Abstract
An efficacious vaccine is needed to control Chlamydia trachomatis infection. In the murine model of C. muridarum genital infection, multifunctional mucosal CD4 T cells are the foundation for protective immunity, with antibody playing a secondary role. We previously identified four Chlamydia outer membrane proteins (PmpE, PmpF, PmpG and PmpH) as CD4 T cell vaccine candidates using a dendritic cell-based immunoproteomic approach. We also demonstrated that these four polymorphic membrane proteins (Pmps) individually conferred protection as measured by accelerated clearance of Chlamydia infection in the C57BL/6 murine genital tract model. The major outer membrane protein, MOMP is also a well-studied protective vaccine antigen in this system. In the current study, we tested immunogenicity and protection of a multisubunit recombinant protein vaccine consisting of the four Pmps (PmpEFGH) with or without the major outer membrane protein (MOMP) formulated with a Th1 polarizing adjuvant in C57BL/6, Balb/c and C3H mice. We found that C57BL/6 mice vaccinated with PmpEFGH+MOMP elicited more robust cellular immune responses than mice immunized with individual protein antigens. Pmps elicited more variable cellular immune responses than MOMP among the three strains of mice. The combination vaccine accelerated clearance in the three strains of mice although at different rates. We conclude that the recombinant outer membrane protein combination constitutes a promising first generation Chlamydia vaccine construct that should provide broad immunogenicity in an outbred population.
Keywords: Chlamydia, Immunoproteomics, Recombinant protein, T cell antigens, Vaccine
INTRODUCTION
Globally over 106 million cases of sexually transmitted Chlamydia trachomatis infections occur annually [1]. C. trachomatis infection in women is of particular concern because ascending infection causes pelvic inflammatory disease (PID) which heals with scar formation resulting in infertility, ectopic pregnancy and chronic pain. Public health programs to control C. trachomatis genital infection appear to be failing as case rates continue to rise [2]. New approaches, such as an effective vaccine, are needed if control is to be achieved.
To date, efforts to develop a vaccine for Chlamydia have been only partially successful. The results from early human trials with inactivated, whole-organism vaccines suggest that development of protective immunity is feasible. While the trials achieved short-term protection, further development was halted when primates experienced enhanced ocular disease upon re-infection possibly due to vaccine induced immunopathology [3, 4]. As such, the focus shifted to a subunit vaccine design that may bypass damaging immunopathologic responses [5, 6].
The major outer membrane protein (MOMP) was the first subunit vaccine tested in animal models and has been a focus of subunit vaccine development for many years [7–9]. However, experimental vaccines based on the purified recombinant MOMP protein in various animal models have not achieved complete nor consistent protection [10, 11]. One possibility for the incomplete success of MOMP-based vaccines is that other antigens in addition to MOMP are required.
A major obstacle in developing a successful Chlamydia subunit vaccine has been the identification of antigens that induce protective cell-mediated immune (CMI) responses, as immunity to Chlamydia depends on CMI responses, especially mucosal CD4 Th1 polarized cytokine responses that accelerate clearance of infection [5, 12, 13]. Antibodies appear to play a more limited role than CD4 T cells [14]. Protective antigens are presented to T cells by MHC molecules following intracellular processing and identifying MHC-bound microbial epitopes can now be empirically determined by immunoproteomic techniques [5]. The determination of the genome sequence for Chlamydia [15, 16], along with the advances in mass spectrometry, provide the experimental means for identifying MHC-presented peptides.
We previously used an immunoproteomic approach to identify MHC class II-bound Chlamydia peptides from C. muridarum-infected murine DCs [17]. We detected MHC class II-bound peptides derived from four Pmps (PmpE, PmpF, PmpG and PmpH). Molecular study using tetramer technology confirmed that the MHC bound peptides are the dominant in vivo antigens induced by C. muridarum infection as determined by long lived expansion of CD4 Th1 cells [18]. A PmpG vaccine epitope in particular persisted on splenic antigen presenting cells for at least 6 months [19]. Vaccination with PmpE, PmpF, PmpG, PmpH or MOMP individually provided significant protection as measured by accelerated clearance in the murine C57BL/6 genital tract C. muridarum infection model. Moreover, the level of protection conferred by PmpG, PmpE or PmpF was comparable to that conferred by immunization with recombinant MOMP [20].
In the present study we compare a multisubunit vaccine composed of five recombinant outer membrane proteins (PmpEFGH+MOMP) formulated with a Th1 polarizing adjuvant DDA/MPL in three genetically distinct strains of mice (C57BL/6, C3H and Balb/c). We evaluated immune responses and shedding post vaccination and infection challenge in these different mouse strains.
MATERIALS AND METHODS
Chlamydia
C. muridarum strain Nigg (the mouse pneumonitis strain) was grown in HeLa 229 cells as previously described [21]. Elementary bodies (EBs) were purified and quantified as described elsewhere [22]. Purified EBs were aliquoted and stored at −80°C in sucrose-phosphate-glutamic acid (SPG) buffer and thawed immediately before use. Heat-killed EBs (HK-EB) were prepared by heating at 56°C for 30 min.
Preparation of C. muridarum recombinant proteins
The production and purification of recombinant proteins PmpG-1(PmpG), PmpE/F-2 (PmpF), PmpE, PmpH and MOMP have been described previously [20, 23, 24]. Briefly, the gene fragments of pmpG (aa 25–500), pmpF (aa 25–575), pmpE (aa 26–600), PmpH (aa 26–600) from C. muridarum were generated by PCR and cloned into vector pET32a (Novagen) for expression. The ompA gene encodes the MOMP protein without first 22 amino acids, was cloned into the vector pET30a. Plasmids containing pmpE, pmpF, pmpG, pmpH and ompA were transformed into Escherichia coli strain BL21 (DE3) (Stratagene). The expressed PmpE, PmpF, PmpG, PmpH and MOMP proteins, with N-terminal His tag were purified with a nickel column by using the His-Bind purification system (Qiagen). Lipopolysaccharide (LPS) was removed by adding 0.1% Triton-114 to the wash buffers during purification [25].
Mice
Female C57BL/6 (H2b), Balb/c (H2d) and C3H/HeNCrl (C3H) (H2k) mice were purchased from Charles River Canada (Saint Constant, Canada) and used at 6–8 wk of age. The mice were housed under pathogen-free conditions at the Animal Facility of the Jack Bell Research Center. All experiments were performed in strict accordance with University of British Columbia guidelines for animal care and use.
Adjuvant
The adjuvant DDA/MPL (dimethyldioctadecylammonium bromide and monophosphoryl lipid A) was prepared as previously described [20]. Briefly DDA (Avanti Polar Lipids product no. 890810P) was mixed into 10 mM Tris buffer at pH 7.4 to a concentration of 3.3 mg/ml and heated to 80°C while being stirred continuously on a magnetic hot plate for 20 min and then cooled to room temperature. MPL (Avanti Polar Lipids product no. 699800P) was suspended in distilled water (dH2O) containing 0.2% triethylamine to a concentration of 1 mg/ml. The MPL solution was heated in a 70°C water bath for 30 s and then vortexed for 60 s. The heating and vortexing procedure was repeated three times. The MPL was mixed with DDA at 1:3 volume ratio immediately before use. The emulsion consisted of 250 μg DDA and 25 μg MPL per 100 μl.
Immunization
Two mouse trials were conducted in this study. In the first trial, five groups of C57BL/6 mice were immunized with the following recombinant proteins formulated with DDA/MPL: (1) PmpG, (2) MOMP, (3) a combination of PmpE, PmpF, PmpG and PmpH (PmpEFGH), or (4) a combination of PmpEFGH plus MOMP (PmpEFGH +MOMP). The PmpG (5 μg), MOMP (5 μg), PmpEFGH (1.25 μg for each protein) or PmpEFGH+MOMP (1 μg for each protein) was diluted in 100 μl of 10 mM Tris buffer (pH 7.4) and mixed by briefly vortexing with 100 μl DDA-MPL for each dose of immunization. A group of mice immunized with phosphate-buffered saline (PBS) was used as a negative control. Mice were immunized three times subcutaneously at the base of the tail at 2-week intervals. Two weeks after the last immunization, mice in each group were sacrificed to isolate splenocytes for lymphocyte multicolor flow cytometry, and enzyme-linked immunospot (ELISPOT) assays. Four weeks after the last immunization, mice in each group were intravaginally challenged with 1,500 inclusion-forming units (IFU) of live C. muridarum for evaluation of protection against infection.
In the second trial, the protective efficacy against infection conferred by the vaccine PmpEFGH+MOMP was evaluated in the three mouse strains, C57BL/6, Balb/c and C3H mice. For each mouse strain, three groups were set up: (1) the PmpEFGH+MOMP group, (2) the PBS group as a negative control and (3) the prior Cm infection group as a positive control in which mice were previously intravaginally infected with 1,500 inclusion-forming units (IFU) of live C. muridarum. The vaccine formulation and immunization course in this trial were the same as those in the first trial. Two weeks after the last immunization, mice were sacrificed to harvest spleens for evaluation of Chlamydia specific immune responses. Four weeks after the last immunization, mice in each group were intravaginally challenged with 1,500 IFU of live C. muridarum for evaluation of protection against infection.
Genital tract infection challenge and C. muridarum quantification
Three weeks after the final immunization or 7 weeks after live C. muridarum genital, mice were injected subcutaneously with 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Pharmacia and Upjohn). One week after Depo-Provera treatment, mice were challenged intravaginally with 1,500 IFU of C. muridarum. Cervicovaginal washes were taken at selected dates after infection. Samples were vortexed in 0.5 ml of SPG buffer and stored at −80°C until analysis. Quantitative shedding of Chlamydia was measured by infection of Hela 229 cell monolayers with a titrated volume of the sample suspension. The plates were centrifuged at 1600 g for 30 min at room temperature followed by incubation at 37 °C for 30 min. Infection medium was then replaced with fresh medium containing 1 μg/ml cycloheximide (Sigma-Aldrich), and the cells were incubated at 37 °C for 24 hours. Hela cells were fixed in methanol containing 0.3% H2O2 for 30 minutes and inclusions were visualized by staining with anti-EB mouse polyclonal antibody (Homemade), followed by peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Code: 715-035-150) and a 3,3′-diaminobenzidine (DAB) substrate (Vector Laboratories).
Multiparameter flow cytometry
Two weeks after the final immunization or eight weeks after live genital C. muridarum immunization, mice were sacrificed and splenocytes were stimulated with 2 μg/ml antibody to CD28 and HK-EBs (5 × 105 IFU/ml) in complete RPMI 1640 for 4 h at 37°C. Brefeldin A was added at a final concentration of 1 μg/ml, and cells were incubated for an additional 12 h before intracellular cytokine staining. The protocol of intracellular cytokine staining was previously described [24]. We acquired 200,000 live lymphocytes per sample by using a Fortessa flow cytometer and analyzed the data by using the FlowJo software program (Tree Star).
ELISPOT assay
Two weeks after the final immunization, the IFN-γ ELISPOT assay was performed as described previously [26]. Briefly, 96-well filtration plates (Millipore, Catalog no. MAHAS4510) were coated overnight at 4°C with 2 μg/ml of murine IFN-γ-specific monoclonal antibody (clone R4-6A2; BD Pharmingen). Splenocytes isolated from each group were added to the coated plates at 106 cells per well in the presence of HK-EBs (5 × 105 IFU/ml). After 20 h of incubation at 37°C and 5% CO2, the plates were washed completely and then incubated with biotinylated murine IFN-γ-specific monoclonal antibodies (clone XMG1.2; BD Pharmingen) at 2 μg/ml. This was followed by incubation with streptavidin-alkaline phosphatase (BD Pharmingen) at a 1:1,000 dilution. The spots were visualized with a substrate consisting of 5-bromo-4-chloro-3-indolyl phosphate and Nitro Blue Tetrazolium (Sigma-Aldrich).
Statistical analysis
Data were analyzed with the aid of the GraphPad Prism software program. The Kruskal–Wallis test was performed to analyze data for C. muridarum sheddings from multiple groups, and the Mann–Whitney U test was used to compare medians between pairs. Comparison of cytokine productions as determined by multiparameter flow cytometry and ELISPOT assay between two groups were analyzed using two-tailed t test. A P value <0.05 was considered significant. Data are presented as means ± SEM.
RESULTS
Vaccination with multiple outer membrane proteins either as pooled recombinant antigens or as single antigens accelerates clearance of C. muridarum genital tract infection
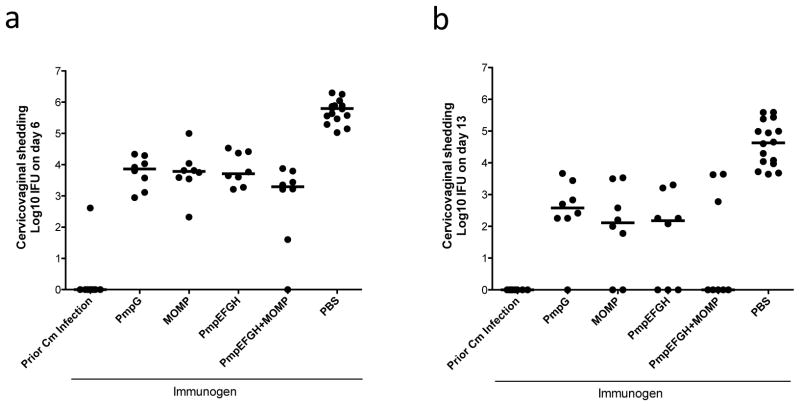
We previously evaluated the protective efficacy of individual Pmps (PmpE, PmpF, PmpG and PmpH) with the adjuvant DDA/MPL in the C57BL/6 genital tract infection model and all four proteins individually elicited protection as measured by accelerated clearance [20]. In this study, we tested the efficacy of a multisubunit formulation consisting of the four Pmps with or without MOMP. We included the PmpG alone and the MOMP alone groups for comparison. We formulated vaccine with DDA/MPL. Four weeks after the final immunization, mice were challenged intravaginally with C. muridarum. Cervicovaginal washes were taken at day 6 and day 13 after infection, and bacterial shedding was measured. Mice immunized with PBS were used as a negative control, and mice previously infected with live C. muridarum intravaginally were used as a positive control. On day 6 and 13, as shown in Fig 1a and b, mice recovered from a previous genital Chlamydia infection exhibited excellent protection. All vaccine groups demonstrated a significant reduction in Chlamydia shedding compared to the PBS group (P < 0.001). At day 13 (Fig 1b), no Chlamydia shedding was detected in the live EB group and the majority (five of 8) of mice in the PmpEFGH+MOMP group also resolved infection at day 13 although this was not statistically different from the PmpG, MOMP and PmpEFGH groups.
Figure 1.
Vaccine elicited protection against C. muridarum genital tract infection in C57BL/6 mice after immunization with different vaccine formulations in DDA/MPL adjuvant. Four weeks after the final immunization, mice were challenged intravaginally with 1,500 IFU of C. muridarum. Cervicovaginal washes were taken at day 6 (a), day 13 (b) post infection, and bacterial shedding was measured by inclusion counts on HeLa 229 cells. Mice immunized with PBS were used as a negative control, and mice previously infected with 1,500 IFU of live C. muridarum intravaginally were used as a positive control. Horizontal bar represents median value for each group. All vaccine groups significantly reduced Chlamydia shedding compared to the PBS group P value <0.001.
Multiple recombinant outer membrane protein vaccine induces more robust multifunctional Chlamydia-specific IFN-γ and TNF-α CD4 T cell responses than the single recombinant protein vaccine
We next assessed the magnitude and quality of Chlamydia specific T cell responses in C57BL/6 mice following immunization with the different vaccines. Two weeks after the final immunization, mouse splenocytes from each group were harvested. IFN-γ- or TNF-α-producing CD4 T cells were recalled by stimulation with HK-EBs and analyzed by multiparameter flow cytometry. As shown in Fig 2a, splenocytes from mice following vaccination with different antigens exhibited different levels of IFN-γ- and TNF-α responses. The PmpEFGH+MOMP immune splenocytes exposed to HK-EBs developed the highest frequency of IFN-γ- and TNF-α-secreting CD4 cells. The multiple recombinant outer membrane proteins groups, PmpEFGH and PmpEFGH+MOMP groups demonstrated statistically significantly higher frequency of EB-specific IFN-γ- or TNF-α-secreting CD4 cells than the single recombinant MOMP (P < 0.01) and PmpG groups (P < 0.05).
Figure 2.
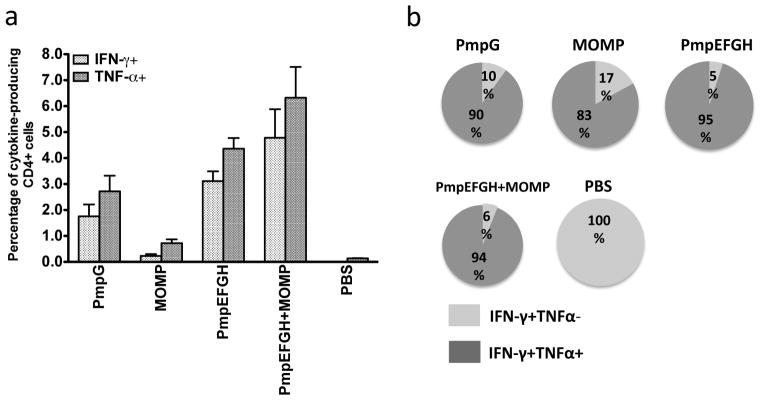
C. muridarum -specific cytokine responses after immunization with different vaccine formulations in C57BL/6 mice. Two weeks after the final immunization, splenocytes from different vaccine groups were harvested and stimulated with HK-EB (5×105 IFU/ml). IFN-γ- or TNF-α-producing CD4 T cells were analyzed by multiparameter flow cytometry as described in Materials and Methods. (a) Percentage of IFN-γ- or TNF-α-producing CD4 T cells from each vaccine group. The results are expressed as means ± SEM for groups of six mice. (b) Fraction of IFN-γ single- and IFN-γ/TNF-α double-positive cells in total IFN-γproducing CD4 T cells. All vaccine formulations most commonly elicited double positive cytokine producing CD4 T cells.
Multiparameter flow cytometry allows simultaneous analysis of multiple cytokines at the single-cell level. The total of IFN-γ- producing cells can be IFN-γ single or IFN-γ- and TNF-α double positive cells. Figure 2b pictorially represents the fractions of IFN-γ single or IFN-γ- and TNF-α double positive cells among the total of IFN-γ- producing CD4 T cells in the different vaccine groups. We observed that the IFN-γ+ TNF-α+ double-positive CD4 T cells encompassed 95, 94, 90, 83, and 0% of the total IFN-γ-producing CD4 T cells in the PmpEFGH, PmpEFGH+MOMP, PmpG, MOMP and PBS groups, respectively. The results demonstrate that the vaccine antigens formulated in the adjuvant DDA/MPL induced a high frequency of IFN-γ and TNF-α-double-positive CD4 T cells, a previously identified correlate of C. muridarum immunity [27].
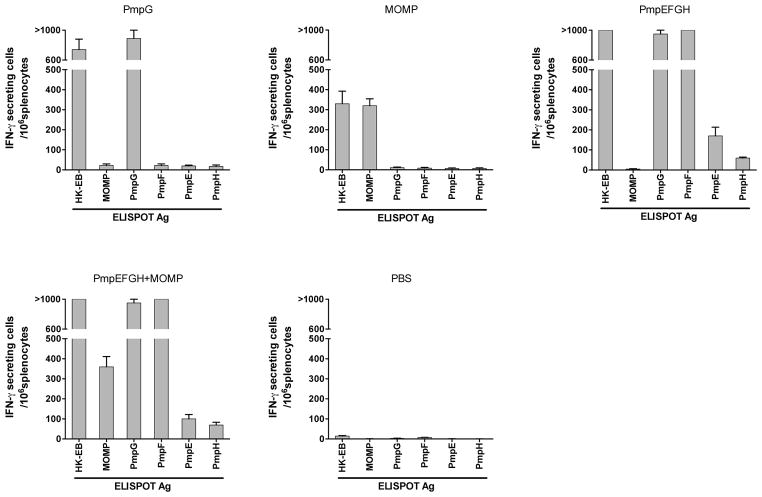
We next performed ELISPOT assays to detect IFN-γ responses against individual antigens in responses to immunization. Splenocytes from different vaccine groups were harvested and exposed to individual recombinant protein PmpE, PmpF, PmpG, PmpH and MOMP. HK-EB was included as a positive control. As shown in the Fig 3, splenocytes from the PmpG or the MOMP immunization groups showed potent IFN-γ responses only to the corresponding recombinant protein as well as to HK-EB. The PmpEFGH with or without MOMP groups exhibited profiles characterized by very strong responses to PmpG, PmpF and HK-EB, and weak responses to PmpE and PmpH. As expected MOMP responses were observed in the PmpEFGH+MOMP but not the PmpEFGH group. The PBS group exhibited very low background levels, indicating that IFN-γ –secreting cells are Chlamydia antigen-specific. Taken together, these results show that PmpG, PmpF and MOMP are the dominant protein antigens that induce IFN-γ responses among C57BL/6 mice.
Figure 3.
C. muridarum antigen-specific IFN-γ responses after immunization with different vaccine formulations determined by ELISPOT assay in C57BL/6 mice. The vaccine immunogen is shown alone each figure panel. Two weeks after the final immunization, mouse splenocytes from different vaccine groups were harvested and stimulated in vitro for 20 h with 5×105 IFU/ml of HK-EB or 1 μg/ml of recombinant protein as indicated as the ELISPOT Ag along the x-axis. The results are expressed as means ± SEM for groups of six mice. MOMP, PmpG and PmpF are immunodominant among the antigens tested in C57BL/6 mice.
Vaccine immunogens induce superior or similar Th1 immune responses as those elicited by live Chlamydia infection in different mouse strains
All the T cell antigens we studied were originally identified in C57BL/6 mice. We next evaluated immunogenicity in two additional mouse strains with different MHC backgrounds: Balb/c and C3H and compared the results to those observed in C57BL/6. Mice immunized with PBS were used as a negative control and mice previously genitally infected with live EB as a positive control. Two weeks after the final immunization or six weeks after live EB infection, splenocytes from the three strains with four different vaccinations were stimulated with HK-EBs. IFN-γ- or TNF-α-producing CD4 T cells were analyzed by multiparameter flow cytometry. As shown in Fig 4, C57BL/6 mice immunized with PmpEFGH+MOMP developed the highest IFN-γ- and TNF-α responses against HK-EBs among the three mouse strains. Compared to C57BL/6 mice, immunized C3H mice showed less balanced IFN-γ- and TNF-α responses. Immune Balb/c mice induced the lowest and least balanced cytokine responses, especially for IFN-γ. Within the same mouse strain Th1 immune response levels after vaccination with the PmpEFGH+MOMP vaccine were immunologically superior or similar to those elicited by live Chlamydia at six weeks after infection.
Figure 4.
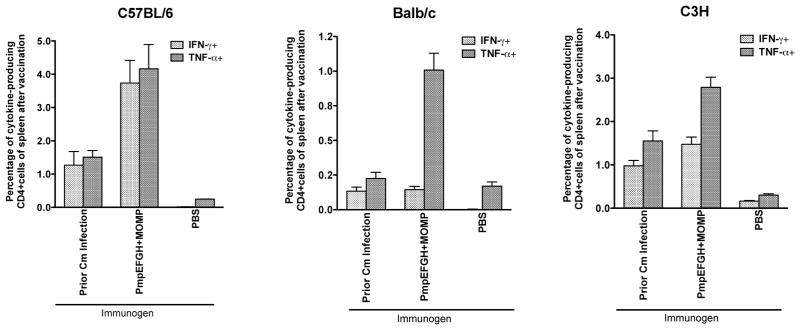
C. muridarum -specific cytokine responses after immunization with different vaccine formulations in three different inbred strains of mice (C57BL/6, Balb/c, C3H). Two weeks after the final immunization or six weeks after live EB infection, mouse splenocytes were harvested and stimulated with HK-EB (5×105 IFU/ml). IFN-γ- or TNF-α-producing CD4 T cells were analyzed by multiparameter flow cytometry. The results are expressed as means ± SEM for groups of four mice. The vaccine formulations were variably immunogenic among the three tested strains of mice.
Vaccination with PmpEFGH+MOMP elicits IFN-γ responses to the individual outer membrane proteins vary dependent on the mouse strain
We performed the ELISPOT assay to detect the IFN-γ responses to the five Chlamydia outer membrane proteins after immunization with the PmpEFGH+MOMP vaccine in the three mouse strains. Splenocytes were harvested and individually exposed to the recombinant proteins PmpE, PmpF, PmpG, PmpH and MOMP as well as HK-EB. As shown in Fig 5, C57BL6 and C3H mice immunized with the PmpEFGH+MOMP vaccine induced strong IFN-γ responses to all five outer membrane proteins. PmpG induced the strongest IFN-γ responses in both C57BL/6 and C3H mice but not in Balb/c where PmpF was dominantly immunogenic. Interestingly, MOMP was similarly immunogenic in all three strains of mice. These results suggest that the PmpEFGH+MOMP, as a multi-component vaccine, provides broad immunogenicity for CD4 T cell recognition among different MHC genetic backgrounds.
Figure 5.
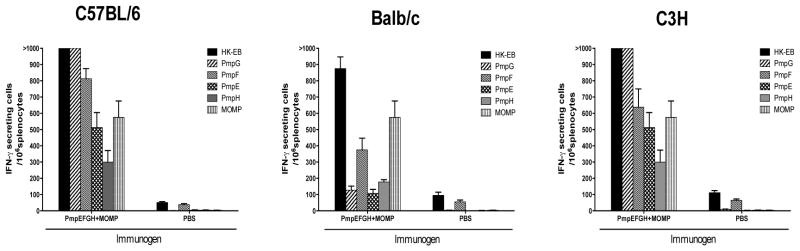
C. muridarum individual antigen-specific IFN-γ responses determined by ELISPOT assay in C57BL/6, Balb/c and C3H mice after immunization with PmpEFGH+MOMP. Two weeks after the final immunization, mouse splenocytes were harvested and stimulated in vitro for 20 h with 5×105 IFU/ml of HK-EB, or 1 μg/ml of indicated recombinant proteins. The results are expressed as means ± SEM for groups of four mice. All three mouse strains immunized with the PmpEFGH+MOMP vaccine elicited IFN-γ responses to five individual outer membrane proteins with Pmps displaying different patterns based on the genetic background and MOMP similar immunogenic in the three strains of mice.
The effect of vaccine on the clearance rate of C. muridarum infection among the three strains of mice
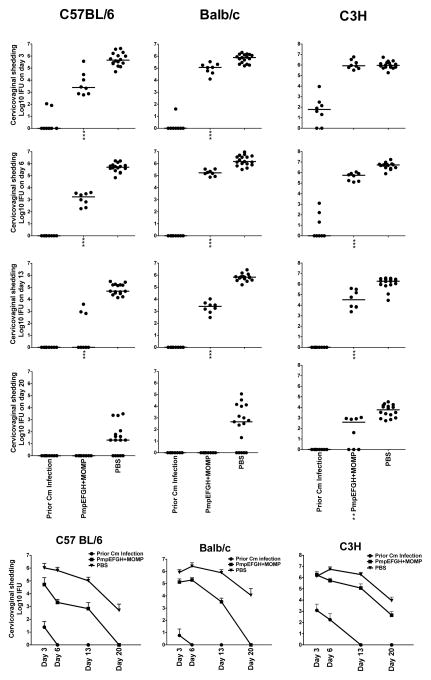
We next evaluated protection against C. muridarum infection as measured by clearance post challenge. Chlamydia inclusion titers in the cervicovaginal washes were tested at day 3, 6, 13 and 20. As shown in Fig. 6, C57BL/6 mice immunized with PmpEFGH+MOMP exhibited a 2 log10 reduction of Chlamydia shedding as early as day 3-post challenge compared to the PBS group. At day 13, five of 8 the C57BL/6 mice immunized with PmpEFGH+MOMP resolved infection. Compared to C57BL/6 mice, Balb/c mice immunized with vaccines developed less-efficient protective immunity. Although C. muridarum sheddings in Balb/c mice immunized with PmpEFGH+MOMP was statistically significantly lower than that in the PBS group (P < 0.001), no mouse resolved the infection by day 13. C3H mice had the slowest rate of clearance of Chlamydia infection among the three tested mouse strains. The different clearance kinetics in response to vaccine in the three mouse strains was similar to that observed in the live EB group suggesting innate differences in vaccine and infection susceptibility.
Figure 6.
Vaccine elicited protection against C. muridarum genital tract infection in C57BL/6, Balb/c or C3H mice immunized with different vaccine formulations. Four weeks after the final immunization or eight weeks after live EB infection, mice were challenged intravaginally with 1,500 IFU of C. muridarum. Cervicovaginal washes were taken at day 3, 6, 13 and 20 post infection and Chlamydia shedding was measured by inclusion forming units (IFU) on HeLa 229 cells. Mice immunized with PBS were used as a negative control, and mice infected with 1,500 IFU of live C. muridarum intravaginally were used as a positive control. (a) Individual Chlamydia sheddings for each group. The bar represents the median value. **, P<0.01; ***, P<0.001 vs the PBS group. (b) Infection curves for each group. The bar represents the mean Chlamydia IFU ± SD. The effect of vaccine on the rate of clearance differed among the strains of mice.
DISCUSSION
Studies in animal models and human infections have revealed that immunity to Chlamydia relies mainly on CD4 Th1 T cells producing IFN-γ. B cells and antibody play a more limited role by enhancing CD4 T cell priming and preventing spread of infection [18]. Clearance of infection from mucosal epithelial cells depends on local cellular immune responses [28, 29]. Selection of molecularly defined antigens for a subunit vaccine that stimulate CD4 Th1 cells is an essential first step in the current design effort. We applied immunoproteomic approaches to identify the MHC class II-bound peptides using C. muridarum infection of bone marrow derived dendritic cells. Polymorphic membrane family of proteins (Pmps) commonly generated MHC class II binding epitopes using this methodology. Pmps belong to a group of proteins known as type V autotransporters that may be involved in microbial attachment to host cells [30, 31]. The genomes of C. trachomatis and C. muridarum encode nine different Pmps (PmpA to PmpI) [15, 16]. The identification of Pmps as T cell antigens suggests that these outer membrane proteins may have advantages over other proteins for preferential processing and presentation to the CD4 T cells. MOMP is also known as a highly immunogenic protein that elicits measurable protection against challenge infection in several animal model systems [7, 32, 33]. In general antigenic analysis of Chlamydiae has revealed that proteins vary in whether they contain T cell epitopes, B cell epitopes or both. A Chlamydia genome wide study recently identified 42 immunogenic proteins [34]. Sixteen proteins (38%) contained CD4 T cell epitopes only, 21 (50%) contained B cell epitopes only and 5 (12%) contained both T and B cell epitopes. MOMP contains both B and T cell epitope [35, 36] whereas Pmps generally contain only T cell epitopes [37]. We hypothesized that combining Pmps with MOMP may result in a vaccine with greater protection than Pmp or MOMP alone.
Recombinant proteins alone as subunit vaccines are poor inducers of cell mediated immunity and the selection of an adjuvant is extremely important. Using PmpG protein as a model antigen, we previously tested a series of adjuvants [20]. Alum was not a suitable adjuvant in part because it failed to induce Th1 polarized CD4 T cell responses. While both CpG oligodeoxynuleotide (CpG-ODN) and DDA/MPL are potent Th1-polarizing adjuvants, which activate antigen-presenting cells through Toll-like receptor 9 (TLR9) and TLR4 respectively. However, immunization with PmpG formulated with CpG-ODN failed to provide protection against Chlamydia infection, whereas immunization with PmpG formulated with DDA/MPL reduced infection by 2~3 log10 [24].
In this study, we tested four Pmps (PmpEFGH) plus MOMP formulated with DDA/MPL as a novel Chlamydia multisubunit recombinant vaccine in three strains of mice (C57BL/6, C3H and Balb/c). In C57BL/6 mice the multisubunit vaccine (PmpEFGH+MOMP) produced the best overall protection as measured by accelerated clearance (P <0.001 versus the PBS group) although this was not statistically different from the single and multi antigen groups perhaps due to the limited number of animals tested (Figure 1). The multisubunit vaccine was significantly more immunogenic than the single antigen groups (P <0.01versus MOMP and P <0.05 versus PmpG) and induced a robust multifunctional CD4 T cell responses previously identified as a correlate of protection in the C. muridarum genital tract model [27]. The multisubunit vaccine induced strong CD4 T cell responses to PmpG, PmpF and MOMP whereas the single antigen group induced CD4 T cell responses only to itself. Overall, the multisubunit vaccine was more immunogenic and tended to clear infection faster than the single antigen vaccine groups.
Since the Chlamydia CD4 T cell antigens were originally discovered in C57BL6 mice it is important to demonstrate that mice with different MHC backgrounds are capable of responding to these antigens. Accordingly, we evaluated the multisubunit vaccine in C57BL/6, Balb/c and C3H mice. Overall the multisubunit vaccine was at least as immunogenic as live C. muridarum infection in the three strains of mice. Interestingly, the TNFα and IFN-γ cytokine response in Balb/c mice was significantly unbalanced compared to the other two strains of mice. Balb/c mice are known to have reduced IFN-γ responses to C. muridarum infection than C57BL/6 [22]. Balb/c responded with the lowest IFN-γ responses to the individual Pmp antigens compared to the other two strains with PmpF emerging as immunodominant. In C57BL/6 and C3H, PmpG was immunodominant. In all three strains PmpE and PmpH were relatively immunorecessive. In distinction MOMP was similarly immunogenic in all three strains of mice (figure 5). The induction of a robust Chlamydia-specific T cell response in different strains may imply that the vaccine antigens contain assorted epitopes that are recognized by different MHC haplotypes. The higher level of immune responses may be due to the presence of more epitopes recognized by some MHCs or superior innate immune reactivity in some strains. The multisubunit vaccine accelerated clearance of C. muridarum genital infection in all three strains compared to the PBS group (p<0.001) but was least effective in C3H. Interestingly the rate of clearance of live C. muridarum infection exhibited similar differences among the three strains as did vaccine suggesting that innate factors are important to both infection resistance and vaccine immunity. Our previous study showed that genetically determined differences in C. muridarum infection are associated with differences in early innate cytokine profiles, dendritic cell functions and cellular response patterns [38].
The assessment of protection against C. trachomatis-induced pathologies is a key consideration in Chlamydia vaccine development. In this study we did measured visually apparent hydrosalpinx in murine oviduct as a surrogate of the pathologic outcome eight weeks after genital infection challenge with C. muridarum in the different vaccine groups. We observed that the majority of mice developed hydrosalpinx in all groups and no significant differences were observed between the vaccine groups and the PBS group. Similar pathologic results were reported by Hansen et al using the adjuvant DDA/TDB with MOMP as an antigen that induced robust protection against Cm genital infection in the mouse model [32].
It is known that tissue damage from a Chlamydia infection is caused by the inflammatory response [39–41]. C. muridarum infection of the murine genital tract causes early inflammatory damage to oviductal tissue that is mediated by neutrophils and dependent on local activation of TLR-2 driven by active replication of the organism [42, 43]. Human C. trachomatis tissue damage on the other hand, appears to be driven by the T cell immune response dependent on cycles of reinfection or of long duration infection [44]. For these reasons, the C. muridarum genital tract model is unlikely to be predictive of human C. trachomatis immunopathology. Currently we are planning to carry out a systematic analysis of the effect of vaccination on tissue pathology using the primate model where vaccine induced immunopathology was initially defined.
In conclusion, a multisubunit T cell vaccine based on five Chlamydia outer membrane proteins including PmpE, PmpF, PmpG, PmpH and MOMP induces protective immunity as measured by enhanced clearance in three strains of mice. Broad immunogenicity and possible synergy among the multiple outer membrane proteins may explain the efficacy of PmpEFGH+MOMP as a Chlamydia vaccine. We propose assessment of the multisubunit vaccine for protection against infection and immunopathology using C. trachomatis orthologs in a non-human primate genital tract model [45].
Acknowledgments
This work was supported by grant from the National Institute of Health (Grant No. R01A1076483).
We are grateful to David Ko and Gayle Thornbury in the Terry Fox Laboratory at the British Columbia Cancer Research Centre for providing flow cytometry services and technical assistance.
Footnotes
This work was supported by National Institutes of Health Grant R01AI076483
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Starnbach MN, Roan NR. Conquering sexually transmitted diseases. Nat Rev Immunol. 2008;8:313–7. doi: 10.1038/nri2272. [DOI] [PubMed] [Google Scholar]
- 2.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 3.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–7. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967;63(Suppl):1615–30. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 5.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 6.Stanberry LR, Rosenthal SL. Progress in vaccines for sexually transmitted diseases. Infect Dis Clin North Am. 2005;19:477–90. xi. doi: 10.1016/j.idc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. Journal of immunology. 2009;182:8063–70. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect Immun. 2001;69:6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw J, Grund V, Durling L, Crane D, Caldwell HD. Dendritic cells pulsed with a recombinant Chlamydial major outer membrane protein antigen elicit a CD4(+) type 2 rather than type 1 immune response that is not protective. Infect Immun. 2002;70:1097–105. doi: 10.1128/IAI.70.3.1097-1105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal S, Barnhart KM, Wei Q, Abai AM, Peterson EM, de la Maza LM. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–65. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 12.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. Journal of immunology. 1997;158:3344–52. [PubMed] [Google Scholar]
- 14.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to Chlamydial genital tract reinfection. Journal of immunology. 2005;175:7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–9. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 16.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karunakaran KP, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. Journal of immunology. 2008;180:2459–65. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- 18.Li LX, McSorley SJ. B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Chlamydia muridarum Genital Tract Infection. PLoS pathogens. 2013;9:e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RM, Yu H, Kerr MS, Slaven JE, Karunakaran KP, Brunham RC. PmpG303-311, a protective vaccine epitope that elicits persistent cellular immune responses in Chlamydia muridarum-immune mice. Infect Immun. 2012;80:2204–11. doi: 10.1128/IAI.06339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun. 2012;80:1510–8. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Xing Z, Brunham RC. GM-CSF transgene-based adjuvant allows the establishment of protective mucosal immunity following vaccination with inactivated Chlamydia trachomatis. Journal of immunology. 2002;169:6324–31. doi: 10.4049/jimmunol.169.11.6324. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, HayGlass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. Journal of immunology. 1996;156:4338–44. [PubMed] [Google Scholar]
- 23.Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. Journal of immunology. 2009;182:1602–8. doi: 10.4049/jimmunol.182.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun. 2010;78:2272–82. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clinical biochemistry. 1997;30:455–63. doi: 10.1016/s0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 26.Ioannou XP, Griebel P, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. J Virol. 2002;76:9002–10. doi: 10.1128/JVI.76.18.9002-9010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Karunakaran KP, Kelly I, Shen C, Jiang X, Foster LJ, et al. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. Journal of immunology. 2011;186:3615–21. doi: 10.4049/jimmunol.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. Journal of immunology. 2012;188:1896–904. doi: 10.4049/jimmunol.1102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayarapu K, Kerr M, Ofner S, Johnson RM. Chlamydia-specific CD4 T cell clones control Chlamydia muridarum replication in epithelial cells by nitric oxide-dependent and -independent mechanisms. Journal of immunology. 2010;185:6911–20. doi: 10.4049/jimmunol.1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molleken K, Schmidt E, Hegemann JH. Members of the Pmp protein family of Chlamydia pneumoniae mediate adhesion to human cells via short repetitive peptide motifs. Molecular microbiology. 2010;78:1004–17. doi: 10.1111/j.1365-2958.2010.07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molleken K, Becker E, Hegemann JH. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS pathogens. 2013;9:e1003325. doi: 10.1371/journal.ppat.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen J, Jensen KT, Follmann F, Agger EM, Theisen M, Andersen P. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital Chlamydial infection in a mouse model. J Infect Dis. 2008;198:758–67. doi: 10.1086/590670. [DOI] [PubMed] [Google Scholar]
- 33.Andrew DW, Hafner LM, Beagley KW, Timms P. Partial protection against Chlamydial reproductive tract infection by a recombinant major outer membrane protein/CpG/cholera toxin intranasal vaccine in the guinea pig Chlamydia caviae model. Journal of reproductive immunology. 2011;91:9–16. doi: 10.1016/j.jri.2011.06.100. [DOI] [PubMed] [Google Scholar]
- 34.Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9969–74. doi: 10.1073/pnas.1101756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baehr W, Zhang YX, Joseph T, Su H, Nano FE, Everett KD, et al. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4000–4. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz L, Demick KP, Petersen JW, Polka M, Rudersdorf RA, Van der Pol B, et al. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from infected humans. Journal of immunology. 1996;157:4554–67. [PubMed] [Google Scholar]
- 37.Coler RN, Bhatia A, Maisonneuve JF, Probst P, Barth B, Ovendale P, et al. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS immunology and medical microbiology. 2009;55:258–70. doi: 10.1111/j.1574-695X.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang X, Shen C, Yu H, Karunakaran KP, Brunham RC. Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection. Immunology. 2010;129:556–66. doi: 10.1111/j.1365-2567.2009.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belay T, Eko FO, Ananaba GA, Bowers S, Moore T, Lyn D, et al. Chemokine and chemokine receptor dynamics during genital Chlamydial infection. Infect Immun. 2002;70:844–50. doi: 10.1128/IAI.70.2.844-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799–806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patton DL, Kuo CC. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–56. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 42.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, et al. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis. 2005;32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 43.Darville T, O’Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in Chlamydial genital tract infection. Journal of immunology. 2003;171:6187–97. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 44.Stephens RS. The cellular paradigm of Chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 45.Wolner-Hanssen P, Patton DL, Holmes KK. Protective immunity in pig-tailed macaques after cervical infection with Chlamydia trachomatis. Sex Transm Dis. 1991;18:21–5. doi: 10.1097/00007435-199101000-00005. [DOI] [PubMed] [Google Scholar]