Abstract
As the most common reported communicable disease in North America and Europe, Chlamydia trachomatis is the focus of concerted public health control efforts based on screening and treatment. Unexpectedly control efforts are accompanied by rising reinfection rates attributed in part to arresting the development of herd immunity. Shortening the duration of infection through the testing and treatment program is the root cause behind the arrested immunity hypothesis and because of this a vaccine will be essential to control efforts. Advances in Chlamydia vaccinomics have revealed the C. trachomatis antigens that can be used to constitute a subunit vaccine and a vaccine solution appears to be scientifically achievable. We propose that an accelerated C. trachomatis vaccine effort requires coordinated partnership among academic, public health and private sector players together with a commitment to C. trachomatis vaccine control as a global public health priority.
The Public Health Problem
Today Chlamydia trachomatis is the commonest reported bacterial infection in the United States as it is in many other developed countries [1]. Chlamydia is even more important in developing countries and globally WHO estimates that 92 million sexually transmitted infections occur annually with most infections occurring in the most impoverished parts of the world where control programs are virtually absent [2]. Because untreated infection in women causes long term problems with reproduction such as infertility and ectopic pregnancy Chlamydia has been the focus of public health control programs for nearly two decades [3]. In 2002 the estimated tangible costs of C. trachomatis illness in the United States exceeded $2.6 billion [3]. Globally the costs are uncalculated.
C. trachomatis normally infects the single cell columnar layer of epithelium of the endocervix of women and urethra of men. At the mucosal site intense inflammation characterized by erythema, edema and mucous discharge causes mucopurulent cervicitis in women and non-gonococcal urethritis in men. Despite initiating local inflammation, C. trachomatis infection remains subclinical in 70-90% of women and 30-50% of men [4]. Asymptomatically infected women on vaginal speculum examination can show signs of mucopurulent endocervical discharge, hypertrophic cervical ectopy and friability (that is, easily induced bleeding of the cervical epithelium)[5]. When women are symptomatic they may complain of dysuria, abnormal vaginal discharge, abnormal menstrual bleeding, postcoital bleeding and lower abdominal pain. In some untreated women infections spread along the epithelial surface through the endometrium to the fallopian tubes to cause pelvic inflammatory disease (PID), infertility, ectopic pregnancy and chronic pelvic pain (Figure 1). Spread along epithelial surfaces results in clinical PID (approximately 30% of clinical PID cases are due to C. trachomatis)[6] or occurs silently to cause infertility or ectopic pregnancy. Over 50% of women with infertility or ectopic pregnancy due to Chlamydia do not recall a history of PID [7, 8]. Because C. trachomatis infections are commonly subclinical screening at risk persons is at the core of Chlamydia control efforts.
Figure 1.
Infection of the female genital tract with Chlamydia trachomatis.
C. trachomatis elementary bodies infect the columnar epithelial cells of the cervix, and can ascend to infect the endometrium and the fallopian tubes, causing pelvic inflammatory disease which can lead to infertility or ectopic pregnancy. The inflammatory reaction is characterized by an influx of macrophages and neutrophils and the formation of immune inductive sites in the submucosa containing B cells, T cells, dendritic cells and macrophages [15].
National screening recommendations for C. trachomatis infection have been in place for nearly two decades in many countries [3]. Despite wide scale roll out of Chlamydia control programs in the United States, Canada and Scandinavia reported case counts of Chlamydia have not exhibited sustained declines [9]. In 2000 in the United States nearly 710,000 cases of Chlamydia were reported while in 2009 over 1.2 million infections were reported and 2.8 million infections were estimated to have annually occurred that year [1]. The question as to whether public health control programs have failed to interrupt Chlamydia transmission has been studied in detail in British Columbia (BC) Canada which has had a population wide control program in place since 1994 [10]. Over the course of the BC control program Chlamydia case rates initially declined but since 1998 have risen to levels approximately 50% above what was seen prior to the introduction of the program (Figure 2). Over the course of the control program reinfection rates significantly increased at approximately 5% per year with reinfection significantly more common among young women [10].
Figure 2.
Shown are case rates for C. trachomatis infection (blue) among women between the ages of 15 to 39 years and clinical PID (red) among women between the ages of 14 to 44 in the province of British Columbia, Canada between the years 1994 and 2009.
Reasons for rising case rates are likely multifactorial and include expansion of the screening program into higher risk populations and improved sensitivity of diagnostic tests. In BC expansion of screening and improved sensitivity of diagnostic tests accounted for less than half of the annual increase in rates. Instead it has been hypothesized that the control program is treating individuals at earlier and earlier stages of infection before the development of protective immune responses thereby perturbing the development of herd immunity. This has been called the arrested immunity hypothesis [11]. Even before these epidemiological observations were made experimental studies in the murine model of Chlamydia genital infection had already demonstrated that early treatment prevented the acquisition of protective immunity [12].
The goal of public health efforts in control of Chlamydia has been to improve the reproductive heath of women and reassuringly clinical PID rates have substantially declined during the control era. For instance in BC PID rates have declined over 80% during the 18 years of the control program (Figure2)[13]. Since Chlamydia disease pathogenesis is immune mediated [14] the control program also is arresting the development of immunopathology following infection which is the cause of PID and its sequelae.
The unexpected response of Chlamydia to control efforts suggests that its immunology determines its epidemiology. Clearly the response of C. trachomatis to seek and treat public health and medical control efforts suggests that vaccine development is the next essential step for control. The goal of a successful C. trachomatis vaccine would be to prevent the acquisition and transmission of infection and to prevent the development of inflammatory disease sequelae. Similar to the HPV vaccine a C. trachomatis vaccine would likely be first offered to school-aged girls.
Immunology
C. trachomatis is both an immunizing and immunologically sensitizing infectious disease [14]. Immunity to C. trachomatis natural infection is incomplete and long to acquire likely due to a number of immune evasion mechanisms [15]. Such mechanisms include antigen variation of the principal protective antigens (allelic variation of the major outer membrane protein [MOMP] and phase variation of the polymorphic membrane proteins [Pmps]), and a developmental cycle responsive to the cytokine environment of the host cell enabling the establishment of a persistent form associated with reduced expression of the major protective antigens. Chlamydia replication within epithelial cells puts the organism in a protected sanctuary shielded from immune effectors and likely represents its most elite strategy for immune evasion. This ‘bag of dirty little tricks’ of organismal pathobiology means that vaccine immunity will need to outdo natural immunity for a vaccine solution to successfully control C. trachomatis.
In humans mounting evidence supports the acquisition of natural immunity despite the major immune evasion mechanisms exploited by Chlamydia. Infection is less common among older than younger individuals including sex workers and among sex workers resistance to infection is related to duration of prostitution independent of age suggesting the development of acquired immunity [16]. The time to clear infection takes many months suggesting that the development of immune effector mechanisms takes months to acquire perhaps as a consequence to immune evasion [17]. HIV infection among sex workers prevents the development of immunity [16] and risk for PID is increased by repeated Chlamydia infection and by the loss of CD4 T cells among HIV infected women [18]. The prevalence of cervical antibody to Chlamydia is inversely correlated with shedding of the organism from the cervix [19]. Production of interferon gamma to Chlamydia antigen by peripheral blood mononuclear cells correlates with reduced risk of infection [20].
C. trachomatis infection of non human primates replicates key attributes of human C. trachomatis infection such as long duration to acquisition of immunity, partial resistance to reinfection and tissue pathology preferentially occurring in hosts that have been immunologically sensitized. In primates primary cervical infection generates significant resistance to reinfection[21]. Resistance is partial rather than complete in the sense that the duration and magnitude of infection is substantially attenuated. Repeated infection directly into the primate fallopian tube incites immunopathology characterized by inflammation and adhesion formation, the hallmarks of PID and its sequelae [22].
The immunological mechanism for resistance has been best studied in rodent models of Chlamydia infection which in general support human data[23]. Rodent models are based on C. muridarum infection in the mouse and C. caviae infection in the guinea pig. However, since immunity arises very quickly in the rodent model which does not appear to be the case in humans there needs to be caution in generalizing mechanistic results from rodent models to human infection.
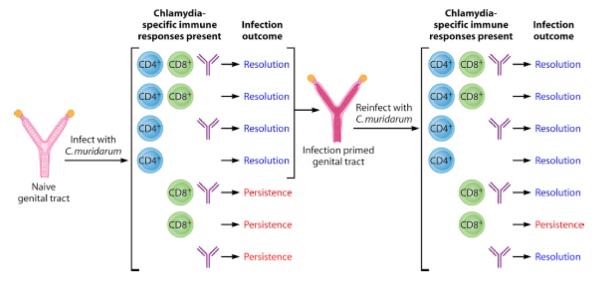
The murine C. muridarum model has demonstrated that CD4 T cells are necessary and sufficient to resolve primary infection while either CD4 T cells or antibody is necessary and sufficient to resolve reinfection [23](see Figure 3). The reason(s) behind the differences for immunity to primary infection and reinfection is unclear and its implications for vaccine design is yet to be elucidated. The mechanism for antibody mediated immunity appears to be dependent on Fc receptors [24]. These findings suggest that both CD4 T cell and antibody will be needed for a vaccine and that native conformation and surface localization will be important antigen properties.
Figure 3.
Shown are the relationship between Chlamydia specific immune responses and infection outcomes among primarily and reinfected animals [23].
In the murine model systemic and subepithelial CD4 T cells that secrete interferon gamma and tumor necrosis factor alpha correlate with protection [23] and the loss of CD4 T cells from the subepithelial site correlates with susceptibility to reinfection [25]. CD4 T cells directly inhibit epithelial cell growth of Chlamydia by contact dependent up regulation of epithelial cell indoleamine 2,3 dioxygenase and inducible nitric oxide synthase and T cell secretion of Plac8 explaining the essential nature of local mucosal immunity to Chlamydia [26]. Chlamydia antibody amplifies T cells responses by augmenting dendritic cell antigen presentation especially at low antigen levels found during early reinfection [27]. The immunological mechanism for tissue damage is less understood but may involve weak CD4 Th1 cell responses and over exuberant CD8 T cell responses consistent with the observation that loss of CD4 cells due to HIV infection increases the risk of C. trachomatis PID [18, 28].
Chlamydia Vaccines
Although vaccines have a long history in Chlamydia research the last human vaccine trial occurred nearly 50 years ago [29]. Initial approaches were entirely based on empiric Pasteurian principles of isolate, inactivate and inject. Shortly after C. trachomatis was first isolated in egg yolk sac in China in 1959 the organism was chemically inactivated and parenterally delivered in a variety of oil in water adjuvants. Trials were conducted in children at risk for trachoma because a sibling with the disease was living in the same household. The best of these trials showed that up to 70% of the children were protected against disease but that immunity waned with time and was no longer detectable three years after vaccination [30]. The vaccine formulations were studied in greater detail in non human primates. These studies demonstrated that the best protection required the highest concentration of organism suggesting that the preparations were of limited immunogenicity. Furthermore protection was most marked when the same strain was used for vaccine and challenge. Finally when breakthrough infections occurred in non human primates vaccinated with heterologous strains the inflammatory pathology was exacerbated. The investigators suggested that protective immunity was induced by type specific antigens and immunopathology was induced by antigens shared among the strains [30]. A more rational, designed based approach based on modern principle of vaccinomics is currently yielding a new path toward a subunit based Chlamydia vaccine [31].
More than a decade after these initial studies were completed the Chlamydia type specific antigen was identified as the major outer membrane protein (MOMP) and this protein became the near exclusive focus of vaccine development for nearly 20 years [32]. However MOMP is an integral membrane protein notoriously difficult to prepare in its native conformation. Denatured recombinant MOMP failed as a vaccine in multiple animal model systems including primates, mice, guinea pigs and sheep [29]. However MOMP can be enriched in its native conformation when the organism has its cytosolic proteins extracted with detergent leaving a protein rich insoluble shell termed the Chlamydia Outer Membrane Complex (COMC). Immunization of mice, guinea pigs, sheep and primates with COMC produced immunity characterized by reduced duration and intensity of shedding suggesting that native conformation was essential to MOMP efficacy as a vaccine antigen[33-36]. Purifying MOMP with selective detergents and columns yielded a native trimeric structure which produced excellent protection in mice [37] but only partial protection in primates [38]. These results suggested that conformationally intact proteins in addition to MOMP found in the COMC may be necessary for a successful vaccine.
Vaccinomics
Genomics has transformed Chlamydia vaccinology with the introduction of three new unbiased approaches to discovery of antigens relevant to vaccine design. Mining of the whole genome by reverse vaccinology through bioinformatic analysis allowed prediction of novel genes coding for membrane or secreted proteins, or proteins with homology to known virulence factors. Chlamydia secrete several proteins into the host cells cytoplasm which are expected to be preferentially loaded onto major histocompatibility complex (MHC) class I molecules for recognition by CD8 T cells. However, because murine studies demonstrate that CD8 T cells play little or no role in protective immunity, secreted Chlamydia proteins are not currently seen as priority vaccine candidates. Rather identification of proteins that load onto MHC class II molecules for CD4 T cell recognition or Chlamydia outer membrane molecules recognized by antibody are lead candidates for a subunit vaccine. In one study 120 Chlamydia proteins were identified, expressed in E. coli and analyzed for their ability to be recognized by sera of infected people or by CD4 T cells of experimentally infected mice [39]. The analysis led to the discovery of 21 novel antigens inducing antibodies and 16 proteins recognized by CD4 T cells of infected animals. Surprisingly the majority of T cell proteins were not recognized by antibody. A subgroup of the T cell proteins was shown to be protective in the animal models and when four of them were combined they made a partially efficacious vaccine. A second approach used genome wide screening of human antibodies to over 80% of the expressed C. trachomatis proteome [40]. Most of the 719 tested proteins were recognized by sera from at least one subject among the 99 naturally infected women studied. However, only 27 proteins were recognized by 50% or more of the subject sera and were suggested as potential vaccine candidates although the observation that proteins with T cell sites do not substantially overlap with B cell proteins raises concern that this approach may not identify T cell antigens. The third approach identified T cell antigens via determining Chlamydia proteins which generated peptides binding to MHC class II molecules during natural infection of dendritic cells [41]. Twenty seven proteins generated over 70 peptides which bound to class II MHC molecules. Several of these proteins were shared between C. muridarum and C. trachomatis immunoproteome. Seven proteins containing MHC class II binding peptides produced protective immune responses in the murine model of infection and when five of them were combined they generated a partially protective vaccine [42].
Vaccinomics emphasized the role for a new family of outer membrane protein antigens which had undergone only limited characterization during the pre-genomic era. This new family of outer membrane proteins is called the polymorphic membrane protein family (Pmps)[43, 44] and like MOMP they are selectively enriched in the COMC[45, 46]. Pmps are thought to be involved in Chlamydia host cell interaction such as attachment [47] and are commonly immunogenic in infected humans [48]. There are nine members of the Pmp family that are phase variable in expression[49]. Three members are also allelically variable [50] including one member whose sequence variability accurately maps to the biological variation of serovar correlated disease expression [51]. Four Pmp members are selectively enriched in T cell antigen sites and are partially protective in murine models of infection [52]. Antibody to one member of the Pmp family is neutralizing in cell culture [47].
Successful bacterial vaccines that are based on subunit components such as capsule, toxin or outer membrane vesicles often require adjuvants and can be limited by the conformational state of the vaccine antigen. Live attenuated bacterial vaccines overcome these problems which are inherent in molecular vaccines. Thus the discovery that a C. trachomatis strain that has lost the 7.5kb plasmid is attenuated in virulence allowed for exploration of such a strain as a potential vaccine. Studies in a primate trial of trachoma demonstrated for the first time in the modern vaccine era an approach that produced protection against both infection and disease [53]. Unfortunately efficacy was limited to primates that shared a common MHC class II allele suggesting that specific antigens presenting via MHC class II to CD4 T cells are vitally important to immunity and prevention of inflammatory pathology.
To complete the rational development of a Chlamydia subunit vaccine will require further knowledge about the reasons why subunit vaccines produce only partial immunity, the role for and mechanisms of antibody mediated immunity and elucidation of adjuvant technologies that engender polarized CD4 T cell together with antibody responses. Fortunately these gaps in knowledge are being rapidly bridged with advances in human immunology that allow for tackling questions of the difference between protective immunity and pathology caused by the immune response [31, 54]. Novel adjuvants and vaccine delivery technologies enable selective induction of the protective response while avoiding immunopathology [55]. Particularly promising approaches to a Chlamydia vaccine include immunization with conformationally intact outer membrane proteins in the form of COMC or the use of outer membrane vesicles containing recombinantly expressed C. trachomatis outer membrane proteins [56]. The recent development of a genetic transformation system for C. trachomatis [57] may allow the design of a C. trachomatis strain that constitutively over expresses protective CD4 T cell antigens such as the phase variable Pmps. Such genetically engineered C. trachomatis strains may be useful in preparing antigenically enhanced COMC as vaccine antigen.
Conclusions
This review aims to be a call to action to mobilize all the actors of the global vaccine community around a problem that is defeating modern medicine. The development of a Chlamydia vaccine today is scientifically tractable: genomics made available virtually all potential antigens; several animal and in vitro models are available to prioritize them; the advances in immunology provided novel adjuvants and antigen delivery systems; clinical trials to test vaccine efficacy are feasible. Therefore now that science is not the bottleneck any longer, we must focus on the non scientific obstacles. The main obstacle is the low priority on the public health agenda for Chlamydia vaccine development.
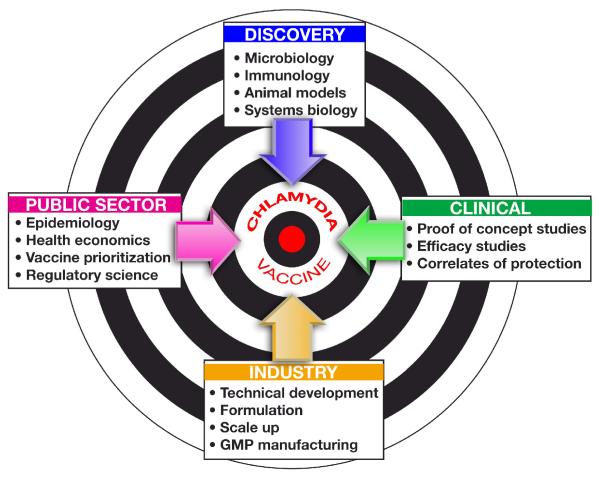
We need a vision able to mobilize the passion of scientists in different disciplines to work together and form an enterprise that can tackle a project that no one can do alone. The enterprise must involve experts in microbiology, biotechnology, immunology, animal models and systems biology to tackle vaccine discovery; experts in epidemiology and health economics to define the medical need and provide the rationale for vaccine recommendation to the public sector; experts in clinical trials, Good Clinical Practices and regulatory sciences to define the feasibility of clinical trials, the endpoints for clinical proof of concepts and efficacy studies; experts in technical development, adjuvants, formulation and Good Manufacturing Practices to develop the vaccine(s) to be tested in clinical studies. Finally, once proof of concept in humans is achieved, a private industrial partner needs to take leadership to industrialize the process, get regulatory approval and commercialize the vaccine (Figure 4). In conclusion, an increase in the priority of a C. trachomatis vaccine is the first important step in mobilizing the global vaccine community and the private sector around a problem that has become scientifically tractable. With a coordinated vaccinology approach we predict that the science is in place for a C. trachomatis vaccine to reach human trials within five years.
Figure 4.
Successful development of a C. trachomatis vaccine requires organizing activities across four key sectors. We propose the creation of a global public health enterprise dedicated to this purpose.
Highlights.
We review the epidemiological evidence supporting the need for a Chlamydia vaccine
We review the immunological basis for Chlamydia immunity
We review the vaccinomic approaches taken to develop a Chlamydia vaccine
We propose that the development of a Chlamydia vaccine requires global commitment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].CDC Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep. Apr 1;60(12):370–3. [PubMed] [Google Scholar]
- [2].Global prevalence and incidence of selected curable sexually transmitted infections: Overview and estimates. World Health Organization; 2001. [PubMed] [Google Scholar]
- [3].Johnson RE, Newhall WJ, Papp JR, Knapp JS, Black CM, Gift TL, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections--2002. MMWR Recomm Rep. 2002 Oct 18;51(RR-15):1–38. quiz CE1-4. [PubMed] [Google Scholar]
- [4].Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003 Dec 18;349(25):2424–30. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- [5].Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, et al. Mucopurulent cervicitis--the ignored counterpart in women of urethritis in men. N Engl J Med. 1984 Jul 5;311(1):1–6. doi: 10.1056/NEJM198407053110101. [DOI] [PubMed] [Google Scholar]
- [6].Brunham RC, Binns B, Guijon F, Danforth D, Kosseim ML, Rand F, et al. Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis. 1988 Sep;158(3):510–7. doi: 10.1093/infdis/158.3.510. [DOI] [PubMed] [Google Scholar]
- [7].Brunham RC, Maclean IW, Binns B, Peeling RW. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–82. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- [8].Brunham RC, Binns B, McDowell J, Paraskevas M. Chlamydia trachomatis infection in women with ectopic pregnancy. Obstet Gynecol. 1986 May;67(5):722–6. doi: 10.1097/00006250-198605000-00022. [DOI] [PubMed] [Google Scholar]
- [9].Rekart ML, Brunham RC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008 Apr;84(2):87–91. doi: 10.1136/sti.2007.027938. [DOI] [PubMed] [Google Scholar]
- [10].Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005 Nov 15;192(10):1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- [11].Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis. 2008 Jan;35(1):53–4. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- [12].Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999 Oct;180(4):1252–8. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- [13].Brunham RC, Rekart ML. Considerations on Chlamydia trachomatis disease expression. FEMS Immunol Med Microbiol. 2009 Mar;55(2):162–6. doi: 10.1111/j.1574-695X.2008.00509.x. [DOI] [PubMed] [Google Scholar]
- [14].Rekart ML, Gilbert M, Meza R, Kim PH, Chang M, Money DM, et al. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis. Oct 24; doi: 10.1093/infdis/jis644. [DOI] [PubMed] [Google Scholar]
- [15].Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005 Feb;5(2):149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- [16].Brunham RC, Kimani J, Bwayo J, Maitha G, Maclean I, Yang C, et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996 Apr;173(4):950–6. doi: 10.1093/infdis/173.4.950. [DOI] [PubMed] [Google Scholar]
- [17].Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis. 2005 Mar 15;191(6):907–16. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- [18].Kimani J, Maclean IW, Bwayo JJ, MacDonald K, Oyugi J, Maitha GM, et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis. 1996 Jun;173(6):1437–44. doi: 10.1093/infdis/173.6.1437. [DOI] [PubMed] [Google Scholar]
- [19].Brunham RC, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983 Mar;39(3):1491–4. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cohen CR, Koochesfahani KM, Meier AS, Shen C, Karunakaran K, Ondondo B, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon- gamma. J Infect Dis. 2005 Aug 15;192(4):591–9. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- [21].Wolner-Hanssen P, Patton DL, Holmes KK. Protective immunity in pig-tailed macaques after cervical infection with Chlamydia trachomatis. Sex Transm Dis. 1991 Jan-Mar;18(1):21–5. doi: 10.1097/00007435-199101000-00005. [DOI] [PubMed] [Google Scholar]
- [22].Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997 Jun;65(6):2175–82. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. Mar;79(3):986–96. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moore T, Ekworomadu CO, Eko FO, MacMillan L, Ramey K, Ananaba GA, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003 Aug 15;188(4):617–24. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- [25].Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991 Apr;59(4):1346–51. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J Immunol. Feb 15;188(4):1896–904. doi: 10.4049/jimmunol.1102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998 Aug 1;161(3):1439–46. [PubMed] [Google Scholar]
- [28].Igietseme JU, He Q, Joseph K, Eko FO, Lyn D, Ananaba G, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009 Sep 15;200(6):926–34. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Longbottom D. Chlamydial vaccine development. J Med Microbiol. 2003 Jul;52(Pt 7):537–40. doi: 10.1099/jmm.0.05093-0. [DOI] [PubMed] [Google Scholar]
- [30].Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978 Apr-Jun;5(2):73–7. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- [31].Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathog. Dec;7(12):e1002344. doi: 10.1371/journal.ppat.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–76. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tan TW, Herring AJ, Anderson IE, Jones GE. Protection of sheep against Chlamydia psittaci infection with a subcellular vaccine containing the major outer membrane protein. Infect Immun. 1990 Sep;58(9):3101–8. doi: 10.1128/iai.58.9.3101-3108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Batteiger BE, Rank RG, Bavoil PM, Soderberg LS. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J Gen Microbiol. 1993 Dec;139(12):2965–72. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- [35].Campos M, Pal S, O’Brien TP, Taylor HR, Prendergast RA, Whittum-Hudson JA. A chlamydial major outer membrane protein extract as a trachoma vaccine candidate. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1477–91. [PubMed] [Google Scholar]
- [36].Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997 Aug;65(8):3361–9. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect Immun. 2001 Oct;69(10):6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009 Jun 15;182(12):8063–70. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc Natl Acad Sci U S A. Jun 14;108(24):9969–74. doi: 10.1073/pnas.1101756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol. Aug 1;185(3):1670–80. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- [41].Karunakaran KP, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol. 2008 Feb 15;180(4):2459–65. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- [42].Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun. Apr;80(4):1510–8. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Grimwood J, Stephens RS. Computational analysis of the polymorphic membrane protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb Comp Genomics. 1999;4(3):187–201. doi: 10.1089/omi.1.1999.4.187. [DOI] [PubMed] [Google Scholar]
- [44].Nishimura K, Tajima N, Yoon YH, Park SY, Tame JR. Autotransporter passenger proteins: virulence factors with common structural themes. J Mol Med (Berl) May;88(5):451–8. doi: 10.1007/s00109-010-0600-y. [DOI] [PubMed] [Google Scholar]
- [45].Tanzer RJ, Hatch TP. Characterization of outer membrane proteins in Chlamydia trachomatis LGV serovar L2. J Bacteriol. 2001 Apr;183(8):2686–90. doi: 10.1128/JB.183.8.2686-2690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu X, Afrane M, Clemmer DE, Zhong G, Nelson DE. Identification of Chlamydia trachomatis outer membrane complex proteins by differential proteomics. J Bacteriol. Jun;192(11):2852–60. doi: 10.1128/JB.01628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia RC, Tan C, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1894–9. doi: 10.1073/pnas.0508983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tan C, Hsia RC, Shou H, Haggerty CL, Ness RB, Gaydos CA, et al. Chlamydia trachomatis-infected patients display variable antibody profiles against the nine-member polymorphic membrane protein family. Infect Immun. 2009 Aug;77(8):3218–26. doi: 10.1128/IAI.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tan C, Hsia RC, Shou H, Carrasco JA, Rank RG, Bavoil PM. Variable expression of surface-exposed polymorphic membrane proteins in in vitro-grown Chlamydia trachomatis. Cell Microbiol. Feb;12(2):174–87. doi: 10.1111/j.1462-5822.2009.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gomes JP, Nunes A, Bruno WJ, Borrego MJ, Florindo C, Dean D. Polymorphisms in the nine polymorphic membrane proteins of Chlamydia trachomatis across all serovars: evidence for serovar Da recombination and correlation with tissue tropism. J Bacteriol. 2006 Jan;188(1):275–86. doi: 10.1128/JB.188.1.275-286.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stothard DR, Toth GA, Batteiger BE. Polymorphic membrane protein H has evolved in parallel with the three disease-causing groups of Chlamydia trachomatis. Infect Immun. 2003 Mar;71(3):1200–8. doi: 10.1128/IAI.71.3.1200-1208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J Immunol. 2009 Feb 1;182(3):1602–8. doi: 10.4049/jimmunol.182.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. Oct 24;208(11):2217–23. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. Mar;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Paul WE. Bridging innate and adaptive immunity. Cell. Dec 9;147(6):1212–5. doi: 10.1016/j.cell.2011.11.036. [DOI] [PubMed] [Google Scholar]
- [56].Berlanda Scorza F, Colucci AM, Maggiore L, Sanzone S, Rossi O, Ferlenghi I, et al. High yield production process for Shigella outer membrane particles. PLoS One. 7(6):e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. Sep;7(9):e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]