markers (Bio-Rad, Hercules, CA). The gelatinolytic activities were quantified by Bio-Rad densitometer and Quantity One Quantification Software (Bio-Rad, Hercules, CA).
Reverse zymography
Aortic proteins were separated by electrophoresis on 12.5% SDS-PAGE copolymerized with 1.6% gelatin and 0.16 μg/ml MMP-2. After electrophoresis, the SDS was removed from the gel by washing in 2.5% Triton X-100 for 2 hours. The gels were incubated at 37oC overnight in development buffer and stained with 0.5% Coomassie brilliant blue G-250 (Sigma) for 2 hours, and destained in gel-destaining buffer (40% methanol and 10% glacial acetic acid) until the background was clear. The molecular sizes of gelatinolytic activity were determined by comparison to Molecular Weight Markers (Bio-Rad). The band intensities were quantified by Bio-Rad densitometer and Quantity One Quantification Software (Bio-Rad).
Statistical analyses
Data are presented as mean ± SE. Life table analysis was used for the Kaplan-Meier survival curve. Statistical significance (p<0.05) for all other variables was determined by ANOVA.
RESULTS
Doxycycline prolongs the lifespan of MFS-like mice
In preliminary analysis, we performed necropsy on all Marfan mice that died. We found that 10/10 deaths were related to blood loss from ruptured ascending aneurysms. The aim of the study was to investigate whether doxycycline could prevent MFS-associated aortic rupture and extend the lifespan of mgR/mgR mice [5]. Accordingly doxycycline or placebo (water) was administered to mgR/+ dams in order to transmit the drug via the breast milk to homozygous mgR pups and their wild type littermates. This treatment lasted between P1 and P28 and was followed by direct administration of either water or water containing doxycycline to the two experimental samples after weaning. Mortality was recorded in both groups, while mice that survived past 7 months were sacrificed. Homozygous mice treated with doxycycline at 100 mg/kg lived 132 ± 14.6 days (n=16), significantly longer than the untreated mice that survived 79 ± 6.7 days (n=30) (p<0.01). These results demonstrated that doxycycline can significantly prolong the lifespan of MF mice.
MMP-2 and MMP-9 expression in aortas
We then examined MMP-2 and MMP-9 levels in the aorta of wild type and mgR/mgR mice that were sacrificed at 6 weeks of age. Zymographic analysis showed that MMP-2 and MMP-9 levels were increased in mgR homozygous mouse aorta compared to wild type control mice (Figure 2). These findings suggested that MMP-2 and MMP-9 could play a role in the pathogenesis of aneurysm disease in this mouse model of MFS. In order to determine if the doxycycline treatment impacted MMP-2 and MMP-9 production, we examined MMP-2 and MMP-9 production in doxycycline treated mgR homozygous mice. The MMP-2 and MMP-9 levels were significantly reduced in doxycycline treatment (Figure 2). Levels of tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1 and TIMP-2) are not significantly different among WT, or mgR/mgR with or without doxycycline treatment (Figure 3). The data suggested that doxycycline may prevent aneurysm rupture by inhibiting MMP-2 and MMP-9 production.
Figure 2.

Gelatin zymographic analysis of MMP-2 and MMP-9 in the mouse thoracic aortas. Mouse thoracic aortas from wild type control, mgR/mgR, and doxycycline-treated mgR/mgR mice harvested at 6 weeks of age (n=5 mice/group). Aortic proteins were extracted and separated by electrophoresis on a 10% SDS-PAGE containing 0.8% gelatin. Band intensities (gelatinolytic activities) were measured by densitometer. Relative expression of MMP-9 and MMP-2 in wild type control, mgR/mgR, and doxycycline-treated mgR/mgR mouse aortas was analyzed. *, # p < 0.05 (*relative to wild type controls, # relative to untreated mgR/mgR mice). A representative gel is shown.
Figure 3.
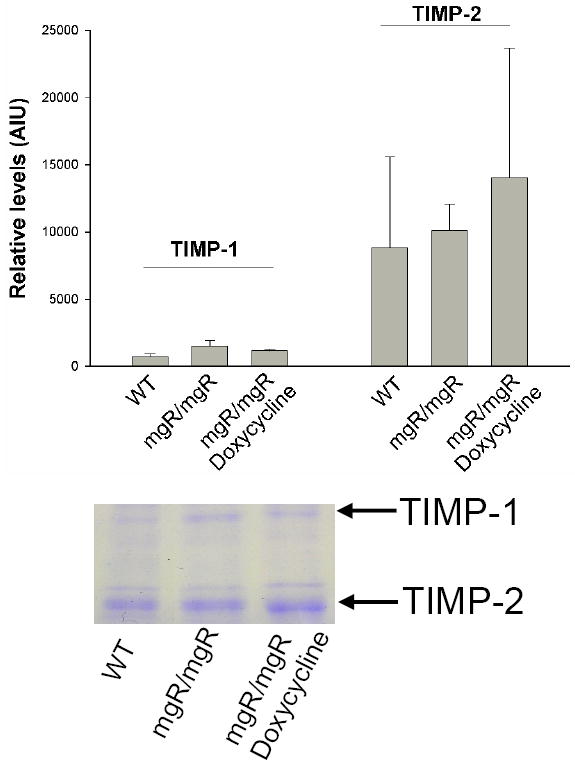
Reverse zymographic analysis of TIMP levels in the mouse thoracic aortas. Mouse thoracic aortas from wild type control, mgR/mgR, and doxycycline-treated mgR/mgR mice harvested at 6 weeks of age (n=5 mice/group). Aortic proteins were separated by electrophoresis on a 12.5% SDS-PAGE containing 1.6% gelatin, 0.16 μg/ml MMP-2. A representative reverse zymogram shows the bands representing gelatin preserved by TIMP-1 and TIMP-2 (indicated by arrows). Band intensities were measured by densitometer. Relative expression of TIMP-1 and TIMP-2 in wild type control, mgR/mgR, and doxycycline-treated mgR/mgR mouse aortas was analyzed.
Histological Changes
MMPs contribute directly to the degradation and remodeling of ECM. Therefore, it is possible that doxycycline treatment can prevent elastic fiber fragmentation. We further studied histological changes in aorta. The number of breaks in the elastin was quantified by an observer unaware of the genetic background or treatment of the mice. Connective tissue staining of aortic sections from mgR/mgR mice showed disruption and fragmentation of medial elastic fibers (Figure 4c), while wild type controls showed intact medial elastic lamella (Figure 4b). Histological analysis revealed that aortic elastic lamella in doxycycline-treated mgR/mgR mice was indistinguishable from that in the wild type control mice (Figure 4d). Quantification of the number of breaks in the elastic lamellae shows significantly fewer breaks in the elastin among the docycycline-treated mgR/mgR mice compared to untreated mgR/mgR mice (Figure 4a). We therefore conclude that doxycycline can significantly alter the course of the disease.
Figure 4.

Elastic fiber degradation in the wild type (WT) and mgR/mgR mice. a) elastin breaks per field under 40x magnification in WT, mgR/mgR, and doxycycline-treated mgR/mgR mice (n=3 aortas/group). *, # p < 0.05 (*relative to wild type controls, # relative to untreated mgR/mgR mice). (b-d) representative trichrome stainings of aortic tissue of WT (b), mgR/mgR (c), and doxycycline-treated mgR/mgR (d) mice at 6 weeks age. Arrows indicates breaks in elastic fibers.
DISCUSSION
Aneurysms of the ascending aorta are the main cardiovascular complication of MFS. In this study, we have used mgR/mgR mice, a well-characterized mouse model of MFS [5,6], in order to examine the potential of doxycycline, a nonspecific MMP inhibitor, to prevent aneurysm rupture. To understand the mechanism of action of doxycycline in this model, we assessed MMP-2 and MMP-9 levels and changes in the aortic histopathology. Doxycycline treatment was able to delay aneurysm rupture and prolong the mgR homozygous mouse lifespan. MMP-2 and MMP-9 levels were found to be significantly higher in the thoracic aorta of mgR/mgR mice than in wild type littermates. Connective tissue staining showed that doxycycline treatment prevented or delayed elastic fiber fragmentation and lowered MMP-2 and MMP-9 expression in mgR homozygous mice.
Fibrillin-1 is the principal structural component of extracellular microfibrils, that confer mechanical properties to connective tissues, alone or in association with elastin in the elastic fibers [26]. Fibrillin-1 mutations are responsible for the pleiotropic manifestations of MFS, which include the life-threatening complications of the cardiovascular system [1]. Several mouse models of MFS have been created that have provided new insights into aortic disease progression in MFS [4]. The mgR/mgR mouse model, in particular, was the first to show that fibrillin-1 deficiency promotes a series of secondary cellular events that exacerbate the progression of aneurysmal disease leading to dissection [5,6]. These pathogenic events include localized aortic calcium deposition, inflammatory cell infiltration, fibrosis, increased expression of MMPs, and elastic lamellae degradation. Consistent with these findings, Marque et al. have shown that reduced expression of fibrillin-1 in mgR/mgR mice leads to severe elastic network fragmentation but no change in cross-linking suggesting that fragmentation of the medial elastic network was not related to a defect in early elastogenesis [27]. Likewise, Guo et al have reported that addition of aortic extracts from mgR/mgR mice to cell culture systems stimulates the expression of MMPs and macrophage chemotaxis [10]. Other mouse models have correlated the emergence of pulmonary and cardiac valve manifestations with higher than normal TGF-β activity [7,28]. Finally, a recent study using the C1039G/+ mouse model of MFS has demonstrated that TGF-β antibodies or the angiotensin II type I receptor (AT1) antagonist losartan can both effectively rescue aneurysm progression [8].
Our study expands knowledge of the factors contributing to vascular disease in MFS by demonstrating that MMP production is increased in mgR/mgR mice. These observations confirm the findings of Bunton et al. who reported elevated tissue MMP levels in another model of MFS[6]. Furthermore, the efficacy of doxycycline, an MMP inhibitor, in reducing MMP levels and elastin degradation and improving survival demonstrates the role of MMPs in progression of MFS. The study suggests that MMP inhibition is a strategy that deserves consideration in patients with MFS. MMPs are a family of Ca2+-activated, Zn2+-dependent endopeptidases that are able to degrade components of ECM by their concerted actions. The elastin and collagen degradation in abdominal aortic aneurysm tissue is mediated by members of the MMP family, especially MMP-9 and MMP-2 [29-31]. MMP-9 is one of the most abundant elastolytic proteinases secreted by human AAA tissues. It is primarily produced by aneurysm-infiltrating macrophages at the sites of tissue damage and its expression appears to correlate with increasing aneurysm diameter [32,33]. MMP-2 expression is elevated in human AAAs and is primarily the product of resident mesenchymal cells [34]. Both of these enzymes are required for experimental aneurysm induction in the abdominal aorta of the mouse [14]. Some studies suggest that upregulation of MMP-2 in vascular smooth muscle cells plays a primary role in MFS aneurysm development [15,16]. Bunton et al and Neptune et al. have respectively shown greater expression of MMPs in the aorta and lung tissue of mgR/mgR mice [6,7]. Our current study showed that MMP-2 and MMP-9 production was significantly increased in mgR homozygous mice compared to normal control mice. TIMP-1 levels showed more variability than TIMP-2 levels; there were no differences in TIMP levels among the groups. The effectiveness of the MMP inhibitor doxycycline in decreasing MMP-2 and MMP-9 production and prolonging survival demonstrate, for the first time, that MMPs play an important etiologic role in the progression of thoracic aneurysms in MFS.
Doxycycline has been used to treat several conditions associated with elevated MMPs. A number of studies have shown that doxycycline can inhibit experimental AAAs and reduce MMP expression in aneurysm tissues [21,35,36]. Work from our laboratory has shown that doxycycline at standard therapeutic serum concentrations inhibits MMP-2 production from cultured human aortic SMC and AAA tissue explants [24]. Doxycycline inhibits aneurysm growth in a murine aneurysm model [22]. The precise mechanism by which doxycycline inhibits MMPs remains to be fully elucidated. A recent study has shown that doxycycline, at pharmacologically achievable nontoxic doses, inhibits TGF-β-induced MMP-9 production and activity through the Smad and MAPK signaling pathway in corneal epithelial cells [37]. A study by Yoshimura et al. demonstrated that JNK is a proximal signaling molecule in the pathogenesis of AAAs and selective inhibition of JNK in vivo not only prevented the development of AAAs but also caused regression of established mouse AAAs [38]. Preliminary data from one of our laboratories indicates that JNK signaling is altered in the aortic smooth muscle cells of mice with MFS (FR unpublished data).
In conclusion, this study provides the proof of principle that doxycycline can inhibit MMP-2 and MMP-9 production and aortic elastic fiber fragmentation, thus delaying aneurysm rupture, in this mouse model of MFS. While MMP inhibition appears to be the primary mechanism through which doxycycline protects the aorta, further work is needed to determine if doxycycline may also affect upstream mediators of matrix metabolism such as TGF-β and JNK. Since losartan blocks the Smad2 phosphorylation, i.e. TGF-β signaling, doxycycline and losartan may work together to block matrix degradation in MFS. Because of the safety profile of the tetracyclines and the potential lethality of MFS, prospective clinical trials should be considered to determine if doxycycline can delay progression of aortic disease in patients with MFS.
Figure 1.
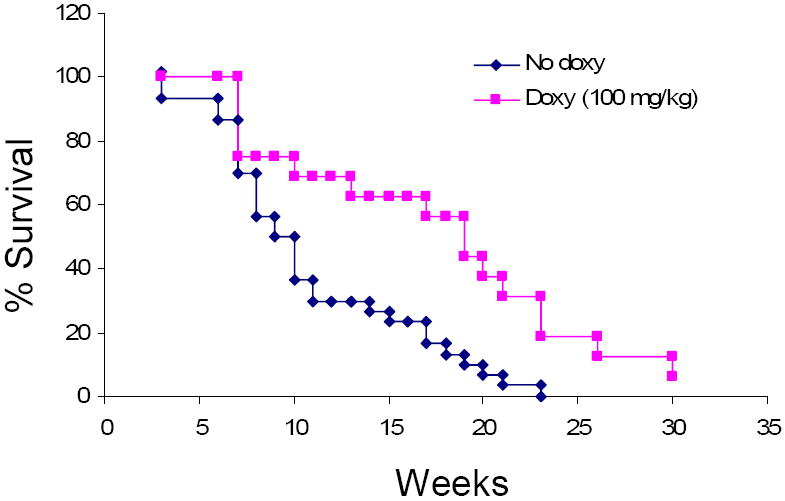
The effect of doxycycline on the survival of mgR/mgR mice. Mice were treated with doxycycline (doxy) (100 mg/kg) or regular water (no doxy) daily, started at the age of 1 day. Life table analysis shows improved survival in mice treated with doxycycline (P=0.0009).
Acknowledgments
This study was supported by National Institute Health grant 5RO1HL62400-02 to BTB and P01 AR-049698 to FR and HCD.
Abbreviations
- MFS
Marfan syndrome
- MMPs
matrix metalloproteinases
- AAAs
abdominal aortic aneurysms
- SMC
smooth muscle cell
- ECM
extracellular matrix
- doxy
doxycycline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (fbn1) in the marfan syndrome and related disorders. Hum Mol Genet. 1995;4 Spec No:1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez F, Dietz HC. Marfan syndrome: From molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007 doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of marfan syndrome. Circulation research. 2001;88:37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of tgf-beta activation contributes to pathogenesis in marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 8.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booms P, Pregla R, Ney A, Barthel F, Reinhardt DP, Pletschacher A, Mundlos S, Robinson PN. Rgd-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: A potential factor in the pathogenesis of the marfan syndrome. Human genetics. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- 10.Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgr marfan mouse model and a gxxpg-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- 11.Palombo D, Maione M, Cifiello BI, Udini M, Maggio D, Lupo M. Matrix metalloproteinases. Their role in degenerative chronic diseases of abdominal aorta. J Cardiovasc Surg (Torino) 1999;40:257–260. [PubMed] [Google Scholar]
- 12.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaitre V, Soloway PD, D’Armiento J. Increased medial degradation with pseudoaneurysm formation in apolipoprotein e-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation. 2003;107:333–338. doi: 10.1161/01.cir.0000044915.37074.5c. [DOI] [PubMed] [Google Scholar]
- 14.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 16.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with marfan’s syndrome. Circulation. 1998;98:II331–337. discussion II337-338. [PubMed] [Google Scholar]
- 17.Agan S, Sonmez S, Serdar M. The effect of topical doxycycline usage on gingival crevicular fluid mmp-8 levels of chronic and aggressive periodontitis patients: A pilot study. International journal of dental hygiene. 2006;4:114–121. doi: 10.1111/j.1601-5037.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 18.Gorska R, Nedzi-Gora M. The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and mmp-8, mmp-9, and timp-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Archivum immunologiae et therapiae experimentalis. 2006;54:419–426. doi: 10.1007/s00005-006-0047-6. [DOI] [PubMed] [Google Scholar]
- 19.de Bri E, Lei W, Svensson O, Chowdhury M, Moak SA, Greenwald RA. Effect of an inhibitor of matrix metalloproteinases on spontaneous osteoarthritis in guinea pigs. Advances in dental research. 1998;12:82–85. doi: 10.1177/08959374980120012601. [DOI] [PubMed] [Google Scholar]
- 20.Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, van Hinsbergh VW, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- 21.Curci JA, Petrinec D, Liao S, Golub LM, Thompson RW. Pharmacologic suppression of experimental abdominal aortic aneurysms: Acomparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg. 1998;28:1082–1093. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- 22.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: Comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 23.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: A randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Xiong W, Baca-Regen L, Nagase H, Baxter BT. Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells. J Vasc Surg. 2003;38:1376–1383. doi: 10.1016/s0741-5214(03)01022-x. [DOI] [PubMed] [Google Scholar]
- 25.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of cd4(+) t cells and ifn-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 26.Dietz HC, Ramirez F, Sakai LY. Marfan’s syndrome and other microfibrillar diseases. Adv Hum Genet. 1994;22:153–186. doi: 10.1007/978-1-4757-9062-7_4. [DOI] [PubMed] [Google Scholar]
- 27.Marque V, Kieffer P, Gayraud B, Lartaud-Idjouadiene I, Ramirez F, Atkinson J. Aortic wall mechanics and composition in a transgenic mouse model of marfan syndrome. Arterioscler Thromb Vasc Biol. 2001;21:1184–1189. doi: 10.1161/hq0701.092136. [DOI] [PubMed] [Google Scholar]
- 28.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. Tgf-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, Tilson MD. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase b) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14:1315–1320. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 30.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase b) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104:304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: The relationship between mmp-9 expression and aortic diameter. Circulation. 1997;96:2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- 34.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 35.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase- induced rat model of abdominal aortic aneurysm: Preservation of aortic elastin associated with suppressed production of 92 kd gelatinase. J Vasc Surg. 1996;23:336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 36.Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Annals of vascular surgery. 2006;20:228–236. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits tgf-beta1-induced mmp-9 via smad and mapk pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-jun n-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]


