Abstract
Background
Coronary artery calcium (CAC) predicts coronary heart disease (CHD) events and serial measurement of CAC has been proposed to evaluate atherosclerosis progression. We examined whether progression of CAC is a predictor of future CHD events.
Methods and Results
We studied 6,778 persons (52.8% female) aged 45–84 years from the Multi-Ethnic Study of Atherosclerosis. 5,682 persons had baseline and follow-up CAC scans approximately 2.5 ± 0.8 years apart; multiple imputation was used to account for the remainder (n=1,096) missing follow-up scans. Median follow-up duration from the baseline was 7.6 (max=9.0) years. CAC change was assessed by absolute change between baseline and follow-up CAC. Cox proportional hazards regression providing hazard ratios (HR) examined the relation of change in CAC with CHD events, adjusting for age, gender, ethnicity, baseline calcium score, and other risk factors. 343 total and 206 hard CHD events occurred. The annual change in CAC averaged 24.9 ± 65.3 units. Among persons without CAC at baseline (n=3,396), a 5 unit annual change in CAC was associated with an adjusted HR of 1.4 (1.0–1.9) for total and 1.5 (1.1–2.1) for hard CHD. Among those with CAC>0 at baseline HR’s (per 100 unit annual change) were 1.2 (1.1–1.4) and 1.3 (1.1–1.5), respectively. Among participants with baseline CAC, those with annual progression of ≥300 units had adjusted HR’s of 3.8 (1.5–9.6) for total and 6.3 (1.9–21.5) for hard CHD compared to those without progression.
Conclusions
Progression of CAC is associated with an increased risk for future hard and total CHD events.
Keywords: coronary calcification, atherosclerosis, imaging, coronary heart disease
Coronary artery calcium (CAC) is strongly associated with atherosclerotic burden and predicts coronary heart disease (CHD) events and mortality.1–4 CAC scanning has been proposed as a measure to track CHD progression and the effects of risk factor modification on atherosclerosis. 5–6 Multiple retrospective and one prospective study suggests that CAC progression is associated with CHD events.7,8 Recently, in the first follow-up based on a large registry of subjects receiving serial CT scans, Budoff et al. showed progression of CAC to be strongly associated with total mortality.9 Our objective was to examine in large multi-ethnic sample of U.S. adults in a population-based prospective study the relation of CAC progression to CHD incidence.
Methods
Study Population and Definitions
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study of the prevalence, risk factors, and progression of subclinical cardiovascular disease (CVD). A detailed description of the MESA design has been previously published.11 Briefly, 6,814 participants aged 45–84 free of clinical CVD, identified as White, African-American, Hispanic, or Chinese, were recruited from six U.S. communities (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; Los Angeles County, CA) in the 2000–2002 period. Recruitment was based on lists of residents, dwellings, telephone exchanges, lists of Medicare beneficiaries, and referrals by participants. Similar numbers of men and women were recruited according to pre-specified age and race/ethnicity quotas. All participants gave informed consent, and the study protocol was approved by the Institutional Review Board at each site. This report includes 6,778 participants with follow-up for events, of which 5,682 subjects had both baseline (Exam 1) and follow-up (Exam 2 or 3) CT scans and with no interim CHD events. Multiple imputation12,13 was used for the 1,096 participants who did not have a follow-up CAC measure, including 141 individuals who experienced a CHD event prior to their second scan (see statistical methods below).
Measurement of Coronary Artery Calcium
CAC was measured by electron-beam (3 sites) or multi-detector (3 sites) computed tomography. Participants were scanned twice consecutively and scans were read by a trained physician-reader at a centralized reading center (Los Angeles Biomedical Research Institute, Torrance, CA). The methodology for acquisition and interpretation of the scans has been published.14 Briefly, each calcific lesion required a minimum of 3 contiguous pixels with an attenuation threshold of 130 Hounsfield units (HU), after which each lesion was multiplied by a density factor based on the maximum HU within the area (1 for lesions with peak attenuation of 130–199, 2 for 200–299, 3 for 300–399, and 4 for 400 or greater). A total CAC score was obtained by summing individual lesion scores from each of the four arteries where calcium was assessed: left main, left anterior descending, left circumflex, and right coronary artery.
Calcium volume scores14 and Agatston scores15 were based on averaging results from each of the two scans done at the examination, and adjusted using a standard calcium phantom (scanned with the participant) to calibrate X-ray attenuation between measurements conducted on different machines.16 Detectable calcium was defined as a CAC score >0. A second scan was performed on half the cohort (randomly selected) at a second exam (September, 2002–January, 2004) and on the other half at a third exam (March, 2004–July, 2005), averaging 1.6 and 3.2 years after the first scan, respectively (average, 2.5 years). The distribution of CAC in MESA at baseline by age, gender and race has been published.17
Examination Data and Covariates
Information on demographics, smoking, medical conditions, and family history was obtained by questionnaire. Height, weight, fasting total and high density lipoprotein-cholesterol, triglycerides, and glucose levels were determined. Resting blood pressure was measured three times, with the average of the last two measurements used in analysis. We included use of antihypertensive medications and systolic and diastolic blood pressure in risk models. Cholesterol, blood pressure, and diabetes medications were determined by questionnaire and from medication containers. Diabetes was defined as a fasting glucose ≥7.0 mmol/l (126 mg/dl), or use of insulin or oral hypoglycemic medications.
Follow-up
The cohort was followed for incident CHD and CVD events for a median of 7.6 (max=9.0) years following the performance of the baseline CT scan (4.8 years following the second scan for the complete case analysis [see below]). At intervals of 9–12 months, a telephone interviewer inquired about interim hospital admissions, cardiovascular diagnoses, and deaths. An adjudication committee received copies of all death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses, and conducted next-of-kin interviews for out of hospital cardiovascular deaths. Records were obtained on 98% of reported hospitalized CVD events.
CHD endpoints included myocardial infarction, angina, resuscitated cardiac arrest, and CHD death. Angina required clear documentation of chest pain or anginal equivalent and evidence of reversible myocardial ischemia, obstructive coronary artery disease or a positive stress test. Definite fatal CHD required an MI within 28 days prior to death, resuscitated cardiac arrest, chest pain within 72 hours before death, or a history of CHD and absence of a known non-atherosclerotic or non-cardiac cause of death. Hard CHD included myocardial infarction and fatal CHD. Two physicians from the MESA study events committee independently reviewed all medical records and death certificates for endpoint classification and assignment of incidence dates. The reviewers were blinded to CT results and used pre-specified criteria. A more detailed description of the MESA follow-up methods is available at www.mesa-nhlbi.org.
Statistical analysis
Separate analyses were done for those with baseline CAC=0 (n=3,396) and those with CAC>0 (n=3,382), given the likelihood that the effect of progression may be different between these two groups. Absolute progression rates were annualized. Analyses were based on Agatston scores to ensure consistency with prior MESA publications (see also discussion below). For all missing information, multiple imputation12,13 using a chained equation approach18 was used to replace each missing value with a set of 100 plausible substitutes that were consistent with the observed values. For persons without follow-up CAC scans (n=1,096), follow-up CAC at exam 2 or 3 was predicted using regression equations based on the observed data, using baseline risk factors, baseline CAC (for those with CAC>0), CHD events, time to CHD event or last follow-up. Prior literature has described the rationale and necessity of including these variables in multiple imputation prediction equations19–21 and imputation with repeated measures.13
Consistent with our analysis models, the follow-up CAC measures were imputed separately for participants with baseline CAC=0 and CAC>0 at the time when they would have been given a second scan (estimated or actual date of follow-up exam), and assumed a linear rate of change in CAC. In those with intervening initial CHD events, the degree of progression (and follow-up time) was based on the date of occurrence of the event used in the analysis, imputing what the follow-up scan score would have been at that time. A conditional (two-part) imputation was done for subjects with CAC=0. The first step predicted the likelihood of any CAC progression using logistic regression; the second part was conditioned on the first and only those who were predicted to have had progression were imputed to have a follow-up CAC score greater than zero. The conditional imputation allowed adequate modeling of the relatively large proportion of participants with baseline CAC=0 who experienced no progression. All reported results, including the descriptive statistics in Table 1, were obtained by averaging the parameter estimates across the multiple datasets and using Rubin’s rules12 to combine the standard errors when applicable.
Table 1.
Descriptive Statistics. Multi-Ethnic Study of Atherosclerosis (MESA)
| Baseline CAC=0 | Baseline CAC>0 | |
|---|---|---|
|
| ||
| Overall (n) | 3,396 | 3,382 |
|
| ||
| Men (n, % male) | 1,243, 36.6% | 1,953, 42.2% |
|
| ||
| Women (n, % female) | 2,153, 63.4% | 1,429, 57.8% |
|
| ||
| Caucasian (n) | 1,125 (33.1%) | 1,489 (44.0%) |
|
| ||
| Black (n) | 398 (11.7%) | 402 (11.9%) |
|
| ||
| Chinese (n) | 1,061 (31.2%) | 819 (24.2%) |
|
| ||
| Hispanic (n) | 812 (23.9%) | 672 (19.9%) |
|
| ||
| Taking lipid-lowering medication | 10.6% | 21.8% |
|
| ||
| Taking anti-hypertensive medication | 28.8% | 45.8% |
|
| ||
| Smoker | ||
| Never | 56.0% | 44.6% |
| Former | 30.8% | 42.5% |
| Current | 13.2% | 12.9% |
|
| ||
| Diabetes | 9.3% | 15.9% |
|
| ||
| Family history of MI or stroke | 37.1% | 48.3% |
|
| ||
| Mean ± standard deviation | ||
|
| ||
| Age (years) | 58.0 ± 9.1 | 66.4 ± 9.5 |
|
| ||
| Total cholesterol, mmol/L (mg/dL) | 5.0 (193.7) ± 0.9 (35.0) | 5.1 (194.6) ± 0.9 (36.4) |
|
| ||
| HDL-C, mmol/L (mg/dl) | 1.4 (52.5) ± 0.4 (15.0) | 1.3 (49.5) ± 0.4 (14.5) |
|
| ||
| SBP (mm Hg) | 122.4 ± 20.5 | 130.8 ± 21.7 |
|
| ||
| DBP (mm Hg) | 71.2 ± 10.3 | 72.6 ± 10.2 |
|
| ||
| CAC at baseline | 0 | 290.8 ± 545.9 |
|
| ||
| Median follow-up duration in years (max) | 7.6 (max=9.0) | 7.6 (max=8.9) |
|
| ||
| Years between exams | 2.5 ± 0.8 | 2.4 ± 0.9 |
|
| ||
| Incidence of CAC in those with CAC=0 (n,%) | 535, 15.8% | |
| Median (25–75%tiles) CAC change/year in those with incident CAC | 2.2 (0.7–5.9) | |
|
| ||
| Progression of CAC in those with CAC>0 (n,%) | 2869, 84.8% | |
| Median (25–75%tiles) of CAC change/year in those with progression of CAC | 28.9 (60.9–529.1) | |
Subjects include both imputed and non-imputed cases; MI=myocardial infarction, HDL-C=high density lipoprotein-cholesterol, SBP=systolic blood pressure, DBP=diastolic blood pressure, CAC=coronary artery calcium
CHD event rates were annualized and reported in terms of events per 1000 person years, overall and according to absolute change categories (no change, and >0 in those without CAC at baseline and no/negative change, .01–99, 100–199, 200–299, and ≥300 in those with CAC at baseline).
For the CAC=0 baseline group, we used Cox regression to model the change in CAC both as a dichotomous variable comparing those with any progression to those who remained at zero, and as a continuous variable for each 5-unit change in progression. For those with CAC>0, in the Cox model with CAC in intervals, we chose intervals of 100 units of change per year compared to those who had less than or equal to zero change, and as a continuous variable in units of 100 change per year. In the imputed analyses, follow-up time was calculated from the time of the baseline scan since progression was imputed in cases of intervening CHD events or where second scans were unavailable; for the complete case analysis, it was calculated from the time of the second scan. Socio-economic status was unrelated to progression, and not included in final models. Models both unadjusted and adjusted for age, gender, ethnicity, and baseline total and HDL-cholesterol, lipid-lowering medication, systolic and diastolic blood pressure, hypertension medication, smoking, diabetes, family history, and baseline CAC score were run. All analyses were conducted with Stata statistical software, version 12.1.
Results
Overall 3,382 (50.1%) of participants had CAC at baseline; 12% of our overall sample were Black, 28% Chinese, 38% White, and 22% Hispanic. Participants with CAC present at the baseline exam were more likely to be older, male, have diabetes, have a family history of MI or stroke, be on lipid-lowering or antihypertensive medication, and to have previously smoked. Of those with baseline CAC=0, 84.2% still had a 0 value at the time of follow-up scan, whereas 15.8% showed progression, with a median progression of 2.2 Agatston units/year. For those with CAC >0, 15.2% did not progress, whereas 84.8% showed progression, with a median progression of 28.9 units/year (Table 1). Among those with baseline CAC scores of 0, 0.001–99, 100–199, 200–299, and ≥300, median annual progression was 2.2, 8.3, 28.4, 44.0, and 103.3 units/year, respectively, for the imputed analysis and 0, 7.9, 27.7, 43.2, and 95.5 units/year, respectively, for the non-imputed analysis. There were 343 total incident CHD events, of which 206 were hard CHD events.
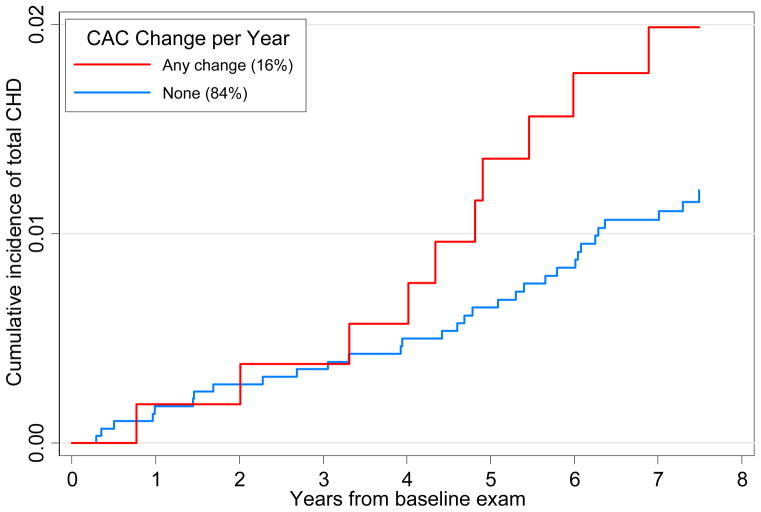
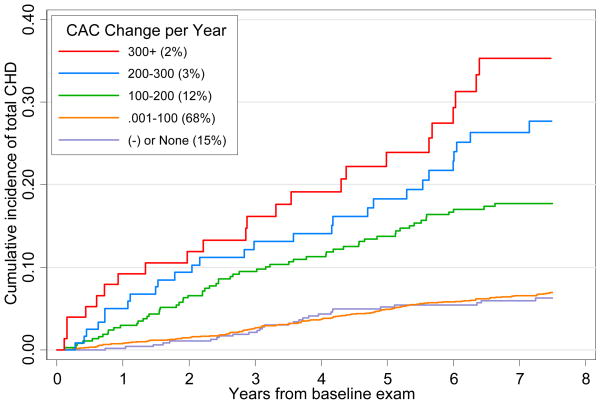
Figure 1 shows the Kaplan Meier failure estimates with incident CHD being significantly greater comparing any to no CAC change among those with baseline CAC=0 (p<0.001 for the log-rank test). Figure 2 shows successively greater cumulative proportions of incident CHD events according to annual CAC change (none, 0.001–99, 100–199, 200–299, and ≥300) among those with baseline CAC>0 (p<0.001 comparing CAC change groups). Kaplan-Meier estimates were also determined for the non-imputed samples (see online-only Data Supplement).
Figure 1.
Kaplan-Meier Plot of Cumulative Incidence of Total CHD Among Persons with CAC=0 at Baseline; p<.001 for log-rank test for equality of survivor function comparing those with any vs. no change in CAC. Numbers in parenthesis indicate the proportion of subjects in each group. Both imputed and nonimputed subjects are included. CAC=coronary artery calcium; CHD=coronary heart disease.
Figure 2.
Kaplan-Meier Plot of Cumulative Incidence of Total CHD Among Persons with CAC>0 at Baseline; p<0.001 for log-rank test for equality of survivor function across CAC change groups: 300+, 200–299, 100–199, 0.001–100, negative or no change. Numbers in parenthesis indicate the proportion of subjects in each group. Both imputed and nonimputed subjects are included. CAC=coronary artery calcium; CHD=coronary heart disease
As shown in Table 2, among those with CAC=0 at baseline, compared to persons with no increase in CAC score, any increases were associated with greater risk for CHD events; those with an increase in CAC had a 1.4-fold greater risk of total and 1.5-fold greater risk for hard CHD events in adjusted analyses. There was overall a 50% greater risk for both total and hard CHD events per increase of 5 units per year of CAC. The analysis for Table 2 was repeated on the non-imputed sample and the results of this comparison are available in the online-only Data Supplement. The imputed models had more precision and statistical power, although results were similar regardless of which analytic strategy was used.
Table 2.
Hazard ratio examining the likelihood of total CHD and hard CHD by progression of CAC among those with CAC=0 at baseline (Multi-Ethnic Study of Atherosclerosis)
| Event rate per 1000 person-years [events/subjects] | Total CHD | Hard CHD |
|---|---|---|
|
| ||
| Absolute Δ in CAC/year | ||
| No change | 1.6, [34/2861] | 1.1, [24/2861] |
| Any progression | 2.6, [10/535] | 1.8, [7/535] |
| Total | 1.8, [44/3396] | 1.3, [31/3396] |
|
| ||
| Hazard Ratio (95% CI) | Total CHD | Hard CHD |
|
| ||
| Unadjusted absolute Δ in CAC/year | [44/3396] | [31/3396] |
| No change | Reference | Reference |
| Any progression | 1.5 (0.7–3.5) | 1.5 (0.6–3.9) |
|
| ||
| Adjusted1 absolute Δ in CAC/year | [44/3396] | [31/3396] |
| No change | Reference | Reference |
| Any progression | 1.0 (0.4–2.3) | 1.1 (0.4–2.8) |
|
| ||
| Unadjusted absolute Δ in CAC/year (per 5 units) | [44/3396] | [: 31/3396] |
| 1.3*(1.1–1.8) | 1.4*(1.1–1.9) | |
|
| ||
| Adjusted1 absolute Δ in CAC/year (per 5 units) | [44/3396] | [31/3396] |
| 1.4*(1.0–1.9) | 1.5*(1.1–2.1) | |
p<0.05,
p<0.01,
p<0.001 (two-tailed);
Adjusted for baseline age, gender, ethnicity, total cholesterol, HDL-cholesterol, lipid lowering medication, systolic and diastolic blood pressure, antihypertensive medication, smoking, diabetes, and family history; Δ=change; analysis includes imputed and nonimputed subjects; follow-up time is calculated from the time of the baseline scan. CHD=coronary heart disease, CAC=coronary artery calcium
In Table 3 the risk of future CHD events by progression of CAC in those with CAC>0 at baseline is compared to the risk in those with no progression of CAC. In participants with CAC increases of 100 units or more per year, there were significant 2–4-fold greater increases in risk for total and hard CHD events in adjusted analyses (3–6-fold increased risk with changes of 300 or more per year).
Table 3.
Hazard ratio examining the likelihood of total CHD and hard CHD by progression of CAC among those with CAC>0 at baseline (Multi-Ethnic Study of Atherosclerosis)
| Event rate per 1000 person-years [events/subjects] | Total CHD | Hard CHD |
|---|---|---|
|
| ||
| Absolute Δ in CAC/year | ||
| No or negative change | 9.7, [33/513] | 5.8, [21/513] |
| .001 to 100 | 9.8, [157/2309] | 5.8, [94/2309] |
| 100 to 200 | 28.4, [62/372] | 15.0, [35/372] |
| 200 to 300 | 39.6, [25/113] | 19.4, [13/113] |
| 300+ | 56.3, [22/75] | 28.3, [12/75] |
| Total | 13.1, [299/3382] | 7.5, [175/3382] |
|
| ||
| Hazard Ratio (95% CI) | Total CHD | Hard CHD |
|
| ||
| Unadjusted absolute Δ in CAC/year | [Model: 299/3382] | [Model: 175/3382] |
| No or negative change | Reference | Reference |
| .001 to 100 | 1.0 (0.6–1.7) | 1.0 (0.5–1.9) |
| 100 to 200 | 3.0‡(1.7–5.4) | 2.6*(1.2–5.8) |
| 200 to 300 | 4.3‡(2.4–8.4) | 3.3†(1.9–8.6) |
| 300+ | 5.9‡(3.0–11.6) | 5.0‡(2.2–11.6) |
|
| ||
| Adjusted1 absolute Δ in CAC/year | [Model: 299/3382] | [Model: 175/3382] |
| No or negative change | Reference | Reference |
| .001 to 100 | 1.0 (0.6–1.7) | 1.0 (0.5–1.9) |
| 100 to 200 | 2.1*(1.1–3.8) | 1.9 (0.8–4.5) |
| 200 to 300 | 2.4*(1.1–5.1) | 2.1 (0.7–6.3) |
| 300+ | 2.8*(1.2–5.4) | 3.0* (1.0–8.9) |
|
| ||
| Unadjusted absolute Δ in CAC/year (per 100 units) | [Model: 299/3382] | [Model: 175/3382] |
| 1.5‡(1.4–1.7) | 1.5‡(1.3–1.6) | |
|
| ||
| Adjusted1 absolute Δ in CAC/year (per 100 units) | [Model: 299/3382] | [Model: 175/3382] |
| 1.2‡(1.1–1.4) | 1.3*(1.1–1.5) | |
p<0.05,
p<0.01,
p<0.001 (two-tailed);
Adjusted for baseline age, gender, ethnicity, total cholesterol, HDL-cholesterol, lipid lowering medication, systolic and diastolic blood pressure, antihypertensive medication, smoking, diabetes, family history, and baseline CAC; Δ=change; analysis includes imputed and non-imputed subjects; follow-up is calculated from the time of the baseline scan. CHD=coronary heart disease, CAC=coronary artery calcium
We also conducted a sensitivity analysis and compared results from the imputed and not imputed (complete case) data. In adjusted analyses, increases of CAC of 300 or greater (vs. no progression) were associated with HR’s for total CHD of 2.8 (1.2–5.4) for imputed and 3.8 (1.5–9.6) for non-imputed analyses, with a 100 unit annual progression of CAC associated with HR’s of 1.2 (1.1–1.4) and 1.3 (1.2–1.5), respectively (Table 4).
Table 4.
Hazard ratio examining the likelihood of total CHD by progression of CAC among those with CAC>0 at baseline (MESA Study), with and without imputed values
| Event rate per 1000 person-years [events/subjects] | Total CHD Imputed | Total CHD Not imputed2 |
|---|---|---|
|
| ||
| Absolute Δ in CAC/year | ||
| No or negative change | 9.7, [33/513] | 7.0, [13/358] |
| .001 to 100 | 9.8, [157/2309] | 11.5, [112/2027] |
| 100 to 200 | 28.4, [62/372] | 28.1, [29/234] |
| 200 to 300 | 39.6, [25/113] | 31.4, [10/72] |
| 300+ | 56.3, [22/75] | 43.4, [9/52] |
| Total | 13.1, [299/3382] | 13.1 [173/2743] |
|
| ||
| Hazard Ratio (95% CI) | Total CHD | Not imputed |
|
| ||
| Unadjusted absolute Δ in CAC/year | [Model: 299/3382] | [Model: 173/2743] |
| No or negative change | Reference | Reference |
| .001 to 100 | 1.0 (0.6–1.7) | 1.6 (0.9–2.8) |
| 100 to 200 | 3.0‡(1.7–5.4) | 3.9‡(2.0–7.5) |
| 200 to 300 | 4.3‡(2.4–8.4) | 4.3†(1.9–9.9) |
| 300+ | 5.9‡(3.0–11.6) | 5.9‡(2.5–13.8) |
|
| ||
| Adjusted1 absolute Δ in CAC/year | [Model: 299/3382] | [Model: 160/2542] |
| No or negative change | Reference | Reference |
| .001 to 100 | 1.0 (0.6–1.7) | 1.7 (0.9–3.2) |
| 100 to 200 | 2.1*(1.1–3.8) | 3.1†(1.5–6.4) |
| 200 to 300 | 2.4*(1.1–5.1) | 3.2*(1.3–7.9) |
| 300+ | 2.8*(1.2–5.4) | 3.8†(1.5–9.6) |
|
| ||
| Unadjusted absolute Δ in CAC/year (per 100 units) | [Model: 299/3382] | [Model: 160/2543] |
| 1.5‡(1.4–1.7) | 1.5‡(1.3–1.6) | |
|
| ||
| Adjusted1 absolute Δ in CAC/year (per 100 units) | [Model: 299/3382] | [Model: 160/2542] |
| 1.2‡(1.1–1.4) | 1.3‡(1.2–1.5) | |
p<0.05,
p<0.01,
p<0.001 (two-tailed);
Adjusted for baseline age, gender, ethnicity, total cholesterol, HDL-cholesterol, lipid lowering medication, systolic and diastolic blood pressure, antihypertensive medication, smoking, diabetes, family history, and baseline CAC; Δ=change; the imputed sample includes both imputed and non-imputed subjects and follow-up time is calculated from the date of the baseline scan; for the not imputed analyses, survival time is calculated as the time between hard CHD and exam 2 or exam 3 depending on whether the follow-up CAC score is from exam 2/3. CHD=coronary heart disease; CAC=coronary artery calcium
Corresponding analysis for subjects with CAC=0 at baseline and for hard CHD are available in the online-only Data Supplement. For those with CAC=0 at baseline, results were similar regardless of which analytic strategy was used. Using only the non-imputed data, for those with CAC=0 at baseline, adjusted absolute changes in CAC of 5 units per year (compared to no change) were associated with a significant 1.4 (1.0–1.9)-fold greater risk for total CHD events, compared to a 1.4 (1.1–1.8)-fold greater risk in non-imputed analyses (table 1a, online Data Supplement). For hard CHD in those with baseline CAC=0, similar differences are seen: 1.5 (1.1–2.1) and 1.5 (1.2–1.9) for increases of 5 units/year in imputed vs. non-imputed analyses (all adjusted models) (Table 2a, online Data Supplement). For those with CAC>0 at baseline, in adjusted analyses, increases of CAC of 300 or greater (vs. no progression) were associated with HR’s for hard CHD of 3.0 (1.01–8.9) for imputed and 6.3 (1.9–21.5) for non-imputed analyses, and per 100 unit increase in continuous analyses, 1.3 (1.10–1.5) and 1.3 (1.1–1.5), respectively (table 3a, online Data Supplement).
Discussion
Serial evaluation of CAC has been proposed for measuring progression of atherosclerosis, and thus to predict CHD in asymptomatic individuals. The most striking findings in the current study were the graded relationships of CAC progression with CHD event risk, strongly suggesting that the functions are linear, with greater progression associated with greater risk. We demonstrated that progression of CAC is associated with total and hard CHD risk; these relationships were attenuated but still significant after adjusting for risk factors and baseline calcium. As shown in Table 3, we demonstrated that those with annual progression of ≥300 units (approximately 2 percent of the cohort) were 3–6.3 more likely to suffer cardiovascular events, whether assessed as total or hard CHD in our cohort. Compared with those without progression of CAC, any progression of CAC in those with CAC=0 at baseline and progression of at least 100 units in those with CAC>0 at baseline, even after adjustment for baseline CAC, were associated with increased CHD event risk, although absolute risks were much lower in those who were initially free of CAC at baseline.
Our results are consistent with earlier retrospective and prospective studies showing that persons who experienced CHD events had also greater progression of CAC7,8 as well as a large recent prospective study demonstrating a strong relation of CAC progression with total mortality.9 We have previously demonstrated9 in a prospective evaluation of 4,609 patients that, after adjusting for baseline score, age, sex, and time between scans, CAC progression (mean progression was 247 in this group) was associated with a 3.34 fold risk of all-cause mortality (HR 3.34; 95% CI: 2.65 to 4.21; p < 0.0001). This result is quite similar to results in the present study, where we demonstrated 3.0–6.3 fold increased risk in hard events for those with available data. In a retrospective follow-up study of 817 persons, CAC progression was the strongest predictor of myocardial infarction.26 Another study measured CAC change in 495 asymptomatic subjects who underwent sequential CT scanning In this study, the relative risk for acute MI for patients exhibiting ≥15% CAC progression was 17.2 (95% CI: 4.1 to 71.2) when compared to that of patients without CAC progression (p<0.0001).7 Also, increased progression of CAC has been recently shown in persons with metabolic syndrome and diabetes, with greater CAC progression also shown to be related to greater CHD event rates.27
Of great interest has been whether risk-reducing therapies such as statins may retard progression of atherosclerosis assessed by serial CAC scanning. Among 615 hyperlipidemic postmenopausal women randomized to intensive versus moderate lipid-lowering with a statin, with baseline and 12-month follow-up CAC assessed by CT, no difference in calcium volume score change was seen between the treatment groups.5 Also, in a clinical trial conducted to evaluate the impact of aggressive lipid-lowering and antioxidant therapy on the progression of CAC in 4,613 asymptomatic persons with baseline and follow-up CT scans at 2 years, while showing no effects of the intervention on progression of CAC, those who sustained a coronary event had a median increase in CAC score of 247 compared to only 4 in those who did not sustain a coronary event at any time during the study. However, the strongest predictor of subsequent CHD events was two-year change in calcium score. 6 Finally, in a randomized study of statins and CAC progression, the mean progression of CAC volume scores, corrected for the baseline CAC volume score, was 27% in the 80-mg atorvastatin group, similar to the 25% in the 10-mg atorvastatin group. CAC progression showed no relationship with on-treatment LDL cholesterol levels.28
Important to understanding factors related to the progression of CAC is the baseline calcium score, a strong predictor of CAC progression.29,30 While baseline CAC can be considered a confounder, it can also be considered part of the causal pathway between CHD risk factors and CAC progression. Persons with higher baseline CAC have a higher risk factor burden and, perhaps, more uncalcified atherosclerotic plaques destined for calcification and, thus, may exhibit greater future CAC progression. Thus, including baseline CAC in the model could account for effects that variables of interest may have had prior to the baseline examination.22 However, in the current study we were able to show that CAC progression predicts CHD events even after adjustment for baseline risk factors and baseline calcium score. Also, in those with CAC=0, while even an annual CAC increase of 5 units/year is associated with greater CHD risk, the risk in this group is low compared to those with CAC at baseline. Other reports also document the low progression and event rate in those with CAC=0 at baseline,6 which indicates a low risk of future CHD events for at least five years.31 However, in those with CAC at baseline, annual progression of CAC of 100 units or greater is associated with substantially higher CHD event rates.
In our study, those with missing follow-up CAC scores had this information imputed based on predicted CAC values from baseline CAC and risk factor relationships. We present both non-imputed (only cases with follow-up) and imputed cases in this study. We believe that multiple imputation was an optimal method for treating these participants because they were likely to have been missing at random (MAR), whereas complete case analysis requires the stringent and unlikely assumption that the values are missing completely at random (MCAR).13,23,24 The follow-up CAC scores were clearly not MCAR because the probability of a participant missing a second scan was related to other observed information (e.g., age and gender) as well as to having experienced an event. For example, for participants with baseline CAC>0, the event rate was 0.8 per 1,000 person-years while in the participants missing second scans the event rate was 2.2 per 1,000 person-years. If the excluded participants were at higher risk, they may have also been more likely to have greater progression of CAC; thus, exclusion of such individuals would have potentially biased the results towards the null. The imputed cases were estimated to have higher average progression than occurred in the observed cases, with respective means and standard deviations of 73.1 ± 104.2 units per year and 44.9 ± 79.8 units per year in the CAC>0 group. The MAR assumption is plausible for the follow-up CAC measures because the study contained information that was related to the probability of a participant not having a second scan, and this information was incorporated in the imputation model. It is worth noting that the results from the imputed data showed no unexpected or major differences when compared to the estimates from data that were not imputed. The event rates in the imputed data were higher than in the complete case analysis models due to the inclusion of a larger number of events (and not surprisingly, these people also had higher baseline risk and progression of CAC). In other words, the complete participant analysis model had to exclude people who had intervening CHD events before receiving a follow-up CAC scan, whereas the imputed models were able to include these events; this was a major advantage of the imputation approach. The main difference between the Cox models was that the complete case analysis results had greater variability and less precision than the imputed results. We believe that multiple imputation by using all available data provides for more accurate estimates of effect sizes, although the substantive consequences of the imputation were relatively unremarkable. Regardless of whether missing values were imputed, similar conclusions emerged about the importance of CAC progression on the likelihood of a CHD event.
Our study includes several strengths and limitations. MESA includes standardized risk factor assessment, standardized protocols for CAC scanning and interpretation, and event ascertainment.11,14 Our data involved progression of CAC measured over a limited time interval (mean, 2.5 years) with the assumption of a linear relation of time with extent of CAC progression, so findings may have differed if longer term progression had been studied (e.g., with additional measures of CAC), if the relation of time to progression were nonlinear, and if a greater follow-up for events had been conducted. This report did not examine relationships with other CVD events such as stroke or heart failure, nor heterogeneity by gender, ethnicity, or comorbidity. Our analyses are also based on Agatston scores to ensure consistency with other MESA publications; however we also ran our primary analyses using volume score and found virtually indistinguishable results (not shown). Also, in MESA a high correlation (≥0.99) is seen between Agatston and volume scores so it is not surprising the results are robust regardless of which score is used.
Based on current guidelines, the clinical utility of a CAC scan is reasonable for risk assessment of low-intermediate, intermediate risk individuals and persons with diabetes.32 With regard to CAC progression, the current study adds important evidence from a large well-characterized prospective study that CAC progression is a significant predictor of CHD events, independent of traditional risk factors and baseline CAC scores.33 More data are needed on whether serial CAC measures would help guide therapy and benefit patients to a greater extent than a single CAC measure, particularly to justify the additional cost and exposure to radiation.34 Some questions include timing of scanning, standardization of scanning and reading, and whom to target for serial scans. Some participants in our study were scanned with two different CT models, but the use of phantoms, standardized reading protocols and inter-scanner comparisons limited the variations caused by this temporal shift in scanner technology during the study.14 It should be noted that radiation exposure has decreased over recent years to below 1 mSv in most cases10; the CT heart scan for CAC is lower in radiation than most other cardiology diagnostic procedures and now comparable to mammography.35 In addition, costs associated with CAC CT scans have also decreased, with current Medicare reimbursement at approximately $100.
Conclusions
We report an association of progression of CAC with incident hard and total CHD events in a large multiethnic cohort with CAC scans averaging 2.5 years apart. While this suggests that serial scans may identify some people at high risk of CHD events, many questions remain about whether targeting those with progression of CAC with a more intense risk factor modification strategy would subsequently decrease CHD outcomes, and if it does, whether it is a cost-effective strategy.
Acknowledgments
Funding Sources:
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures:
Dr. Budoff is a consultant for General Electric. Dr. Wong is a consultant for Re-Engineering Healthcare, Inc. No other authors have any disclosures.
References
- 1.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 3.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–60. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 4.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–8. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann GA, Hecht H, Rumberger JA. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES) Circulation. 2005;112:563–571. doi: 10.1161/CIRCULATIONAHA.104.512681. [DOI] [PubMed] [Google Scholar]
- 6.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Callister TQ, Shaw LJ. Progression of Coronary Artery Calcium and Risk of First Myocardial Infarction in Patients Receiving Cholesterol-Lowering Therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–7. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Raggi P. Coronary artery disease progression assessed by electron beam tomorgraphy. Am J Cardiol. 2001;88:46E–50E. doi: 10.1016/s0002-9149(01)01767-2. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. J Am Coll Cardio Img. 2010;3:1229–36. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Rubin DB. Multiple imputation for non-response in surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 13.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 14.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JC, Detrano R, Kronmal RA, McNitt-Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring Coronary Calcium on CT Images Adjusted for Attenuation Differences. Radiology. 2005;235:403–414. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. The Distribution of Coronary Artery Calcium by Race, Gender and Age--Results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 18.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 19.Graham JW. Missing data analysis: Making it work in the real world. Annu Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–89. [Google Scholar]
- 21.Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects—results from the Multi-ethnic Study of Atherosclerosis (MESA) Circulation. 2007;29;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 23.Schafer JL. Multiple imputation: A primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 24.Giorgi R, Belot A, Gaudart J, Launoy G the French Network of Cancer Registries FRANCIM. The performance of multiple imputation for missing covariate data within the context of regression relative survival analysis. Stat Med. 2008;7:6310–31. doi: 10.1002/sim.3476. [DOI] [PubMed] [Google Scholar]
- 25.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic approach that accounts for inter-scan variability. Am J Roentgenol. 2004;182:1327–32. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 26.Raggi P, Cooil B, Shaw L, Aboulhson J, Takasu J, Budoff M, Callister TQ. Progression of coronary calcification on serial electron beam tomography scanning is greater in patients with future myocardial infarction. Am J Cardiol. 2003;92:827–9. doi: 10.1016/s0002-9149(03)00892-0. [DOI] [PubMed] [Google Scholar]
- 27.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, Guerci A, Jacobs DR, Jr, Kronmal R, Liu K, Saad M, Selvin E, Tracy R, Detrano R. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5:358–66. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Förster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V, Lahiri A, Leischik R, Moshage W, Schartl M, Siffert W, Steinhagen-Thiessen E, Sinitsyn V, Vogt A, Wiedeking B, Erbel R. Effect of Intensive Versus Standard Lipid-Lowering Treatment With Atorvastatin on the Progression of Calcified Coronary Atherosclerosis Over 12 Months. A Multicenter, Randomized, Double-Blind Trial. Circulation. 2006;113:427–437. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- 29.Lee KK, Fortmanm SP, Fair JM, Iribarren C, Rubin GD, Varady A, Go AS, Quertermous T, Hlatky MA. Insulin resistance independently predicts the progression of coronary artery calcium. Am Heart J. 2009;157:939–45. doi: 10.1016/j.ahj.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Wong ND, Kawakubo M, LaBree L, Azen SP, Kiang M, Detrano R. Relation of coronary calcium progression and control of lipids according to National Cholesterol Education Program Guidelines. Am J Cardiol. 2004;94:431–36. doi: 10.1016/j.amjcard.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Min JK, Lin FY, Gidseg GS, Weinsaft JW, Berman DS, Shaw LJ, Rozanski A, Callister TQ. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan. What is the “warranty period” for remaining normal? J Am Coll Cardiol. 2010;55:1110–7. doi: 10.1016/j.jacc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 32.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–22. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 34.McEvoy JW, Blaha MJ, Nasir K, Blumenthal RS, Jones SR. Potential Use of Coronary Artery Calcium Progression to Guide the Management of Patients at Risk for Coronary Artery Disease Events. Curr Treat Options Cardiovasc Med. 2012;14:69–80. doi: 10.1007/s11936-011-0154-5. [DOI] [PubMed] [Google Scholar]
- 35.Nakazato R, Dey D, Gustein A, Le Meunier L, Cheng VY, Pimentel R, Paz W, Hayes SW, Thomson LE, Friedman JD, Berman DS. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr. 2009;3:394–400. doi: 10.1016/j.jcct.2009.10.002. [DOI] [PubMed] [Google Scholar]