Abstract
Background: Treatment options for patients with advanced or metastatic medullary thyroid cancer (MTC) have, in recent years, expanded with the approval of two tyrosine kinase inhibitors (TKIs): vandetanib and cabozantinib. Other agents, including TKIs, are under clinical investigation for MTC. Although patients treated with TKIs are at risk of developing dermatologic adverse events (AE), these untoward events may be mitigated through AE-driven algorithms.
Summary: AE-driven algorithms combine effective nonpharmaceutical and pharmaceutical treatment modalities implemented by a multidisciplinary effort that incorporates nursing interventions, patient education, and referrals to pain-management specialists, podiatrists, and dermatologists, as appropriate. Effective AE prevention and management reduce the need for dose interruptions and modifications, allowing patients the opportunity to derive the maximal benefit from TKI therapy, while maintaining quality of life.
Conclusions: Optimal use of targeted therapies in the treatment of MTC depends on careful patient selection, interdisciplinary communication, and patient education and encouragement to enhance compliance and safety, optimize consistent dosing, and maximize the use of effective therapies.
Introduction
Thyroid cancer is the most common endocrine-related cancer (1) and is classified into three main histologic types: differentiated (papillary, follicular, and Hürthle), medullary, and anaplastic (undifferentiated) (2). The most common forms of thyroid cancer are papillary (80%) and follicular (11%); rarer types include medullary thyroid cancer (MTC) (4%) (2), Hürthle cell (3%), and anaplastic (2%) (3). While MTC is uncommon, it accounts for up to 14% of all thyroid cancer-related deaths (4). Approximately 70–75% of all MTC cases are sporadic and often present as a single unilateral tumor (5).
The five-year relative survival rate for patients with MTC by stage at diagnosis is 98% for stages I and II, 73% for stage III, and 40% for stage IV (3). Lower clinical stage and younger age (<40 years) at diagnosis are strong independent predictors for improved survival (4).
Patients with MTC are susceptible to the development of early metastatic disease. For example, 50–55% of patients with MTC develop local metastases to the lymph nodes, and 20% have metastatic disease to the lung, liver, or bone at diagnosis (6,7).
The main goals of treatment for patients with metastatic disease are optimizing survival and quality of life (QoL). Currently, there is no curative systemic therapy for patients with MTC, and treatment options for patients with recurrent or persistent disease are limited (2,8). Vandetanib is an oral small-molecule multitargeted tyrosine kinase inhibitor (TKI) of the product of the Rearranged during Transfection (RET) gene, vascular endothelial growth factor receptor (VEGFR), and epidermal growth factor receptor (EGFR) signaling pathways (9,10). Another small-molecule TKI, cabozantinib, also inhibits multiple tyrosine kinases, including RET, VEGFR2, as well as mesenchymal-epithelial transition factor (MET) (11,12). The U.S. Food and Drug Administration (FDA) approved vandetanib and cabozantinib for the treatment of advanced MTC in 2011 and 2012 respectively (12,13). Vandetanib also received marketing approval for MTC in Europe and Canada. Cabozantinib has recently been approved for treatment of MTC by the European Medicines Agency for this indication (14). Other TKIs are under clinical investigation for MTC (15), some of which are currently marketed for other indications. TKIs have varying profiles of potential pharmacologic targets that include subtypes of VEGFR and EGFR, as well as other proto-oncogene targets (Table 1) (12,13,15–21).
Table 1.
Incidence of Dermatologic Adverse Events Associated with TKI Treatment in Clinical Trials of Patients with Locally Advanced or Metastatic MTC
| Dermatologic adverse events, % of patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Pharmacologic targets | Authors | Type of study | Type of MTC | No. of patients | All grades | Grade≥3 | |
| Vandetanib | EGFR VEGFR RET BRK TIE2 EPH Src (13) |
Robinson et al. 2010 (16) | Phase II, single arm, 100 mg/d | Hereditary | 19 | Rash | 26 | NR |
| Wells et al. 2010 (17) | Phase II, single arm, 300 mg/d | Hereditary | 30 | Rash | 67 | 3 | ||
| Wells et al. 2012 (18) Caprelsa PI 2012 (13) |
Phase III, vandetanib (300 mg/d) vs. placebo | Hereditary or sporadic | 231 | Rash | 53 | 5 | ||
| Dermatitis acneiform/acne | 35 | 1 | ||||||
| Dry skin | 15 | 0 | ||||||
| Photosensitivity reactions | 13 | 2 | ||||||
| Pruritus | 11 | 1 | ||||||
| Nail abnormalities | 9 | 0 | ||||||
| Cabozantinib | VEGFR1 VEGFR2 VEGFR3 RET MET KIT FLT3 TIE-2 AXL TRKB (12) |
Cometriq PI 2012 (12) Schoffski 2012 (19) |
Phase III, cabozantinib (140 mg/d) vs. placebo | Hereditary or sporadic (12) | 214 | HFSR | 50 | 13 |
| Hair color changes | 34 | 0 | ||||||
| Rash | 19 | 1 | ||||||
| Dry skin | 19 | 0 | ||||||
| Alopecia | 16 | 0 | ||||||
| Erythema | 11 | 1 | ||||||
| Hyperkeratosis | 7 | 0 | ||||||
| Sunitinib | VEGFR1 VEGFR2 VEGFR3 RET RET/PTC subtype1 RET/PTC subtype3 (15) |
De Souza et al. 2010 (20) | Phase II | NS | 25 | HFSR | NR | 17 |
| Sorafenib | VEGFR2 VEGFR3 RET BRAF (15) |
Lam et al. 2010 (21) | Phase II, single arm, 400 mg twice daily | Hereditary or sporadic | 21 | HFSR | 90 | 14 |
| Alopecia | 76 | 0 | ||||||
| Rash (non-HFSR) | 67 | 0 | ||||||
| Nail changes | 48 | 0 | ||||||
| Dry skin/scalp | 48 | 0 | ||||||
| Skin lesions/sores (non-HFSR) | 38 | 0 | ||||||
| Scalp pruritus | 33 | 0 | ||||||
| Photosensitivity | 14 | 0 | ||||||
| Ruddy complexion | 5 | 0 | ||||||
EGFR, epidermal growth factor receptor; HFSR, hand-foot skin reaction; MTC, medullary thyroid cancer; NR, not reported; NS, not specified; RET, Rearranged during Transfection; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
The present article focuses on the assessment and management of TKI-associated dermatologic events in patients with MTC. The rationale and data to support the use of TKIs in MTC, the dermatologic adverse events (AEs) associated with this treatment, and recommendations for prevention, management, nursing assessments, and patient education are described.
Review
Rationale for using TKIs in MTC
In the early 1990s, investigators found that mutations in the RET proto-oncogene are linked to the development of MEN2A, MEN2B, and FMTC (22–25). Somatic mutations in RET are also common in sporadic MTC (26) and correlate with the risk of having lymph-node metastases at diagnosis, persistent disease, and shorter survival (27).
The RET gene encodes a transmembrane receptor tyrosine kinase that has important roles in cell growth, differentiation, and survival (5). The RET receptor is recognized by the persephin, artemin, and neurturin ligands that belong to the glial cell-derived neurotrophic factor family. The RET receptor is expressed by noradrenergic and dopaminergic neurons, thyroid C cells, and adrenal medulla. Gain of function or activating mutations in the RET gene are the primary cause of all hereditary MTC cases and between 25% and 50% of sporadic cases (5).
The vascular endothelial growth factor A (VEGF-A) and its VEGFRs, which have major roles in angiogenesis in normal tissues and malignant tumors, may also be involved in the development and maintenance of sporadic and hereditary MTC (28). VEGF-A and several VEGFRs are overexpressed in MTC biopsy specimens (28,29). In addition, VEGFR2 and EGFR are overexpressed in some metastases, while they are not expressed within the primary tumor, suggesting a role for these receptors in the progression of MTC (30). In preclinical studies using human thyroid tumor cell lines, high VEGF expression correlated with increased tumorigenic potential (31). Increased expression of MET has been observed in some MTC tumors (11,32).
Members of the RAS family of low-molecular weight GTP-binding proteins are important downstream mediators of effects occurring through these tyrosine kinases (33). RAS proteins are essential mediators in a variety of pathways that regulate normal and malignant cell proliferation (33). The RAS pathway, particularly through downstream effects at the RET receptor, has been implicated in MTC (34). In addition, a recent study in which the exomes of 17 sporadic MTCs were sequenced and compared with corresponding findings in an independent cohort of 40 sporadic and hereditary MTCs revealed that approximately 90% of MTCs have mutations in either RET or RAS (35). These mutations were found to be mutually exclusive, and few other types of mutations were identified.
In a phase III randomized double-blind study, vandetanib treatment (300 mg, administered orally, once daily) (13) compared with placebo significantly prolonged progression-free survival (PFS) in patients with symptomatic or progressive locally advanced or metastatic MTC (hazard ratio [HR] 0.46; 95% confidence interval [CI 0.31–0.69]; p<0.001) (18). Patients treated with vandetanib also showed improved objective response rates, disease control rates, and calcitonin and carcinoembryonic antigen biochemical response rates. Overall survival data are not yet available. The most common all-grade AEs (occurring at a frequency of ≥5%) reported for the vandetanib and placebo groups respectively were diarrhea (57% vs. 27%), rash (53% vs. 12%), dermatitis acneiform/acne (35% vs. 7%), nausea (33% vs. 16%), hypertension (33% vs. 5%), fatigue (24% vs. 23%), headache (26% vs. 9%), upper respiratory infection (23% vs. 16%), decreased appetite (21% vs. 12%), and abdominal pain (21% vs. 11%) (13). The study protocol mandated dose reduction from 300 mg/d to 200 mg/d for a grade 3 or 4 dermatologic AE (as well as for grade 3 or 4 gastrointestinal AEs and QTc prolongation) and another reduction to 100 mg/d if an additional grade 3 or 4 AE occurred (18).
Another phase III, double-blind, placebo-controlled study evaluated cabozantinib (140 mg, administered orally, once daily) compared with placebo in patients with actively progressive MTC within 14 months of screening (19). Median PFS was 11.2 months and 4.0 months in the cabozantinib and placebo arms respectively (HR 0.28; [CI 0.19–0.40]; p<0.0001) (12). An improvement in partial response rate was also observed in the group receiving cabozantinib compared with those receiving placebo. No statistically significant difference in overall survival was seen at the planned interim analysis. The most common all-grade AEs in the cabozantinib arm compared with the placebo arm were diarrhea (63% vs. 33%), stomatitis (51% vs. 6%), palmar-plantar erythrodysesthesia syndrome (i.e., hand-foot skin reaction [HFSR]; 50% vs. 2%), decreased weight (48% vs. 10%), decreased appetite (46% vs. 16%), nausea (43% vs. 21%), fatigue (41% vs. 28%), oral pain (36% vs. 6%), hair color changes (34% vs. 1%), dysgeusia (34% vs. 6%), hypertension (33% vs. 4%), constipation (27% vs. 6%), abdominal pain (27% vs. 13%), vomiting (24% vs. 2%), asthenia (21% vs. 15%), and dysphonia (20% vs. 9%) (12).
Dermatologic AEs associated with TKIs
Dermatologic AEs have been well characterized for TKIs that target the VEGFR or EGFR pathways. Patients treated with TKIs may experience cutaneous AEs such as rash, erythema, pruritus, acneiform rash, paronychia, telangiectasia, alopecia, changes in hair growth or pigmentation, skin discoloration, xerosis (dryness), and HFSR (36,37). Incidence and severity of dermatologic AEs vary across the specific targeted therapies used and prescribed doses. While the factors that determine the profile of AEs have not been fully elucidated, risk factors that may be associated with skin reactions subsequent to EGFR inhibition include age, smoking status, and exposure to ionizing and ultraviolet (UV) radiation (e.g., sunlight) (38).
Putative mechanisms
The EGFR, which is thought to play an important role in the proliferation of the basal epidermal layer, is expressed at high levels in the basal and suprabasal layers of the skin, and in the outer layers of hair follicles (37). In experimental systems, inhibition of EGFR signaling in keratinocytes causes growth arrest, initiation of differentiation, and apoptosis. In patients treated with EGFR inhibitors, histologic analysis of the skin has revealed marked downregulation of phosphorylated EGFR and decreased proliferation and premature differentiation of basal keratinocytes (37). Thus, skin AEs associated with EGFR-inhibitor treatment are likely to be linked to the overall inhibition of EGFR signaling in basal keratinocytes, leading to alterations in keratinocyte survival, proliferation, differentiation, migration, and attachment (37,39). EGFR inhibition can also cause an inflammatory response by triggering the release of cytokines and the resultant recruitment and activation of neutrophils, monocytes, and lymphocytes (39). Inflammation is a secondary but important contributor to the development of these dermatologic AEs (39). The pathogenic mechanism for the development of HFSR is thought to involve combined inhibition of the VEGFR and platelet-derived growth factor receptor (PDGFR) proangiogenic pathways, possibly preventing the proper implementation of vascular repair mechanisms in these high-pressure areas (36).
Types of skin AEs linked to specific drugs
Table 1 lists the incidence of dermatologic AEs associated with the TKIs vandetanib, sunitinib, sorafenib, and cabozantinib in clinical trials of patients with locally advanced or metastatic MTC (12,13,15–21). Table 2 presents a grading scale on the severity of these AEs provided in the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) (40).
Table 2.
CTCAE Grading of Selected TKI-Associated Dermatologic AEs*
| Grade | |||||
|---|---|---|---|---|---|
| AE | 1 | 2 | 3 | 4 | 5 |
| Palmar-plantar erythrodysesthesia syndrome (hand-foot skin reaction) Definition: A disorder characterized by redness, marked discomfort, swelling, and tingling in the palms of the hands or the soles of the feet |
Minimal skin changes or dermatitis (e.g., erythema, edema, or hyperkeratosis) without pain | Skin changes (e.g., peeling, blisters, bleeding, edema, or hyperkeratosis) with pain; limiting instrumental ADL | Severe skin changes (e.g., peeling, blisters, bleeding, edema, or hyperkeratosis) with pain; limiting instrumental ADL | — | — |
| Photosensitivity Definition: A disorder characterized by an increase in sensitivity of the skin to light |
Painless erythema and erythema covering <10% BSA | Tender erythema covering 10–30% BSA | Erythema covering >30% BSA and erythema with blistering; photosensitivity; oral corticosteroid therapy indicated; pain control indicated (e.g., narcotics or NSAIDs) | Life-threatening consequences; urgent intervention indicated | Death |
| Acneiform rash Definition: A disorder characterized by an eruption of papules and pustules, typically appearing in the face, scalp, upper chest, and back |
Papules and/or pustules covering <10% BSA, which may or may not be associated with symptoms of pruritus or tenderness | Papules and/or pustules covering 10–30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; associated with psychosocial impact; limiting instrumental ADL | Papules and/or pustules covering >30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; limiting self-care ADL; associated with local superinfection with oral antibiotics indicated | Papules and/or pustules covering any % BSA, which may or may not be associated with symptoms of pruritus or tenderness and are associated with extensive superinfection with IV antibiotics indicated; life-threatening consequences | Death |
National Cancer Institute Common Terminology Criteria for Adverse Events (40).
ADL, activities of daily living; AEs, adverse events; BSA, body surface area; CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events; IV, intravenous; NSAIDs, nonsteroidal anti-inflammatory drugs.
In the placebo-controlled phase III study of patients with MTC, dermatologic AEs of vandetanib were, in decreasing order of incidence, rash, dermatitis acneiform or acne, dry skin, photosensitivity reactions, pruritus, and nail abnormalities (Table 1) (13). Severe skin reactions, such as Stevens–Johnson syndrome, were rare, although some led to death or permanent treatment discontinuation (13). A systematic review and meta-analysis of nine studies of single-agent vandetanib in 2961 patients found a 46.1% incidence of all-grade skin rash and 3.5% high-grade skin rash (41). The same analysis reported that, when compared with controls in randomized studies, patients treated with vandetanib had a 2.43 relative risk ([CI 1.37–4.29]; p=0.002) of developing an all-grade rash. An analysis of the skin toxic effects observed in three clinical trials of patients receiving vandetanib for the treatment of advanced MTC revealed two additional dermatologic AEs: photodistributed erythematous eruptions and skin pigmentation changes (e.g., appearance of gray-blue macules on the face, scalp, or trunk) (42). Discontinuation of therapy was associated with rapid improvement in almost all patients with skin phototoxic effects, and reinitiation of therapy at a lower dose accompanied by strict photoprotection measures was successful in preventing a recurrence of this AE (42). A gradual disappearance of pigmented macules over a three- to six-month period was noted upon treatment discontinuation (42).
Dermatologic AEs associated with cabozantinib treatment in patients with MTC from the placebo-controlled phase III study were, in descending order, HFSR, hair color changes, rash, dry skin, alopecia, erythema, and hyperkeratosis (Table 1) (12).
Data from trials in MTC represent relatively small numbers of patients. Hence, the occurrence and incidence of these skin reactions may not be representative of experience in the overall population of patients treated with TKIs for all forms of cancer.
In registration studies for sunitinib, rash (29%), HFSR (29%), skin discoloration (25%), dry skin (23%), and hair color changes (20%) were among the most common AEs experienced by patients with renal cell carcinomas. Similar dermatologic AEs were reported in patients with gastrointestinal stromal tumors or pancreatic neuroendocrine tumors (43). Warning notices or precautions for dermatologic AEs are not listed in the manufacturer's prescribing information.
In patients with renal cell cancer and hepatocellular cancer, HFSR and rash are the most common dermatologic AEs associated with sorafenib treatment. They are usually grade 1 or 2 according to the National Cancer Institute's CTCAE grading scale and typically occur during the first six weeks of treatment (40,44). In registration studies of sorafenib in patients with hepatocellular carcinoma and renal cell carcinoma, up to about 1% of patients had to permanently discontinue treatment due to HFSR. In the sorafenib prescribing information for dermatologic events reported from multiple clinical trials, erythema occurred in ≥10% of patients, and exfoliative dermatitis, acne, and flushing in 1% to ≤10% of patients (44).
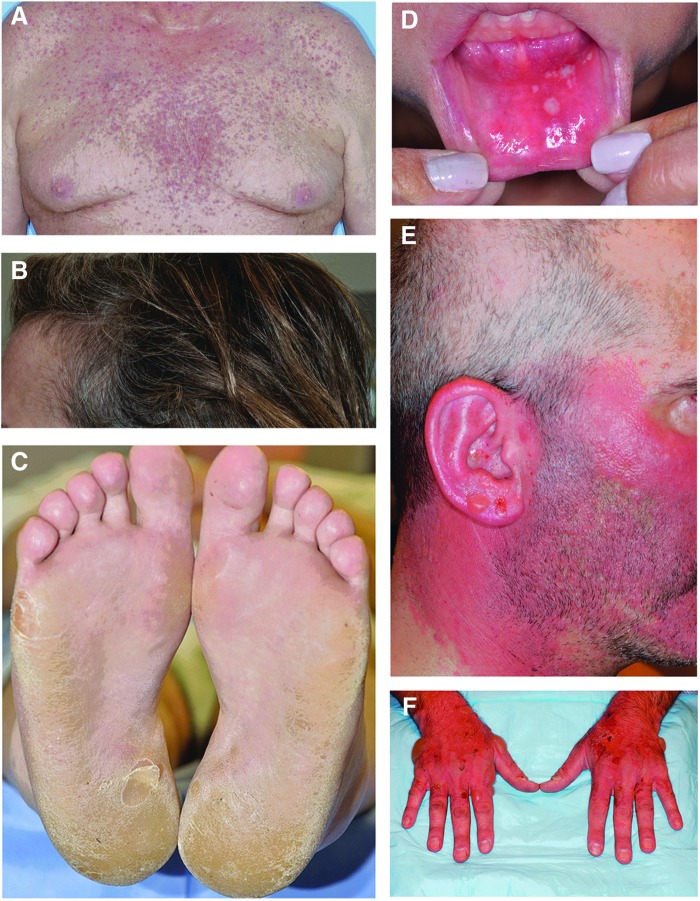
Photographs of representative patients exhibiting some of the skin reactions associated with TKI therapy are shown in Figure 1.
FIG. 1.
Photographs of representative patients exhibiting skin reactions associated with tyrosine kinase inhibitor (TKI) therapy. (A) acneiform rash, grade 2 (vandetanib); (B) gray and curly hair, grade 1 (sunitinib); (C) hand-foot skin reaction, grade 2 (cabozantinib); (D) stomatitis, grade 2 (cabozantinib); (E) photosensitivity, grade 1 (vandetanib); (F) photosensitivity, grade 1 (vandetanib). Photos courtesy of Mario E. Lacouture, MD.
Impact of dermatologic events on QoL
Dermatologic events can cause physical discomfort such as pain, burning, and increased skin sensitivity (45). These symptoms can affect a patient's ability to perform activities of daily living such as eating, grooming, exercise, and work. The effects of EGFR inhibitor-induced dermatologic AEs on QoL (46), including xerosis, rash, pruritus, and paronychia, resulted in decreased QoL, with rash having the highest impact. Younger patients reported decreased overall QoL compared with older patients for the same AEs (46).
Symptoms from ineffectively managed dermatologic AEs may also cause depression, frustration, worry, and withdrawal from certain social activities, thereby affecting a patient's physical, functional, emotional, and social well-being (45). Moreover, HFSR results in decreased QoL due to the associated symptoms of pain and tenderness (47).
Although data are limited, it is important to note the financial impact of managing dermatologic AEs to the economic burden of cancer care. Assessment and management may involve ongoing long-term care from multiple healthcare professionals over multiple visits to the clinic or office.
Management
Guidelines and recommendations
Prior to starting an oncology patient on any type of treatment, a thorough medical history must be taken to document the patient's history of cancer treatments and past skin disorders, their current medication profile, and presence of current skin disorders or symptoms (48). Patients with skin symptoms should be asked specific questions regarding the location, onset, duration, relieving factors, severity, and history of medications (Fig. 2) (49,50).
FIG. 2.
Triage questions that may be used to assess skin reactions during treatment (49,50). Republished with permission of Jannetti Publications, Inc., from Johannsen L 2005 Dermatology nursing essentials: a core curriculum. Dermatol Nurs 17:165–166; via Copyright Clearance Center, Inc.
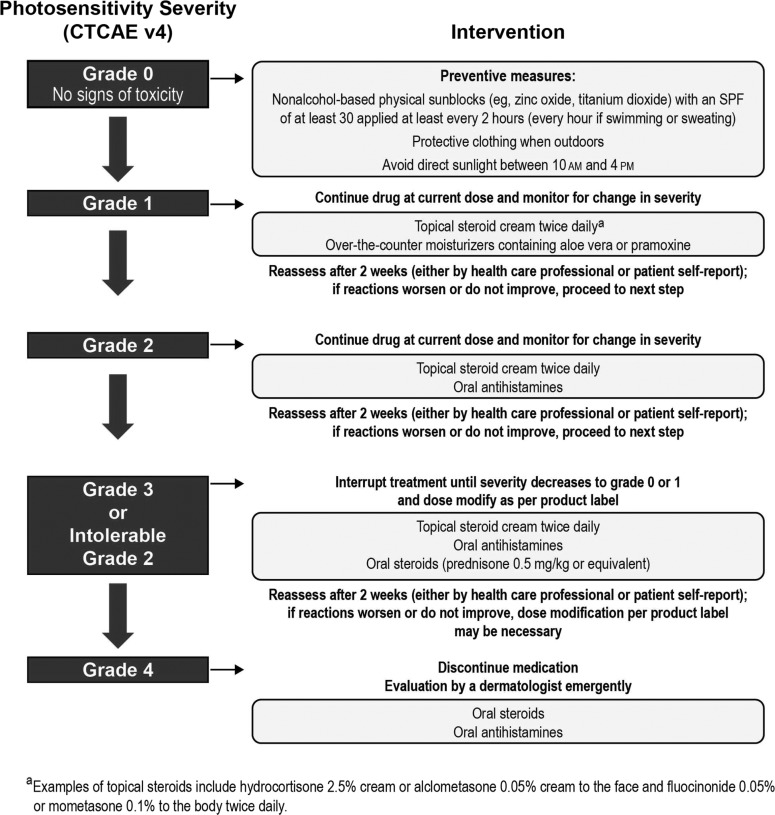
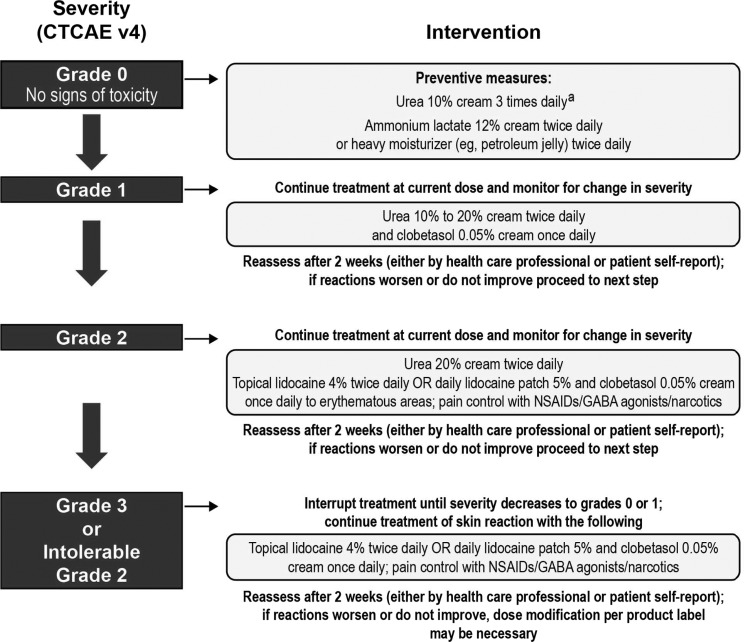
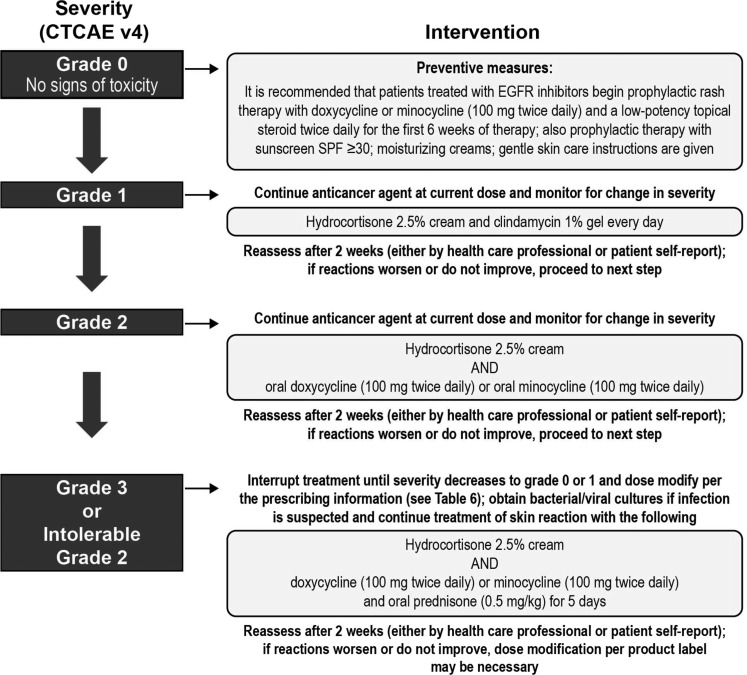
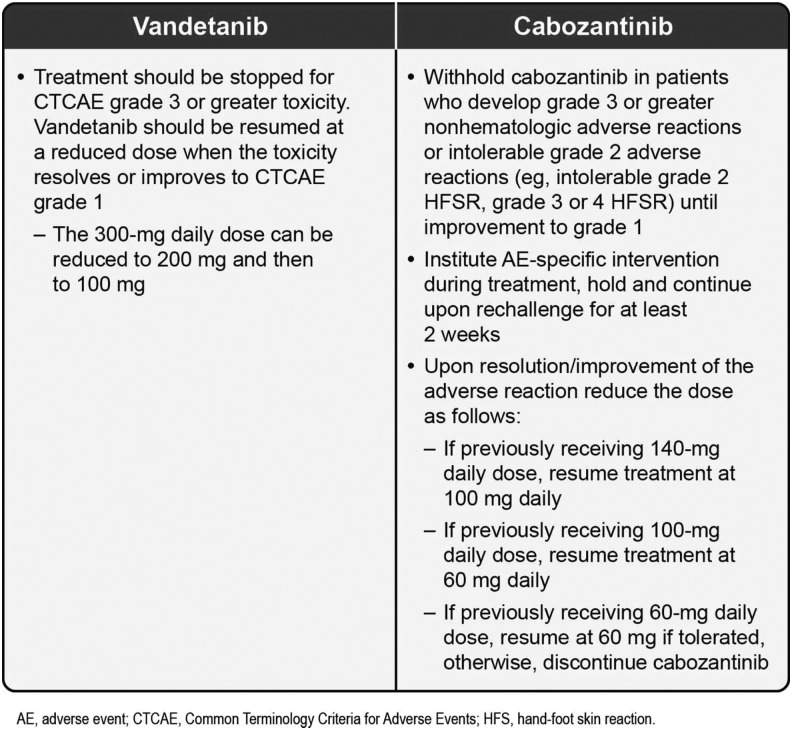
Various recommendations exist for the management of TKI-associated dermatologic AEs (36,38,51–54). Figures 3 (51–53), 4 (55), 5 (38), and 6 (12,13) also provide specific guidance. There have not been any prospective randomized trials to evaluate the best management strategies for these dermatologic AEs in patients with MTC. Recommendations are based on the evaluation of existing data and clinical experience (36). An important message from all these publications is the need for practitioners to develop and consistently implement effective algorithms for AE management (see Figs. 3–5 for examples of effective algorithms for management of photosensitivity, HFSR, and acneiform rash, respectively). Careful management is critical in allowing the patient to gain the most benefit from therapy by avoiding or limiting dose interruptions and modifications (36). However, these considerations must be balanced with the important goal of maintaining patients' QoL by minimizing the negative effects of debilitating AEs, particularly dermatologic AEs. In the case of serious drug-related dermatologic AEs, dose reductions, modifications, or interruptions might be necessary, as recommended by the product prescribing information. For example, in the cabozantinib prescribing information, dose interruptions are recommended for patients experiencing intolerable grade 2, grade 3, or higher grade skin toxicities, and some patients may require dose modifications at treatment reinitiation. These recommendations are described in Figure 6 (12,13).
FIG. 3.
Intervention algorithm for photosensitivity (51–53). CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events; SPF, sun protection factor.
FIG. 4.
Intervention algorithm for hand-foot skin reaction (55). Published in Balagula Y 2010 J Support Oncol 8:149–161. Copyright © 2010; Elsevier. aFrom Ren Z, et al. 2012 Clin Oncol 30: Abstract 4008 (58). GABA, gamma-aminobutyric acid; NSAIDs, nonsteroidal anti-inflammatory drugs.
FIG. 5.
Intervention algorithm for acneiform rash (38). Published in Balagula Y 2011 Int J Dermatol 50:129–146. Copyright © 2011; John Wiley & Sons, Inc. EGFR, epidermal growth factor receptor.
FIG. 6.
Skin toxicities requiring dose interruption and strategies for reintroducing TKI therapy (12,13).
Preventive measures
Results from a randomized study in 95 patients with metastatic colorectal cancer undergoing treatment with the EGFR monoclonal antibody inhibitor panitumumab suggest that preventive therapy may be more effective than reactive therapy in the management of dermatologic AEs with EGFR inhibitors (56). Patients in this study were randomized to pre-emptive treatment, consisting of skin moisturizers, sunscreen, topical steroid, and doxycycline, or reactive treatment begun after dermatologic AE development. The primary objective of the study was to assess the difference in the incidence of protocol-defined grade ≥2 skin AEs between the two groups during the six-week dermatologic treatment period. An approximate 50% reduction in skin AEs was observed in the pre-emptive versus reactive groups (56). Another study has demonstrated the effectiveness of prophylaxis in reducing the incidence or severity of grade ≤2 skin toxicities linked to treatment with the anti-EGFR monoclonal antibody cetuximab in patients with metastatic colorectal cancer (56,57).
Of 868 patients treated with sorafenib for hepatocellular carcinoma, a reduction was seen in the incidence of all-grade HFSR (56% in treatment arm vs. 73.6% in controls; p<0.0001), and a 2.5-fold increase in the median time-to-first onset of HFSR (84 days in the treatment arm vs. 34 days in controls; p<0.001), with the preventive daily use of urea-based cream (58). Figures 4 (55) and 5 (38) provide recommendations for the prevention of HFSR and acneiform rash respectively.
Stomatitis from targeted therapies is characterized by well-defined round plaques <0.5 cm in diameter affecting the lips, tongue, and oral mucosa (59,60). The lesions are very painful and may impair the ability to eat, drink, or speak (59,61). These aphthous-stomatitis–like lesions are treated with topical corticosteroids (e.g., clobetasol 0.05% cream) applied onto the lesions or dexamethasone solution swish and spit, taken three times daily with avoidance of eating or drinking for 30 minutes afterward (59,61). In addition, topical lidocaine 2–4% jelly applied onto the lesions, or one tablespoon or less of lidocaine viscous swished around in the mouth and spat out (taken no more than every 3 h), will help mitigate the pain so that patients can eat or drink (38).
General supportive measures
Supportive measures should include advice for patients on how to manage dry skin. A thick alcohol-free emollient (e.g., Vanicream®, Pharmaceutical Specialties, Inc., Rochester, MN; Eucerin®, Beiersdorf AG, Hamburg, Germany) is recommended for moisturizing dry areas of the body (62,63). When xerotic skin is very dry and scaly, and for xerosis in the palms and soles, moisturizing creams or lotions containing salicylic acid 3–6%, respectively, or ammonium lactate 12%/lactic acid 12% (ammonium lactate topical) are recommended. Figure 7 (49,59,61,64,65) provides measures that may be helpful in preventing and managing xerosis and stomatitis.
FIG. 7.
Supportive and preventive measures in the management of dermatologic adverse events (49,59,61,64,65).
Patient education
Patient and caregiver education is extremely important for the prevention, early recognition, and management of dermatologic AEs. It is recommended that this education start at the beginning and be reinforced throughout treatment by dermatology and oncology specialists. A patient-appropriate guide can be provided describing the common side effects experienced with a particular TKI. Specific instructions on when to call a doctor's office to discuss onset or exacerbation of symptoms should also be provided. Patients should keep documentation of the location, onset, symptoms/quality, treatment, and self-evaluation of all their AEs and bring concomitant medications to the clinic so the healthcare team can determine if they may be causing or worsening AEs. For example, topical and oral retinoids may lead to dryness and may worsen burning or irritation of skin rashes caused by EGFR inhibitors (51).
Nurses have an important role in patient education related to dermatologic AEs. Both healthcare providers and patients may view nurses as spending more time with patients and giving more in-depth treatment instructions than other professionals (66). Therefore, to prevent alteration of anticancer treatment administration and produce optimal results for patient care, nurses must understand the basis of side effects, impact on QoL, and pre-emptive measures, and must be able to provide optimal psychosocial support.
According to one survey, prior to treatment initiation, cancer survivors considered skin irritation and dry skin to be minor concerns compared with AEs such as a weaker immune system, hair loss, and fatigue (67). However, after undergoing treatment, most patients reported that dry skin and skin irritation were important concerns that heavily impacted their lives. Most respondents said that skin AEs were worse than expected, indicating they had either underestimated the potential impact of dermatologic AEs prior to initiation of therapy or had not been sufficiently informed. These data emphasize the importance of patient education.
Conversations with your patients
Conversations between the treatment team and patients should enhance patients' control over disease management. The cause, course, treatment, and management of disease side effects should be discussed (68). Emotional and informational support for patients with cancer is likely to have a beneficial impact on psychological adjustment (68). Such educational interventions may also help increase patient self-esteem and outlook, especially when management strategies are implemented effectively. More data are needed on the psychological needs of patients with dermatologic AEs linked to treatment with targeted therapies to improve the accuracy of assessments and to develop even more effective patient education (69). Oncology nurses provide an easily accessible sounding board for patients to express their psychological as well as physical concerns (70). Therefore, in addition to educating patients on interventions to ease the physical impact of the AEs, nurses can help determine when referral for psychosocial counseling is appropriate.
Patient education on specific topics: protection from UV radiation
Thick application of sunscreen and wearing protective clothing whenever possible should be emphasized, since UV radiation can trigger rash and photosensitivity in patients treated with vandetanib and sorafenib (44,51). Nonalcohol-based physical sunblocks (e.g., zinc oxide, titanium dioxide) with a sun protection factor of 30 that inhibit both UVA and UVB radiation are recommended because they are effective and cause less irritation than other types of sunscreen. To provide the best sun protection, patients should also be advised to be in the shade between 10:00 am and 4:00 pm, and to wear a broad hat and appropriate clothing (65). In addition, patients should be counseled to apply an appropriate amount of sunscreen—at least 35 mL to cover the body of an average adult, and should be repeated every two hours or every hour if swimming or sweating.
Patient education on specific topics: typical timing for development of acneiform rash and HFSR
It is critical to inform patients that both the acneiform rash and HFSR typically develop within the first eight weeks of therapy (71). Knowing that these events peak in severity during this time ensures close follow-up and care during this time, while reassuring patients that they will not endure these toxicities during the entire duration of their therapy.
Summary and Conclusions
The treatment options available for patients with metastatic MTC have been expanded with the FDA approval of vandetanib and cabozantinib. Other TKIs are under clinical investigation for MTC, including some currently marketed for other indications. Patients treated with TKIs are at risk of developing dermatologic AEs, a common type of AE associated with this class of agents. The risk of developing these dermatologic AEs can be reduced and their impact may be diminished through development and implementation of preventive and management algorithms. These algorithms combine nonpharmaceutical and pharmaceutical treatment modalities that are best implemented by a multidisciplinary effort, incorporating nursing interventions, patient education, and referrals to pain-management and dermatologic specialists, as appropriate. Effective AE prevention and management reduces dose interruptions and modifications, enabling patients to derive the most benefit from anticancer therapy, while maintaining a good QoL. Effective management of targeted therapies in the treatment of MTC strongly relies on patient education, interdisciplinary communication, and encouragement to minimize interruption of otherwise effective treatment.
Acknowledgments
We thank Monica Nicosia, PhD, and Antoinette Campo from SCI Scientific Communications & Information (SCI), and Jennifer Steeber, PhD, and Susan Moench, PhD, PA-C, formerly of SCI, who provided medical writing support funded by AstraZeneca LP.
Author Disclosure Statement
Mario E. Lacouture is a consultant for AstraZeneca. Richard T. Kloos is an employee and stockholder in Veracyte, Inc. The remaining authors have no competing financial interests.
References
- 1.Siegel R, Naishadham D, Jemal A.2013Cancer statistics, 2013. CA Cancer J Clin 63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Thyroid Carcinoma v2.2013. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed August21, 2013)
- 3.Hundahl SA, Fleming ID, Fremgen AM, Menck HR.1998A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 4.Roman S, Lin R, Sosa JA.2006Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107:2134–2142 [DOI] [PubMed] [Google Scholar]
- 5.Lodish MB, Stratakis CA.2008RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer: molecular genetics and therapeutic advances. Expert Rev Anticancer Ther 8:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landa I, Robledo M.2011Association studies in thyroid cancer susceptibility: are we on the right track? J Mol Endocrinol 47:R43–R58 [DOI] [PubMed] [Google Scholar]
- 7.Scollo C, Baudin E, Travagli J-P, Caillou B, Bellon N, Leboulleux S, Schlumberger M.2003Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 88:2070–2075 [DOI] [PubMed] [Google Scholar]
- 8.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr.2009Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612 [DOI] [PubMed] [Google Scholar]
- 9.Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M.2002ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62:7284–7290 [PubMed] [Google Scholar]
- 10.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF.2002ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 62:4645–4655 [PubMed] [Google Scholar]
- 11.Hart CD, De Boer RH.2013Profile of cabozantinib and its potential in the treatment of advanced medullary thyroid cancer. Onco Targets Ther 6:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cometriq [package insert] 2012South San Francisco, CA: Exelixis, Inc [Google Scholar]
- 13.Caprelsa [package insert] 2013Wilmington, DE: AstraZeneca Pharmaceuticals LP [Google Scholar]
- 14.European Medicines Agency approves COMETRIQ. Available at www.ema.europa.eu/ema/index.jsp?curl=/pages/medicines/human/medicines/002640/human_med_001726.jsp (accessed March30, 2014)
- 15.Sherman SI.2011Targeted therapies for thyroid tumors. Mod Pathol 24:S44–S52 [DOI] [PubMed] [Google Scholar]
- 16.Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R.2010Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 95:2664–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M.2010Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ.2012Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoffski P, Elisei R, Müller S, Brose MS, Shah MH, Licitra LF, Jarzab B, Medvedev V, Kreissl M, Niederle B, Cohen EEW, Wirth LJ, Ali HY, Hessel C, Yaron Y, Ball DW, Nelkin B, Sherman SI, Schlumberger Mfor the EXAM Study Group 2012An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline [abstract]. J Clin Oncol 30:Abstract 5508 [Google Scholar]
- 20.De Souza JA, Busaidy N, Zimrin A, Seiwert TY, Villaflor VM, Poluru KB, Reddy PL, Nam J, Vokes EE, Cohen EE.2010Phase II trial of sunitinib in medullary thyroid cancer (MTC) [abstract]. J Clin Oncol 28:Abstract 5504 [Google Scholar]
- 21.Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, Sammet S, Hall NC, Wakely PE, Jr, Vasko VV, Saji M, Snyder PJ, Wei L, Arbogast D, Collamore M, Wright JJ, Moley JF, Villalona-Calero MA, Shah MH.2010Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28:2323–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA., Jr1993Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2:851–856 [DOI] [PubMed] [Google Scholar]
- 23.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L.1993Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363:458–460 [DOI] [PubMed] [Google Scholar]
- 24.Hofstra RMW, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Hoppener JW, van Amstel HK, Romeo G.1994A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367:375–376 [DOI] [PubMed] [Google Scholar]
- 25.Carlson KM, Dou S, Chi D, Scavarda N, Toshima K, Jackson CE, Wells SA, Jr, Goodfellow PJ, Donis-Keller H.1994Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A 91:1579–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh DJ, Learoyd DL, Andrew SD, Krishnan L, Pojer R, Richardson AL, Delbridge L, Eng C, Robinson BG.1996Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 44:249–257 [DOI] [PubMed] [Google Scholar]
- 27.Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A.2008Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93:682–687 [DOI] [PubMed] [Google Scholar]
- 28.Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL.2010Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20:863–871 [DOI] [PubMed] [Google Scholar]
- 29.Bunone G, Vigneri P, Mariani L, Buto S, Collini P, Pilotti S, Pierotti MA, Bongarzone I.1999Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 155:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Antona C, Pallares J, Montero-Conde C, Inglada-Perez L, Castelblanco E, Landa I, Leskela S, Leandro-Garcia LJ, Lopez-Jimenez E, Leton R, Cascon A, Lerma E, Martin MC, Carralero MC, Mauricio D, Cigudosa JC, Matias-Guiu X, Robledo M.2010Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer 17:7–16 [DOI] [PubMed] [Google Scholar]
- 31.Viglietto G, Maglione D, Rambaldi M, Cerutti J, Romano A, Trapasso F, Fedele M, Ippolito P, Chiappetta G, Botti G.1995Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene 11:1569–1579 [PubMed] [Google Scholar]
- 32.Papotti M, Olivero M, Volante M, Negro F, Prat M, Comoglio PM, DiRenzo MF.2000Expression of hepatocyte growth factor (HGF) and its receptor (MET) in medullary carcinoma of the thyroid. Endocr Pathol 11:19–30 [DOI] [PubMed] [Google Scholar]
- 33.Downward J.2003Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3:11–22 [DOI] [PubMed] [Google Scholar]
- 34.Gomez K, Varghese J, Jimenez C.2011Medullary thyroid carcinoma: molecular signaling pathways and emerging therapies. J Thyroid Res 2011:815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop J, Westra WH, Wood LD, Hruban RH, Tufano RP, Robinson B, Dralle H, Toledo SP, Toledo RA, Morris LG, Ghossein RA, Fagin JA, Chan TA, Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N, Nelkin BD, Ball DW.2013Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab 98:E364–E369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP.2008Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 13:1001–1011 [DOI] [PubMed] [Google Scholar]
- 37.Bianchini D, Jayanth A, Chua YJ, Cunningham D.2008Epidermal growth factor receptor inhibitor-related skin toxicity: mechanisms, treatment, and its potential role as a predictive marker. Clin Colorectal Cancer 7:33–43 [DOI] [PubMed] [Google Scholar]
- 38.Balagula Y, Garbe C, Myskowski PL, Hauschild A, Rapoport BL, Boers-Doets CB, Lacouture ME.2011Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol 50:129–146 [DOI] [PubMed] [Google Scholar]
- 39.Tsimboukis S, Merikas I, Karapanagiotou EM, Saif MW, Syrigos KN.2009Erlotinib-induced skin rash in patients with non-small-cell lung cancer: pathogenesis, clinical significance, and management. Clin Lung Cancer 10:106–111 [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute Common Terminology Criteria for Adverse Events v 4.0. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed April3, 2014)
- 41.Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME.2012Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab 97:1125–1133 [DOI] [PubMed] [Google Scholar]
- 42.Giacchero D, Ramacciotti C, Arnault JP, Brassard M, Baudin E, Maksimovic L, Mateus C, Tomasic G, Wechsler J, Schlumberger M, Robert C.2012A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch Dermatol 148:1418–1420 [DOI] [PubMed] [Google Scholar]
- 43.Sutent [package insert] 2013New York, NY: Pfizer, Inc [Google Scholar]
- 44.Nexavar [package insert] 2013Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc [Google Scholar]
- 45.Wagner LI, Lacouture ME.2007Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologist's perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology (Williston Park) 21:34–36 [PubMed] [Google Scholar]
- 46.Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, Rosenbaum SE, Lacouture ME.2010Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 116:3916–3923 [DOI] [PubMed] [Google Scholar]
- 47.Sibaud V, Dalenc F, Chevreau C, Roche H, Delord JP, Mourey L, Lacaze JL, Rahhali N, Taieb C.2011HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist 16:1469–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dest VM.2009Systemic therapy-induced skin reactions. In: Haas M, Moore-Higgs GJ. (eds) Principles of Skin Care and the Oncology Patient. Pittsburgh, PA: Oncology Nursing Society [Google Scholar]
- 49.Witt ME, Young M.2009Common drug reactions with cutaneous manifestations. In: Haas M, Moore-Higgs GJ. (eds) Principles of Skin Care and the Oncology Patient. Pittsburgh, PA: Oncology Nursing Society [Google Scholar]
- 50.Johannsen LL.2005Skin assessment. Dermatol Nurs 17:165–166 [PubMed] [Google Scholar]
- 51.Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, Myskowski PL, Paul J, Perlis CS, Saltz L, Spencer S.2009NCCN Task Force Report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Cancer Netw 7:5–21 [DOI] [PubMed] [Google Scholar]
- 52.Lacouture ME, Anadkat MJ, Bensadoun R-J, Bryce J, Chan A, Epstein JB, Eaby-Sandy B, Murphy BA MASCC Skin Toxicity Study Group 2011Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 19:1079–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu PA, Balagula Y, Lacouture ME, Anadkat MJ.2011Prophylaxis and treatment of dermatologic adverse events from epidermal growth factor receptor inhibitors. Curr Opin Oncol 23:343–351 [DOI] [PubMed] [Google Scholar]
- 54.Lipworth AD, Robert C, Zhu AX.2009Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77:257–271 [DOI] [PubMed] [Google Scholar]
- 55.Balagula Y, Lacouture ME, Cotliar JA.2010Dermatologic toxicities of targeted anticancer therapies. J Support Oncol 8:149–161 [PubMed] [Google Scholar]
- 56.Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M.2010Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28:1351–1357 [DOI] [PubMed] [Google Scholar]
- 57.Scope A, Agero ALC, Dusza SW, Myskowski PL, Lieb JA, Saltz L, Kemeny NE, Halpern AC.2007Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 25:5390–5396 [DOI] [PubMed] [Google Scholar]
- 58.Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, Song T, Zhou W, Wang H, Yang W, Wang X, Yang Y, Shi L, Bai Y, Ye S-L.2012A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma [abstract]. J Clin Oncol 30:Abstract 4008 [DOI] [PubMed] [Google Scholar]
- 59.de Oliveira MA, Martins e Martins F, Wang Q, Sonis S, Demetri G, George S, Butrynski J, Treister NS.2011Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol 47:998–1003 [DOI] [PubMed] [Google Scholar]
- 60.Martins F, de Oliveira MA, Wang Q, Sonis S, Gallottini M, George S, Treister N.2013A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol 49:293–298 [DOI] [PubMed] [Google Scholar]
- 61.Nicolatou-Galitis O, Nikolaidi A, Athanassiadis I, Papadopoulou E, Sonis S.2013Oral ulcers in patients with advanced breast cancer receiving everolimus: a case series report on clinical presentation and management. Oral Surg Oral Med Oral Pathol Oral Radiol 116:e110–e116 [DOI] [PubMed] [Google Scholar]
- 62.Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME.2007Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 12:610–621 [DOI] [PubMed] [Google Scholar]
- 63.Eaby B, Culkin A, Lacouture ME.2008An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs 12:283–290 [DOI] [PubMed] [Google Scholar]
- 64.Memorial Sloan–Kettering Cancer Center. General guidelines to care for your dry skin. Available at www2.mskcc.org/patient_education/_assets/downloads-english/131.pdf (accessed April3, 2014)
- 65.Schneider J.2002The teaspoon rule of applying sunscreen. Arch Dermatol 138:838–839 [DOI] [PubMed] [Google Scholar]
- 66.Lindblad AK, Kjellgren KI, Ring L, Maroti M, Serup J.2006The role of dermatologists, nurses and pharmacists in chronic dermatological treatment: patient and provider views and experiences. Acta Derm Venereol 86:202–208 [DOI] [PubMed] [Google Scholar]
- 67.Gandhi M, Oishi K, Zubal B, Lacouture ME.2010Unanticipated toxicities from anticancer therapies: survivors' perspectives. Support Care Cancer 18:1461–1468 [DOI] [PubMed] [Google Scholar]
- 68.Helgeson VS, Cohen S.1996Social support and adjustment to cancer: reconciling descriptive, correlational, and intervention research. Health Psychol 15:135–148 [DOI] [PubMed] [Google Scholar]
- 69.White KJ, Roydhouse JK, Scott K.2011Psychosocial impact of cutaneous toxicities associated with epidermal growth factor receptor-inhibitor treatment. Clin J Oncol Nurs 15:88–96 [DOI] [PubMed] [Google Scholar]
- 70.Oishi K.2008Clinical approaches to minimize rash associated with EGFR inhibitors. Oncol Nurs Forum 35:103–111 [DOI] [PubMed] [Google Scholar]
- 71.Dranitsaris G, Vincent MD, Yu J, Huang L, Fang F, Lacouture ME.2012Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Ann Oncol 23:2103–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]