Abstract
Background: There remain a small number of patients with papillary thyroid cancer (PTC) who suffer recurrence, metastases, or death. While mutation of the BRAF gene, corresponding to the constitutively active BRAFV600E protein, has been associated with worse clinical outcomes in thyroid cancer, the reasons underlying this observation are presently unknown. Disruption of endogenous host immune surveillance and promotion of tumor immune escape is one mechanism by which BRAFV600E tumors may achieve more aggressive behavior. This study evaluated the relationship between BRAFV600E status and known strategies of tumor-mediated immune suppression.
Methods: Tissue sections of PTC tumors from 33 patients were evaluated by immunohistochemistry for tumor-expressed suppressive ligands and enzymes and effector and suppressor populations of tumor-infiltrating immune cells. Presence of BRAFV600E was evaluated by direct DNA sequencing of PTC specimens and the results correlated with tumor-expressed molecules and tumor-infiltrating immune cell populations, as well as patient characteristics and pathologic findings.
Results: BRAFV600E tumors more often express high levels of immunosuppressive ligands programmed death ligand 1 (53% vs. 12.5%) and human leukocyte antigen G (41% vs. 12.5%) compared to BRAF wild-type tumors. There was no association between indoleamine 2,3-dioxygenase 1 expression and BRAFV600E status. Furthermore, BRAFV600E tumors demonstrate both lower CD8+ effector to FoxP3+ regulatory T cell, and CD68+ pan-macrophage to CD163+ M2 macrophage ratios, indicating relative increases in suppressive T cell and macrophage components, respectively.
Conclusions: Overall, BRAFV600E PTC tumors display a broadly immunosuppressive profile and evidence of disturbed host tumor immune surveillance that may contribute to the poorer outcomes observed in this subset of patients with thyroid cancer.
Introduction
Thyroid cancer is the most common endocrine malignancy, and its incidence has increased almost threefold in recent decades (1–4). Papillary thyroid cancer (PTC) is the predominant histologic subtype (85–90%), and has a 10-year survival rate of greater than 90% (5,6). However, 15–30% of patients will have recurrent disease, and 5–10% will have distant metastasis, for whom 5-year survival is only 50–55% (7,8).
Along with clinical features that portend a worse prognosis such as age, tumor invasiveness, and metastasis (6), certain genetic mutations may influence thyroid cancer progression. One mutation in the v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) gene, corresponding to the constitutively active BRAFV600E protein, is present in approximately 45% of PTC (9–11) and has been associated with greater tumor size and invasion, lymph node metastasis, recurrence, and mortality (9–13), although not in all studies (14,15).
The mechanisms underlying aggressive PTC continue to be elucidated and may include tumor-mediated immune suppression. It is now established that host immune cells can recognize and eliminate malignant cells, but that cancers evolve mechanisms to escape immune destruction, including downregulation of antigen recognition, expression of immune-inhibitory ligands, and recruitment of suppressor immune cell populations. Both the expression of immunosuppressive molecules and specific patterns of tumor infiltrating leukocytes (TIL) have been shown to predict cancer recurrence and survival in solid malignancies (16–22).
Recently, studies have shown that the BRAFV600E protein mutation is associated with immunosuppressive mechanisms in PTC, such as indoleamine 2,3-dioxygenase 1 (IDO) (23), human leukocyte antigen G (HLA-G) (24), and programmed death ligand 1 (PD-L1) expression (25). Studies of TIL in other cancer types have shown that specific patterns predict cancer behavior, such as the correlation between a low CD8+ effector T cell to FoxP3+ suppressor T cell ratio with greater tumor aggressiveness and worse patient outcomes (17,19–21). Whether or not these mechanisms of immune suppression occur in isolation or as part of a broader pattern of immune suppression in BRAFV600E PTC has not been well studied. We hypothesized that BRAFV600E is associated with a general suppressive immune profile and disruption of host tumor immune surveillance that could help explain the poorer outcomes observed in this subset of patients with thyroid cancer.
Materials and Methods
Tissue samples
PTC specimens from patients with thyroid cancer with anonymized clinical data were obtained from the University of Southern California (USC) Keck Medical Center Tissue Bank (Institutional Review Board [IRB] protocol HS-11-00215). A pathologist specializing in thyroid cancer reviewed each case to confirm the diagnosis of PTC. Contralateral lobe normal tissue was collected and evaluated in parallel as control tissue when available.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue sections were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval (0.01 M citrate, pH 6.0) followed by treatment with 3% H2O2 for 10 minutes to block endogenous peroxidase activity. Sections were incubated overnight at 4°C with primary antibodies against CD3 (PC3/188; Santa Cruz Biotechnology, Santa Cruz, CA), CD8 (C8/144B, Dako, Carpinteria, CA), CD68 (PG-M1, Dako), CD163 (10D6, Abcam, Cambridge, MA), Arginase-1 (H-52, Santa Cruz), FoxP3 (236A/E7, Novus, Littleton, CO), HLA-G (4H84, Santa Cruz), PD-L1 (4059, ProSci, Inc., Poway, CA), or IDO (ab55305, Abcam). Secondary antibody staining and antigen detection with 3,3'-diaminobenzidine was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin, dehydrated, and mounted. Antibodies for detection of immune cell populations and markers of tumor immune suppression used in this study were previously validated by Russell et al. (19). Positive controls for immunohistochemistry analyses included lymph node or spleen tissues from healthy patients and/or patients with cancer with active disease for immune cell populations (CD3, CD8, CD68, CD163), liver parenchyma (Arg-1), human placental tissues (HLA-G, PD-L1, IDO), and head and neck carcinomas with known overexpression or loss of classic and nonclassic major histocompatibility complex (MHC) class I molecules (19). Negative controls included immunohistochemistry (IHC) using isotype control primary antibodies, secondary antibody in the absence of primary antibody, and known negative tissues for target antigens, including nonlymphoid tissues and lymphoid tissues from healthy donors obtained under IRB-approved protocol HS-11-00215. Correct cellular localization of positive staining was confirmed by pathologists A.L.E. and A.J.C. Hematoxylin and eosin (H&E)-stained sections were provided by the Keck Medical Center Translational Pathology Core.
Scoring of immune markers
An immune infiltrate scoring system to evaluate cancer specimens previously developed in our laboratory was adapted for PTC (19). Areas of tumor and associated TIL intratumorally or at the invading margin were identified on H&E-stained sections, with areas of obvious lymphoid follicle arrangement, necrosis, or hemorrhage excluded. Positively stained leukocytes for CD3, CD8, CD68, FoxP3, CD163, or Arginase-1 were counted in five representative high-powered fields (hpf) for each tumor section (hpf; 400× magnification). Tumor expression of HLA-G, IDO, or PD-L1 was assessed qualitatively as low, moderate, or high. Two independent observers scored each section and the results were pooled, with rare disagreements resolved by a third evaluator.
BRAF mutational analysis
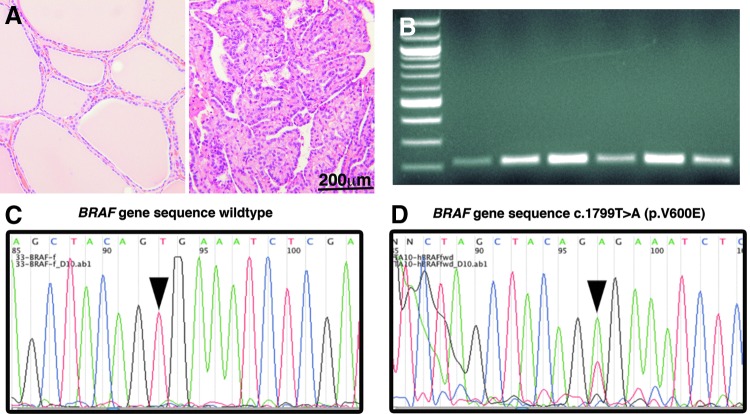
Tumor DNA was isolated from FFPE sections by excision of tumor tissue and DNA purification performed using a Qiagen QIAmp FFPE kit (Qiagen, Valencia, CA). Human BRAF exon 15 was amplified by PCR (forward: TCATAATGCTTGCTCTGATAGGA, reverse: GGCCAAAAATTTA ATCAGTGGA) as described previously (26). PCR DNA amplicons electrophoresed on 1.5% agarose were extracted and purified using a Qiagen MiniElude Gel Extraction kit and sequenced at the USC Genomics Core Facility. Tumor DNA sequences were evaluated for the c.1799T>A mutation in the BRAF gene (Fig. 1) encoding the BRAFV600E mutant protein. Given the rarity and uncertain significance of other BRAF mutation, cases without a BRAF 1799T>A transversion were considered wild-type (BRAFWT).
FIG. 1.
Assessment of BRAFV600E in papillary thyroid carcinoma specimens by polymerase chain reaction (PCR) and nucleotide sequencing. Hematoxylin and eosin staining of thyroid tissue is shown for (A) normal thyroid tissue and papillary thyroid carcinoma (400× original magnification). (B) Separation of PCR products of the BRAF gene by gel electrophoresis. Direct sequencing of purified PCR project shows (C) a single thymine corresponding to nucleotide 1799 representing the wild-type (WT) allele, and (D) an adenosine peak at the same sequence location demonstrating the BRAF c.1799T>A mutation that encodes the BRAFV600E protein (right panel).
Statistical methods
Data are shown as mean±standard error of the mean (SEM) unless otherwise stated. High expression of immunosuppressive markers was defined as the highest quartile of qualitative scores and the correlation with BRAF status analyzed using the Fisher's exact test. The two-sided unpaired Student's t test was used to compare the mean positively stained immune cells per hpf, or the mean value for the CD8+/FoxP3+ or CD68+/CD163+ ratios, respectively, in BRAFV600E and BRAFWT samples. Regression analyses were performed to evaluate markers of immune suppression as predictors of BRAF status, and to evaluate these immune suppression markers in combination with BRAF status as predictors of lymph node metastasis, TNM stage, or tumor invasion, using SPSS Statistics 21.0 (IBM SPSS, Armonk, NY). All other statistical tests were performed using GraphPad Prism 6.0 software (La Jolla, CA) and figures were produced using GraphPad and Abobe Photoshop (San Jose, CA).
Results
Patient characteristics
Tumor specimens from 33 patients with PTC were retrospectively obtained and analyzed. Clinical and pathologic data are summarized in Table 1. Female patients constituted 87.8% (29/33) of the samples. The median age of the patients was 49 years (range, 22–75), with 30.3% (10/33) being younger than 45 years of age. Primary tumors were noninvasive (TNM 1 or 2) in 26 (78.8%) and invasive (TNM 3 or 4) in 7 (21.2%) cases. In these studies, 17 of 33 (51.5%) tumor samples were positive for the BRAF mutation. Lymph node metastases at initial surgery were present in 12 of 33 (35.2%) patients, 9 of whom were in the BRAFV600E group compared to 3 in BRAFWT group (p=0.07). Other than this trend, presence of BRAFV600E was not associated with tumor size, invasion, or any demographic parameter. No patient had known distant metastases at the time of initial surgery.
Table 1.
Clinical and Pathologic Data for Thirty-Three Subjects with Papillary Thyroid Cancer
| Total(n=33) | BRAFWT(n=16) | BRAFV600E(n=17) | ||
|---|---|---|---|---|
| Characteristics | No. (%) | No. (%) | No. (%) | p-Valuea |
| Sex (female) | 29 (87.8) | 15 (93.8) | 14 (82.4) | ns |
| Age (years) | ||||
| Median (range) | 49 (22–75) | 48 (22–72) | 54 (30–75) | nsb |
| <45 years | 10 (30.3) | 4 (25) | 6 (35.3) | |
| >45 years | 23 (69.7) | 12 (75) | 11 (64.7) | ns |
| Primary tumor | ||||
| T1-2 | 26 (78.8) | 12 | 14 | |
| T3-4 | 7 (21.2) | 4 | 3 | ns |
| Lymph node status | ||||
| Nx, N0 | 21 (63.6) | 13 | 8 | |
| N1 | 12 (35.2) | 3 | 9 | 0.07 |
| TNM stage | ||||
| 1 | 20 (60.7) | 10 | 10 | |
| 2 | 2 (6) | 1 | 1 | |
| 3 | 3 (9.1) | 2 | 1 | |
| 4 | 8 (24.2) | 3 | 5 | ns |
Fisher's exact test except as indicated.
Unpaired Student's t-test.
BRAFV600E mutation is associated with the suppressive immune molecules PD-L1 and HLA-G
The BRAFV600E mutation was significantly associated with increased expression of immunosuppressive molecules by PTC cells (Fig. 2). PD-L1 staining showed high expression in 9 of 17 (53%) BRAFV600E specimens, compared with only 1 of 16 (12.5%) BRAFWT tumors (p<0.01). Similarly, 41% of BRAFV600E tumors were positive for HLA-G, whereas only 12.5% were positive in BRAFWT specimens (p<0.05). High IDO expression was more common in BRAFV600E specimens but the difference was not significant.
FIG. 2.
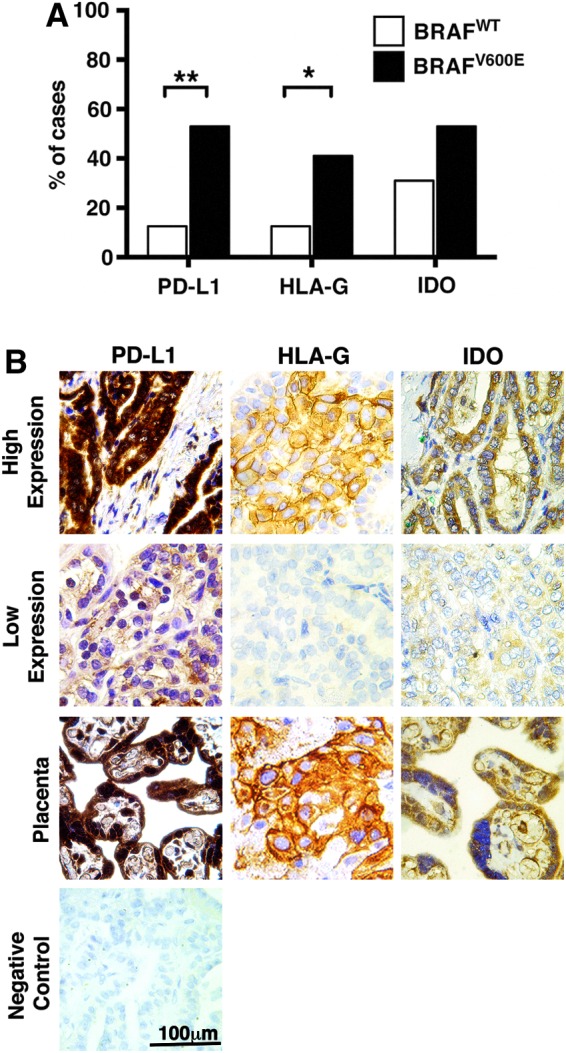
Expression of immunosuppressive mechanisms in BRAFV600E papillary thyroid cancer specimens. (A) Tumors with the BRAFV600E mutation showed a significantly higher rate of PD-L1 (p<0.01) and HLA-G (p<0.05) expression than BRAFWT tumors, while IDO expression, although more frequent in BRAFV600E tumors, was not significantly associated with BRAF status. (B) Representative immunohistochemical staining of PTC specimens, with more intense tumor staining for HLA-G, IDO, and PD-L1 in BRAFV600E compared to BRAFWT PTC. Positive control placental tissue demonstrates specific staining. Negative control PTC tissue demonstrates no staining in the absence of primary antibody. HLA, human leukocyte antigen; PD-L1, programmed death ligand; IDO, indoleamine 2,3-dioxygenase; PTC, papillary thyroid cancer; WT, wild-type.
Immune infiltrates in papillary thyroid carcinoma
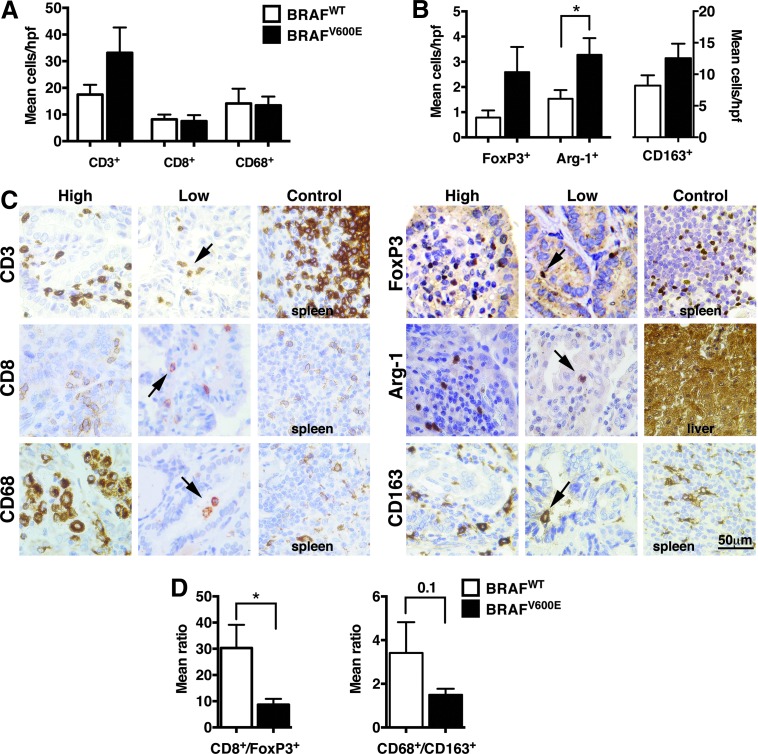
The frequency and quality of tumor infiltrating effector and suppressor immune cell populations was assessed in tumor sections by IHC, as shown in Figure 3. There was a trend toward greater overall T cell infiltration, measured by intratumoral CD3+ cells, in BRAFV600E tumors compared to BRAFWT (p=0.12). While there was a trend toward increased FoxP3+ Treg cells/hpf in BRAFV600E cases, when FoxP3+ cells were measured in relation to intratumoral effector CD8+ T cells, by calculating a CD8+/FoxP3+ cell ratio, there was a signficantly lower CD8+/FoxP3+ cell ratio in BRAFV600E compared to BRAFWT tumors (8.67±2.23 vs. 30.32±8.84, respectively [p<0.05]). Similarly, while neither the mean number of CD68+ (pan-macrophage) nor CD163+ (type M2 macrophage or tumor-associated macrophages [TAM]) immune cell populations varied significantly between groups, a trend toward a lower mean CD68+/CD163+ cell ratio was seen in BRAFV600E versus BRAFWT tumors, 1.49±0.28 versus 3.41±1.41, respectively (p=0.1). Measurement of arginase-1+ myeloid populations, which includes TAM and MDSC, revealed significantly greater intratumoral accumulation of these cells in BRAFV600E versus BRAFWT tumors (3.46±0.67 vs. 1.53±0.35 cells/hpf, respectively [p<0.05]).
FIG. 3.
Correlation of BRAF status with tumor-infiltrating leukocytes (TILs) in papillary thyroid cancer (PTC). Mean (±standard error of the mean [SEM]) cells per high-powered field (hpf) for (A) effector and (B) suppressive immune infiltrates demonstrates greater Arginase-1+ tumor-associated macrophage and myeloid-derived suppressor cell infiltration (mean cells/hpf) in BRAFV600E compared to BRAF wild-type (BRAFWT) tumors (p<0.05). There was a trend toward both higher mean CD3+ (p=0.12) and FoxP3+ (p=0.09) cells/hpf in BRAFV600E compared to BRAFWT specimens, but no other significant finding by immune cell type. (C) Representative immunohistochemical staining of immune cell in PTC specimens, showing examples of extensive or limited immune cell infiltration. (D) In BRAFWT tumors there was a significantly higher ratio of mean CD8+ to FoxP3+ cells/hpf of 30.32±8.84 and 8.67±2.23, respectively (p<0.05) and a trend toward a higher ratio of mean CD68+ to CD163+ of 3.97±2.04 and 0.93±017, respectively (p<0.1), compared to BRAFV600E PTC specimens.
Regression analysis revealed that markers of tumor immune suppression, namely tumor PD-L1, HLA-G, and IDO expression, decreased intratumoral CD8+/FoxP3+ cell ratio, and increased Arg-1+ tumor infiltrating leukocytes, were together, significant predictors of tumor BRAF status χ2[5]=32.88, p<0.001). The model explained 84.1% (Nagelkerke R2) of the variance and correctly classified 90.9% of cases. When analyzed independent of BRAF status, the frequency of the studied immune populations in PTC specimens did not vary significantly between specimens stratified by patient age, TNM stage, tumor invasion, or lymph node metastasis. Logistic regression analyses were performed to evaluate markers of tumor immune suppression and BRAF status as predictors of pathologically aggressive disease. The combined model approached statistically significant prediction (p=0.061) only for lymph node metastasis, and no single factor was independently predictive.
Discussion
The BRAFV600E mutation has been shown to predict PTC aggressiveness and patient prognosis (10,13,27,28). Increased BRAF activity appears to augment numerous cancer pathways, including apoptosis resistance, cell proliferation, and angiogenesis (28), but its immunologic effects in PTC are not well studied. Building upon prior reports of single immune markers in PTC, this investigation evaluated multiple aspects of tumor-induced host immune tolerance, including tumor-expressed factors and infiltrating leukocyte populations, concurrently in PTC specimens to identify overarching patterns of tumor–host immune interactions, specifically in relation to tumor BRAF mutation status. BRAFV600E positivity was associated with an immunosuppressive profile, encompassing direct tumor expression of immunosuppressive ligands and recruitment of suppressor cells.
Examination of PTC expression of immune inhibitory ligands and enzymes found that PD-L1 overexpression was frequently found (53% of cases), confirming the findings of Cunha et al. (25) demonstrating increased PD-L1 staining in thyroid cancer specimens compared with benign thyroid tissue. In addition, the rate of high PD-L1 expression was significantly higher in BRAFV600E tumors. PD-L1 functions to inhibit activated immune cells, such as those directed against tumor expressed antigens, and prevents cytotoxic cell destruction in a variety of ways, including binding with the co-inhibitory receptor, PD-1 (29). Since PD-L1 expression in thyroid cancer has been associated with a number of poor prognostic indicators (30), promising PD-1/PD-L1 pathway antagonistic antibodies currently in clinical trials for other solid maignancies (29,31,32) shoud be considered for the immunotherapy of PTC once clinically available.
HLA-G is a nonclassic and inhibitory MHC class I molecule expressed at immuno-privileged sites and by the placenta to induce maternal/fetal tolerance that is known to inhibit antigen-presenting, natural killer, and T cells (33,34). Aberrant expression of HLA-G is a known mechanism of tumor-mediated immune tolerance and data suggest that HLA-G expression correlates with tumor growth and poorer prognosis (35,36). High HLA-G expression was present in a quarter of PTC cases and was more than three times more frequent in BRAFV600E compared to BRAFWT tumors. In two studies of thyroid cancer that did not analyze mutational status, HLA-G expression was assoicated with tumor size larger than 1 cm (37) and lymph node metastasis (38), respectively. Recently, Smallridge et al. (24) demonstrated an association between increased mRNA expression of HLA-G and BRAFV600E in PTC. Here we confirm the association between HLA-G and BRAFV600E at the protein level and place HLA-G in the context of broader immune suppression within BRAFV600E containing PTC.
The immunosuppressive enzyme IDO was found to be upregulated in nearly a third of PTC specimens, confirming recent results by Moretti et al. (23). Although not statistically significant, high IDO expression was more frequently observed in BRAFV600E tumors, suggesting that IDO inhibitors such as 1-methyltryptophan (39) may be a potential target for future therapeutic interventions for aggressive PTC.
Populations of tumor-infiltrating immune cells were also evaluated in relation to tumor BRAF status. It is now recognized that rather than simply being present, the type, location, functional status, and ratios of immune cells are important to predicting antitumor immunity (17,27). Conflicting reports on the prognostic importance of autoimmune thyroid disease (40–42) may stem in part from not fully elucidating these key factors. Previous evaluations of immune infiltrates in PTC (43–48) have focused on single suppressive immune populations, such as TAM or Treg, finding significant correlations with cancer aggressiveness. However, to our knowledge there has been little data describing multiple immune cell subtypes in PTC tumors, their relative frequencies, and their association with BRAFV600E positivity. A lower CD8+/FoxP3+ cell ratio, a marker of poorer prognosis in other solid malignancies (17), was found in BRAFV600E compared to BRAFWT thyroid cancers (p<0.05). This finding, as well as the observed greater CD3+ cell infiltration seen in BRAFV600E PTC, are consistent with gene expression data of colon cancers suggesting that activating BRAF mutations have greater immune cell infiltration and a reduced intratumoral CD8+ cell to Treg ratio (17,27,49). Similarly, studies in melanoma and murine models demonstrated TIL with a decreased CD8+ to Treg ratio when a BRAF mutation was present, and further showed that inhibition of the enhanced BRAF signaling modulated the cytokine mileu of the tumor microenvironment to promote anti-tumor immunity (50,51). Congruent with these reports, our findings suggest that aberrant constitutive BRAF activity in PTC promotes immune dysfunction by lowering the effector to suppressor cell ratio.
TAM may also be present within tumors and have been noted in thyroid cancers (30,43,46), but study of this population has been limited due to few distinguishing markers for proinflammatory M1 and suppressive M2 phenotypes. While there is no unique marker for M1 macrophages (52), CD163, a hemoglobin scavenger receptor (53), is now recognized as a marker for M2-polarized macrophages (54). While greater intratumoral TAM accumulation was demonstrated with BRAFV600E-induction in a mouse model (55), no association between TAM infiltration and BRAF status was found in a study of human thyroid cancer specimens (46). In this study, quantification of the relative M2 macrophage component by measurement of a CD68+/CD163+ ratio (52) showed that BRAFV600E tumors had a trend toward increased immunosuppressive M2 phenotype or TAMs. This suggests that, like the CD8+/FoxP3+ ratio, comparisons of macrophage subtypes may be more important than either population measured alone. This shift toward suppressive myeloid cell recruitement was further supported by the finding of an increased number of infiltrating arginase-1+ cells in BRAFV600E compared to BRAFWT tumors. Arginase-1 is another immunosuppressive enzyme expressed by TAM and myeloid suppressor cells (56) that can help to identify functional subsets of tumor-infiltrating myeloid cells. Future investigations with possible markers that are specific for M1 macrophages may further elucidate the contributions of these macrophage subsets to the tumor microenvironment.
In this study, while no single immune feature was independently predictive, the composite of tumor expressed PD-L1, HLA-G, and IDO, decreased intratumoral CD8+/FoxP3+ cell ratio, and increased Arg-1+ tumor infiltrating leukocytes, significantly predicted tumor BRAFV600E status, reinforcing the close relationship between BRAFV600E and a general profile of immune suppression. Irrespective of BRAF status, the frequency of TIL populations within PTC specimens stratified by clinical or pathologic characteristics did not vary significantly, though the analyses are preliminary and likely limited by the small sample size. However, logistic regression analysis indicated that presence of the BRAFV600E mutation combined with markers of tumor immune suppression may be predictive of lymph node metastasis (p=0.061). Larger investigations that concurrently assess multiple features of immune suppression may be necessary to demonstrate that the collective presence of BRAFV600E and immunosuppressive features predicts aggressive pathologic features.
These data show for the first time a significant positive association between BRAFV600E and PD-L1 expression, as well as arginase-1+ immune cell infiltrates. Along with increased expression of HLA-G and IDO, and a shift towards immunosupressive Treg and type M2 macrophages in TIL, our analysis of tumor-host immune interactions demonstrates a strongly immunosuppressive profile within the tumor microenvironment of BRAFV600E-positive PTC. While these associations provide preliminary data for the relationship between presence of BRAFV600E and strong immune suppression in PTC, the current study has a small sample size and by its retrospective nature is limited to correlative analyses.
Based on these data, we hypothesize that mutated BRAF protein antigens presented by BRAFV600E PTC cells may increase tumor antigenicity, prompting a greater immune response, as evidenced by the greater T cell infiltration observed in this study, and necessitating that BRAFV600E tumors evolve immunosuppressive mechanisms including PD-L1 and HLA-G expression, and induction or recruitment of suppressive immune cell populations in order to disrupt host immune surveillance and allow escape from immune-mediated tumor destruction. These findings suggest that further investigations using experimental in vitro and in vivo models are warranted to elucidate causative mechanisms by which BRAFV600E action promotes immune suppression and define its contribution to thyroid cancer immune escape.
Acknowledgments
The authors thank Dr. Peter Singer of the Keck Medical Center of USC, Los Angeles, California, for his assistance in the preparation of this manuscript, and Dr. Rikki B. Sevell, Mr. Connor H. Church, and Ms. Lillian Young, all of the Keck Medical Center of USC, for contributions to immunohistochemistry studies. This work was supported in part by the USC Steven's Institute for Innovation Ideas Empowered Program, National Institutes of Health grants 3T32GM067587-07S1 (M.G.L.) and P30CA014089, Department of Defense grant W81XWH-11-1-0466 (A.L.E), Cancer Therapeutics Laboratories, Inc. (Los Angeles, CA), of which A.L.E is a cofounder, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award Number TL1TR000132. Julie K. Jang is a TL1 Trainee awarded under the TL1 (Pre-doctoral) Training Award through Southern California Clinical and Translational Science Institute at University of Southern California, Keck School of Medicine.
Author Disclosure Statement
All authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A.2013Cancer statistics, 2013. CA Cancer J Clin 63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.Amphlett B, Lawson Z, Abdulrahman GO, Jr, White C, Bailey R, Premawardhana LD, Okosieme OE.2013Recent trends in the incidence, geographical distribution, and survival from thyroid cancer in Wales, 1985–2010. Thyroid 23:1470–1478 [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Jemal A, Ward EM.2009Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer Aug 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 5.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR.2003Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885 [DOI] [PubMed] [Google Scholar]
- 6.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM.2009Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 7.Grebe SK, Hay ID.1996Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 5:43–63 [PubMed] [Google Scholar]
- 8.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. (eds) 2013SEER Cancer Statistics Review, 1975–2010, National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2010/ (Last accesed July2, 2014) [Google Scholar]
- 9.Xing M.2013Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V.2013Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikiforov YE.2011Molecular analysis of thyroid tumors. Mod Pathol 24(Suppl 2):S34–43 [DOI] [PubMed] [Google Scholar]
- 12.O'Neill CJ, Bullock M, Chou A, Sidhu SB, Delbridge LW, Robinson BG, Gill AJ, Learoyd DL, Clifton-Bligh R, Sywak MS.2010BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery 148:1139–1145 [DOI] [PubMed] [Google Scholar]
- 13.Li C, Lee KC, Schneider EB, Zeiger MA.2012BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 97:4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu RT, Chen YJ, Chou FF, Li CL, Wu WL, Tsai PC, Huang CC, Cheng JT.2005No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf) 63:461–466 [DOI] [PubMed] [Google Scholar]
- 15.Gouveia C, Can NT, Bostrom A, Grenert JP, van Zante A, Orloff LA.2013Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg 139:1164–1170 [DOI] [PubMed] [Google Scholar]
- 16.Stewart TJ, Abrams SI.2008How tumours escape mass destruction. Oncogene 27:5894–5903 [DOI] [PubMed] [Google Scholar]
- 17.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW.2011The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadun RE, Sachsman SM, Chen X, Christenson KW, Morris WZ, Hu P, Epstein AL.2007Immune signatures of murine and human cancers reveal unique mechanisms of tumor escape and new targets for cancer immunotherapy. Clin Cancer Res 13:4016–4025 [DOI] [PubMed] [Google Scholar]
- 19.Russell SM, Angell TE, Lechner MG, Liebertz DJ, Correa AJ, Sinha UK, Kokot N, Epstein AL.2013Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol 5:24. [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Church CH, Murphy KA, Ohlfest JR, Hu P, Epstein AL.2012Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother Clin Cancer Res 18:4549–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camus M, Tosolini M, Mlecnik B, Pagès F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, Trajanoski Z, Fridman WH, Galon J.2008Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 69:2685–2693 [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA.2012The immune score as a new possible approach for the classification of cancer. J Transl Med 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti S1, Menicali E, Voce P, Morelli S, Cantarelli S, Sponziello M, Colella R, Fallarino F, Orabona C, Alunno A, de Biase D, Bini V, Mameli MG, Filetti S, Gerli R, Macchiarulo A, Melillo RM, Tallini G, Santoro M, Puccetti P, Avenia N, Puxeddu E.2014Indoleamine 2,3-dioxygenase 1 (IDO1) is unregulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J Clin Endocrinol Metab 99:E832–840 [DOI] [PubMed] [Google Scholar]
- 24.Smallridge RC, Chindris AM, Asmann YW, Casler JD, Serie DJ, Reddi HV, Cradic KW, Rivera M, Grebe SK, Necela BM, Eberhardt NL, Carr JM, McIver B, Copland JA, Aubrey Thompson E.2014RNA sequencing identifies multiple fusion transcripts, differentially expressed genes, and reduced expression of immune function genes in BRAF (V600E) mutant vs BRAF wild-type papillary thyroid carcinoma. J Clin Endocrinol Metab 99:E338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, Soares FA, Vassallo J, Ward LS.2013Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer 20:103–110 [DOI] [PubMed] [Google Scholar]
- 26.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA.2002Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- 27.Ogino S, Galon J, Fuchs CS, Dranoff G.2011Cancer immunology—analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 8:711–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing M.2007BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742–762 [DOI] [PubMed] [Google Scholar]
- 29.Afreen S, Dermime S.2014The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther 7:1–17 [DOI] [PubMed] [Google Scholar]
- 30.Cunha LL, Marcello MA, Ward LS.2014The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer 21:R85–R103 [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Gajewski TF, Kline J.2009PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sznol M, Chen L.2013Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19:1021–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva TG, Crispim JC, Miranda FA, Hassumi MK, de Mello JM, Simões RT, Souto F, Soares EG, Donadi EA, Soares CP.2011Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol Histopathol 26:1487–1497 [DOI] [PubMed] [Google Scholar]
- 34.Curigliano G, Criscitiello C, Gelao L, Goldhirsch A.2013Molecular pathways: human leukocyte antigen G (HLA-G). Clin Cancer Res 19:5564–5571 [DOI] [PubMed] [Google Scholar]
- 35.Yie SM, Hu Z.2011Human leukocyte antigen-G (HLA-G) as a marker for diagnosis, prognosis and tumor immune escape in human malignancies. Histol Histopathol 26:409–420 [DOI] [PubMed] [Google Scholar]
- 36.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED.2005HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res 65:10139–10144 [DOI] [PubMed] [Google Scholar]
- 37.Figueiredo Feitosa NL, Oliveira Crispim JC, Zanetti BR, Magalhães PK, Soares CP, Soares EG, Neder L, Donadi EA, Maciel LM.2014HLA-G is differentially expressed in thyroid Tissues. Thyroid 24:585–592 [DOI] [PubMed] [Google Scholar]
- 38.Nunes LM, Ayres FM, Francescantonio IC, Saddi VA, Avelino MA, Alencarrde C, Silva RC, Meneghini AJ, Wastowski IJ.2013Association between the HLA-G molecule and lymph node metastasis in papillary thyroid cancer. Human Immunol 74:447–450 [DOI] [PubMed] [Google Scholar]
- 39.Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P.2009Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 9:445–452 [DOI] [PubMed] [Google Scholar]
- 40.Feldt-Rasmussen U1, Rasmussen AK.2010Autoimmunity in differentiated thyroid cancer: significance and related clinical problems. Hormones (Athens) 9:109–117 [DOI] [PubMed] [Google Scholar]
- 41.Spencer CA.2011Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab 96:3615–3627 [DOI] [PubMed] [Google Scholar]
- 42.Grebe SK.2010Thyroglobulin autoantibodies, thyroid nodules, and new insights into some old questions. Thyroid 20:841–842 [DOI] [PubMed] [Google Scholar]
- 43.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA.2008Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer 15:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR.2010Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab 95:2325–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunha LL, Morari EC, Nonogaki S, Soares FA, Vassallo J, Ward LS.2012Foxp3 expression is associated with aggressiveness in differentiated thyroid carcinomas. Clinics (Sao Paulo) 67:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, Chen X, Wang WQ, Li XY, Xiao JC, Ning G.2012Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid 22:905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS.2012Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid cancer. Endocr Relat Cancer 19:L31–36 [DOI] [PubMed] [Google Scholar]
- 48.Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, Neonakis E, Manoussakis MN, Liapi C.2012Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab 97:1474–1482 [DOI] [PubMed] [Google Scholar]
- 49.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS.2009Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 15:6412–6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Haynes NM, Kinross K, Yagita H, Koya RC, Graeber TG, Ribas A, McArthur GA, Smyth MJ.2013Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest 123:1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y.2006The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 203:1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heusinkveld M1, van der Burg SH.2011Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristiansen M1, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK.2001Identification of the haemoglobin scavenger receptor. Nature 409:198–201 [DOI] [PubMed] [Google Scholar]
- 54.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G.2000Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro and antiinflammatory stimuli. J Leukoc Biol 67:97–103 [PubMed] [Google Scholar]
- 55.Ryder M1, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. 2013Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS One 8:e54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V.2008Tumor induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 222:162–179 [DOI] [PubMed] [Google Scholar]