Abstract
Objectives
The vascular depression hypothesis posits that cerebrovascular burden contributes to the development of depression symptoms in late life. Building on work that suggests that vascular depression is a prodrome for frailty (Paulson & Lichtenberg, 2011), this paper tests a theoretical framework that vascular depression symptoms are an early marker of a broader pattern of decline characterized by more frailty symptoms and shortened lifespan, and that vascular depression symptoms predict mortality through frailty.
Methods
The sample was drawn from the Health and Retirement Study and included 1,361 stroke-free women over the age of 80. Data was included from six biannual waves from 1998 to 2008 (waves 4-9). A vascular depression symptomatology variable was based on CES-D scores and number of cerebrovascular risk factors (hypertension, diabetes, cardiac disease and smoking). Frailty was measured based on wasting, slowness, weakness, fatigue, and falls. Vascular depression and frailty symptoms were modeled using slope and intercept terms. Mortality was modeled using a discrete-time survival term.
Results
The data supported the proposed model (RMSEA=.051; CFI=.971; X2=234.84, p<.001). Higher vascular depression symptom slope and intercept scores significantly predicted higher frailty slope and intercept scores, respectively. Frailty intercept scores significantly predicted mortality. Vascular depression symptoms indirectly predicted mortality through frailty symptoms. A second model testing the competing hypothesis that frailty symptoms lead to vascular depression symptoms and indirectly to mortality was not supported by the data.
Conclusions
Results suggest that vascular depression symptoms are associated with a clinical trajectory that includes greater frailty and shortened remaining lifespan.
Keywords: Longitudinal modeling, Clinical trajectory, Aging, Mood, Disability
“How can we transform to a normal and physiological condition, old age, at present utterly pathological, unless we first understand the most intimate details of its mechanism?” Eli Metchnikoff, 1903
Concurrent with the rapidly increasing numbers of older-old adults in the U.S., the syndrome of frailty is becoming increasingly common. Up to 30% of elders die with, or because of, frailty (Fassbender, Fainsinger, Carson, & Finegan, 2009; Paulson & Lichtenberg, 2011), and frail elders' need for extensive care makes this syndrome among the most costly causes of death (Fassbender et al., 2009). Older women live longer than men, but are at higher risk for disability and depression, placing them at higher risk for frailty (Paulson & Lichtenberg, in press). Risks associated with depression and frailty become exaggerated in late life, making age 80 an important time to reassess patient prognosis and treatment goals. Our objectives were to evaluate a theoretical framework that describes an adverse clinical trajectory among older-old women. The proposed framework specifies a temporal relationship between symptoms of (a) vascular depression, (b) frailty and (c) mortality. We tested this model in a sample of stroke-free women over the age of 80.
The proposed model is based on the well-established finding that high cerebrovascular burden (CVB), characterized here by hypertension, diabetes, cardiac disease, and smoking history, slowly erodes the capacity for adaptive functioning over many years (Flicker, 2010; Pugh & Lipsitz, 2002). The term “adaptive functioning” can be broadly interpreted, but this model emphasizes depression, frailty, and mortality risk—three primary areas of concern for both the aging patient population and the public healthcare system. Although both vascular depression and frailty symptoms (Cawthon et al., 2007; Fried et al., 2001) have been directly associated with heightened mortality rates, the proposed model posits that vascular depression symptoms are an early indicator of a broader pattern of decline that is subsequently characterized by higher rates of frailty symptoms and mortality.
High CVB undermines late-life adaptive functioning in numerous ways, and onset of depression symptoms is chief among those. The vascular depression hypothesis (Alexopoulos et al., 1997) posits that high CVB compromises neurological functioning, particularly in frontostriatal areas, and that as a result, depression symptoms develop. Vascular depression theory has produced two complementary lines of research. One describes how CVB-mediated neurological changes—and prefrontal white-matter hyperintensities in particular—predict higher rates of depression among elders (Coffey, Figiel, Djang, & Weiner, 1990; Sneed, Rindskopf, Steffens, Krishnan, & Roose, 2008). The second line of vascular depression research describes clinically defined vascular depression, in which greater depression symptoms are predicted by comorbidities such as diabetes, hypertension, and cardiac disease (Mast, Neufeld, MacNeill, & Lichtenberg, 2004; Yochim, Mast, & Lichtenberg, 2003). Consistent with these findings, another study from our lab (Paulson, Bowen, & Lichtenberg, under review) found that in the same sample used in the present study, older women with high CVB reported more depression symptoms and were at increased risk for probable depression. While results have been mixed for both neuroradiological (Rainer et al., 2006) and clinical studies (Lyness et al., 1999), both approaches generally support vascular depression theory. Of particular relevance to this synthesis, elders with high CVB and depression have much higher mortality rates than their healthier counterparts (Lavretsky et al., 2010).
In our proposed model, vascular depression symptoms are an early indicator of the decline of brain integrity, with frailty symptoms emerging later as neurological damage continues to accrue. In this way, vascular depression is conceptualized as one entry point into the cycle of frailty. Broadly speaking, frailty symptoms can be viewed as the combined effects of life stress that result in multisystemic dysregulation of homeostatic systems (Clegg, 2011; Fried, Ferrucci, Darer, Williamson, & Anderson, 2004), leading to homeostenosis, or impaired homeostatic maintenance during periods of acute stress (Powell, 1997). In this study, frailty was measured using the Paulson-Lichtenberg Frailty Index (PLFI), a model based on Fried et al.'s (2001) work, and includes wasting, weakness, slowness, fatigue, and falls. Validated using HRS data, this frailty index identifies higher rates of frailty among the older-old, women, minorities, and those with greater medical burden, probable depression, cognitive impairment, and ADL or IADL disability. As in Fried et al.'s research (2001), respondents identified as frail using this index are more likely to experience hospitalization and are at higher mortality risk (Paulson & Lichtenberg, 2011, 2012).
Katz (2004) and others (Mast, 2010) have noted that both late-life depression and frailty share numerous phenomenological and theoretical similarities, including rates of white matter hyperintensities, and that depression, particularly in the context of high CVB, may precede and predict frailty. This hypothesis was supported by Lakey et al.'s (2012) research on middle-aged and older adults. Our earlier study (Paulson & Lichtenberg, in press) using the same sample as this study, concluded that over a 4-year interval, elders with both high CVB and probable depression have a greater risk of both prevalent and incident frailty than did those with either high CVB or probable depression alone. By contrast, a recent review by Mezuk, Edwards, Lohman, Choi, and Lapane (in press) argues that the relationship between depression and frailty is bidirectional. The review was complemented by an empirical study (Mezuk, Lohman, Dumenci, & Lapane, in press) suggesting that frailty and depression are independent constructs, but often overlap in patient populations. Our previous study did not test the competing hypothesis—that frailty predicts vascular depression symptomatology—as suggested by Mezuk et al.'s review examining late life depression and frailty without regard to etiology. It may be, however, that vascular depression, a particular subtype of depression, has a temporal ordering with frailty. Moreover, these past studies have not described the indirect relationships from vascular depression symptomatology through frailty to mortality, as predicted by the proposed model, or conversely, from frailty through vascular depression symptomatology to mortality.
The proposed model describes a dynamic process that develops over a period of years. Accordingly, detection of these effects requires a longitudinal research design that uses large-scale cohort panel data, such as the Health and Retirement Study (HRS). The hypothesis tested in this paper posits that in a sample of stroke-free women over the age of 80, vascular depression symptoms will (a) predict frailty and (b) predict mortality indirectly through frailty. To test the specificity of our model, however, we will also examine whether frailty will (a) predict vascular depression symptoms and (b) predict mortality indirectly through vascular depression symptomatology.
Method
Sample
The HRS is a National Institute on Aging–supported prospective, multistage cohort study of U.S. households (Heeringa & Conner, 1995). The HRS data includes four smaller samples consisting of (a) respondents from the original (1992) HRS who were aged between 51 and 61 that year; (b) the Asset and Health Dynamics of the Oldest Old study, which included participants over age 70 and was merged with the HRS in 1998, and (c) two additional samples selected in 1998 to better represent the population of retired adults in the United States. Most HRS survey data are collected at 2-year intervals.
A subsample of HRS respondents consisting of women who were 80 years or older in 1998 formed the baseline for this study. Respondents were excluded if, in 1998, they reported a history of stroke or were unable to independently respond to survey materials (e.g., the Center for Epidemiological Studies Depression Scale [CES-D], a measure of depressive symptoms). We then incorporated data from the six biennial waves (1998-2008).
Measures
Self-Reported Medical Conditions
Medical data (hypertension, diabetes, history of heart disease) and lifetime history of smoking were collected by self-report. Hypercholesterolemia was not included, because it was not reported in the data at every wave. Incidence of death was identified at each wave. In the case of deaths, the exact date within the 2-year period was not known.
Depressive Symptoms
Depression was measured using a shortened, 8-item form of the original CES-D (Radloff, 1977). Six of the eight items are negatively worded and two are positively worded. Participants are asked to respond “yes” or “no” to each item (“was depressed,” “everything was an effort,” “sleep was restless,” “was happy,” “felt lonely,” “enjoyed life,” “felt sad,” “could not get going”), based on whether or not they had experienced it during the preceding week. Scores ranged from 0 to 8, with higher scores indicating greater depressive symptoms. Using HRS data, the reliability of the 8-item CES-D was adequate, with high Cronbach's alpha based on both normative data (.81-.83; Steffick, 2000) and the sample used in this study (α = .72). This measure has been found to be a valid index of mood in late life (Steffick, 2000) and is broadly used in epidemiological studies of late-life depression (Beekman et al., 1997). Recommended clinical cutoffs for this measure suggest interpreting CES-D scores ≥3 (Steffick, 2000) as indicative of probable clinical depression.
Vascular-Depression Symptoms
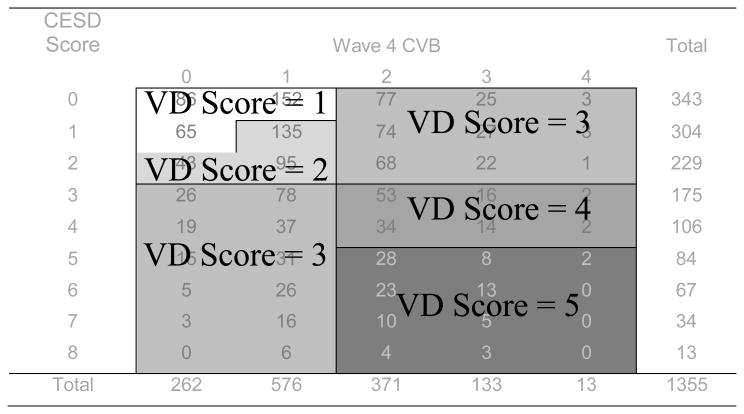
In this study, vascular depression symptomatology was measured as a dimensional variable. In keeping with past work (Paulson & Lichtenberg, 2011), depression symptomatology and CVB were integrated to form a single variable representing vascular depression symptomatology. As displayed in Figure 1, respondents with 0 or 1 cerebrovascular risk factor (CVRF; hypertension, diabetes, cardiac disease, or smoking history) and a CES-D score of 0 or 1 were assigned a score of 1 (description: very healthy). Those with 1 CVRF and CES-D scores of 1 or 2 were assigned a score of 2 (description: healthy), as were those with 0 CVRFs and a CES-D score of 2. Respondents with 2 or more CVRFs or a CES-D score ≥3 were assigned a score of 3 (description: high CVB or probable depression). Respondents with 2 or more CVRFs and CES-D scores of 3 or 4 were assigned a score of 4 (description: mild vascular depression symptoms). Those with more than 2 CVRFs and CES-D scores ≥5 were assigned a score of 5 on the vascular depression symptomatology variable (description: moderate to severe vascular depression symptoms). Vascular depression symptomatology was measured in all six waves from 1998 through 2008.
Figure 1.
Specification of Vascular Depression Symptomatology variable based on CES-D score and number of cerebrovascular risk factors (hypertension, diabetes, cardiac disease, and smoking). Presented are vascular depression symptom scores at baseline (1998; wave 4), though vascular depression symptom scores were calculated at each wave.
Self-Rated Health
Self-rated health was measured by comparing self-rated health reports (1=excellent, 2=very good, 3=good, 4=fair, 5=poor) at adjacent time periods. Positive values indicate health deterioration over a 2-year period. Negative values suggest improved health. Only the baseline (1998) self-rated health information was used.
Functional Independence
Activities of daily living (ADLs) were measured using five criteria: bathing, eating, dressing, walking across a room, and getting in or out of bed. Instrumental activities of daily living (IADLs) were measured using three criteria: using a telephone, taking medication, and handling money. Scores were summed on both indices to indicate the number of items the respondent reported experiencing difficulty completing independently. Higher scores indicate greater disability. Only the baseline (1998) functional independence information was used.
Frailty
In this study, frailty was measured as a dimensional variable. We measured frailty symptomatology using the PFLI (Paulson & Lichtenberg, 2011, 2012), a measure based on Fried et al.'s (2001) conceptualization. Due to differences between the HRS data and Fried's model of frailty, the PFLI includes the following five symptoms: wasting, weakness, slowness, fatigue or exhaustion, and falls. The wasting criterion was met if a respondent reported loss of at least 10% of body weight over a 2-year period. The weakness criterion was met if she endorsed the question, “Because of health problems, do you have any difficulty with lifting or carrying weights over 10 pounds, like a heavy bag of groceries?” The slowness criterion was met if respondents answered in the affirmative to the question, “Because of a health problem, do you have any difficulty with getting up from a chair after sitting for long periods?” The fatigue or exhaustion criterion was met if the respondent answered in the affirmative to the question, “Since we last talked with you in [the last wave], have you had any of the following persistent or troublesome problems: … severe fatigue or exhaustion?” The falls criterion was met if the respondent answered in the affirmative to the question, “Have you fallen down in the past 2 years?” While Fried's criteria include low energy expenditure, this variable was not available in the HRS data. Instead, the frailty-symptom list was modified to include falls, which have been found to be an indirect measure of energy expenditure (Montero-Odasso et al., 2011). None of the frailty items was drawn from the CES-D or from the functional independence scales described above. This frailty index was used as a semicontinuous (discrete count) variable. Complete frailty data were available at the 2000, 2004, and 2008 waves.
Statistical Methodology
Integrated disease models attempt to describe complex interrelationships between disease processes, ultimately representing a complex clinical trajectory. Raykov (2007) a strategy for examining parallel processes, such as developmental processes or disease states, involves the specification of latent variables representing mean values at baseline (intercept) and rate of change over time (slope). This strategy can accommodate missing data, thus preventing bias that results from excluding more vulnerable participants for whom missing data exists (Rabbitt, Lunn, & Wong, 2008). The model employed in this study permits examination of how individuals' baseline scores for a particular variable predict change over time for that same variable. Additionally, this model facilitate examination of how baseline scores and rate of change over time for one variable or process predict baseline scores and rate of change over time for a separate variables or process. Finally, this strategy permits calculation of indirect effects. Examination of these multiple relationships using traditional hypothesis-testing strategies would require numerous analyses, resulting in inflation of type-1 error rates. By comparison to traditional hypothesis testing paradigms, the longitudinal modeling strategy employed in this study is a more parsimonious and robust method of describing longitudinal relationships between parallel, related disease process such as vascular depression and frailty.
Our primary objective was to test the described theoretical model, specifically addressing the hypothesis that vascular depression symptoms are an early indicator of a broader pattern of decline and predict mortality indirectly through frailty. We tested a model of temporal change that uses the intercept-and-slope model (Raudenbush & Bryk, 2002; Raykov & Marcoulides, 2008). For clarity, Table 3 presents the series of relationships represented in this model. First, vascular depression symptoms were modeled using latent variables representing vascular depression symptom score at baseline (vascular depression symptom intercept), and rate of change in the vascular depression symptom score over time (vascular depression symptom slope). Similarly, frailty symptoms were modeled using latent variables representing frailty score at baseline (frailty intercept) and rate of change in frailty score over time (frailty slope). Mortality risk was modeled using a discrete time-survival term (Singer & Willett, 1993) based on mortality data at each wave. Thus, five latent variables were used to represent the primary variables of interest – vascular depression symptoms, frailty, and mortality. As such, this can be described as a fixed-effects model.
Table 3. Hypothesized parameters represented in the theorized model.
| Control Variables | Vascular Depression | Frailty | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Age | ADLs | IADLs | SR Health | Intercept | Slope | Intercept | Slope | ||
| Vascular Depression Symptoms | Intercept | Yes | Yes | Yes | Yes | ||||
| Slope | Yes | Yes | Yes | Yes | Yes | ||||
| Frailty Symptoms | Intercept | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Slope | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Mortality | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
Frailty intercept and slope latent variables were regressed on vascular depression symptom intercept and slope latent variables. Mortality was then regressed on vascular depression slope and intercept latent variables, and on frailty slope and intercept latent variables. Then, the five latent variables representing the primary variables of interest were all regressed on the control variables - self-rated health change, ADLs, IADLs, and age. In this way, the influence of self-rated health, ADLs, IADLs and age were controlled for in the model.
Longitudinal research with older adults is complicated by high attrition rates, in this case reflecting the high rates of mortality and disability. The conventional strategy of excluding respondents with incomplete data systematically underestimates disease severity and incorrectly estimates relationships with outcome variables. The Full Information Maximum Likelihood strategy (FIML; Arbuckle, 1996), was used to estimate parameters using all available information. Specifically, auxiliary variables identified as highly predictive of missing data (self-rated health change, ADLs, IADLs, and age) were used in both models to improve accuracy of parameter estimation (Graham, 2003). In this way, data from all 1,361 respondents with baseline data in the available sample were used, regardless of the availability of complete data on vascular depression symptoms and frailty symptoms. Less than 1% of the sample was missing data on any auxiliary variable. Data were prepared in SPSS V19 and analyses conducted using Mplus (Muthen & Muthen, 2007).
To evaluate the competing hypothesis – that frailty symptoms predict development of vascular depression symptoms and (indirectly) mortality through vascular depression symptomatology – a second structural model was tested wherein the directional pathways from vascular depression symptom to frailty latent variables were reversed. Finally, to ensure that our results were not artifacts produced by the use of FIML or incorporation of auxiliary variables, a mediation model, conceptually analogous to the structural model described above, was used to test the hypothesis that frailty mediates the relationship between vascular depression symptoms and mortality. This model used only CES-D at baseline, frailty at the 2000 wave, and incident mortality between 2002 and 2008. Data used for this mediation model included no missing values.
Results
The final sample included 1,361 respondents with a mean age of 84.12 years (SD=4.10) and 10.85 years of education (SD=3.52; Table 1). The sample was predominantly White. Attrition was primarily a function of mortality: Of the original 1998 sample, 13.2% had died by 2000, 30.7% by 2002, 44.6% by 2004, 57.8% by 2006, and 69.7% by 2008. Frailty rates were higher among respondents with higher vascular depression symptom scores at baseline (Table 2). In multiple regression, frailty score at 2000 was significantly predicted by cardiac disease (B=.29, SE=.09, p<.001) and diabetes (B =.27, SE=.13, p=.04) as reported in 1998. Hypertension (B= .14, SE=.08, p=.07) and smoking history (B=-.08, SE=.08,p=.36) did not significantly predict frailty symptoms. A logistic regression revealed that participants who attrited over the course of the study tended to be older (β=.01, Wald X2=33.39, p<.001, exp(B)=1.01), reported more IADL disability (β=.41, Wald X2=4.36, p<.04, exp(B)=1.51), have more baseline vascular depression symptoms (β=.25, Wald X2=11.80, p=.001, exp(B)=1.29) and reported more baseline frailty symptoms (β=.33, Wald X2=23.55, p<.001, exp(B)=1.39).
Table 1. Description of the sample at baseline (1998).
| Variable | Mean (SD) |
|---|---|
| Age | 84.12 (4.10) |
| Years of Education | 10.85 (3.52) |
| CVB | 1.31 (.92) |
| ADLs | .63 (1.16) |
| IADLs | .23 (.59) |
| Self-Rated Health Change | .23 (1.10) |
| % of Sample | |
|
| |
| Ethnic Distribution | |
| White | 79.7 |
| Black | 14.3 |
| Hispanic | 5.2 |
| Other | 0.8 |
| Medical Diagnoses | |
| Hypertension | 53.8 |
| Diabetes | 11.3 |
| Cardiac Disease | 30.9 |
| Arthritis | 64.8 |
| Pulmonary Disease | 7.2 |
| Cancer | 13.8 |
| Sample size at each wave | |
| 1998 | 1368 |
| 2000 | 1139 |
| 2002 | 899 |
| 2004 | 696 |
| 2006 | 517 |
| 2008 | 365 |
Table 2. Frequencies and mean (SD) frailty scores by Wave 4 vascular depressive symptom score.
| Wave 4 VD Score | Frequency | Description | Mean (SD) Frailty Score | ||
|---|---|---|---|---|---|
|
| |||||
| Wave 5 | Wave 7 | Wave 9 | |||
| 1 | 303 | Very Healthy | 1.58 (1.34) | 1.88 (1.27) | 2.13 (1.26) |
| 2 | 273 | Healthy | 1.18 (1.20) | 2.06 (1.15) | 2.48 (1.05) |
| 3 | 562 | High CVB or Probable Depression | 2.05 (1.28) | 2.31 (1.19) | 2.52 (1.07) |
| 4 | 121 | Vascular Depression, Mild | 2.52 (1.19) | 2.60 (1.16) | 1.77 (1.01) |
| 5 | 96 | Vascular Depression, Moderate-Severe | 2.48 (1.30) | 2.82 (1.14) | 3.08 (1.31) |
The discrete-time survival analysis model (Figure 2) also had good overall fit (RMSEA=.05; CFI=.97; X2=234.84, p<.001). Control variables were omitted from Figure 2 for reasons of visual clarity, but are reported in Table 4. In this model, high vascular depression symptom scores at intercept predicted both a flatter slope in vascular depression symptom scores over the course of the study, and higher frailty symptom intercept scores. Higher vascular depression symptom scores at baseline were significantly predicted by greater ADL disability (β=.47, p<.001) and greater IADL disability (β=.30, p<.005). The rate of change in vascular depression symptoms (vascular depression symptom slope latent variable) was not predicted by any of the control variables. Higher scores on the frailty symptom intercept term were significantly predicted by greater age (β=.23, p<.001) and greater ADL disability (β=.32, p<.001). The frailty symptom slope variable was also not predicted by any control variables, though there was a marginally significant effect for older respondents to experience greater increase in frailty scores over the course of the study (β=.10, p=.06). Higher scores on the mortality term, indicating greater mortality risk, were significantly predicted by greater age (β=.21, p<.001), more ADL disability (β=.10, p<.001), and greater IADL disability (β=.20, p<.001).
Figure 2.
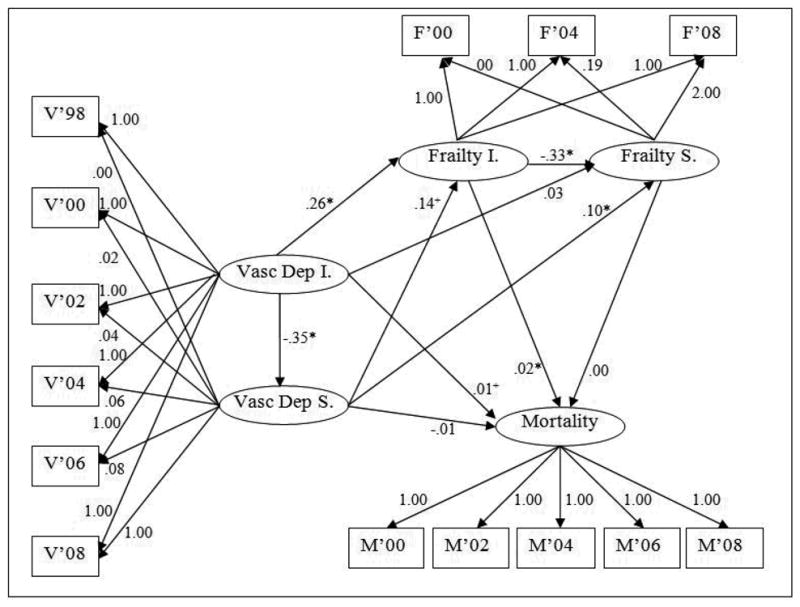
Structural model depicting slope and intercept terms for vascular depression symptoms and frailty symptoms, and a discrete-time survival term reflecting mortality risk predicted by all four factors. Higher baseline vascular depression symptomatology predicts higher baseline frailty symptomatology. Higher rate of vascular depression symptom development predicts higher rate of frailty symptom development. Vascular depression symptoms predict mortality indirectly through frailty. Path-coefficients are unstandardized. +p<.10; *p<.05. V=Vascular Depression Symptoms. Vasc Dep I=Vascular Depression Symptom Intercept. Vasc Dep S=Vascular Depression Symptom Slope. F=Frailty. Frailty I.=Frailty Intercept. Frailty S.=Frailty Slope. M=Mortality.
Table 4. Unstandardized regression coefficients for control variables (age, self-rated health change, ADLs, and IADLs) on vascular depression symptom intercept and slope, frailty intercept and slope, and mortality.
| Latent Var. | Control Var. | β | S.E |
|---|---|---|---|
| VD-Int. | |||
| Age | -0.04 | 0.05 | |
| Self-Rated Health Δ | -0.10 | 0.05 | |
| ADLs | 0.47*** | 0.07 | |
| IADLs | 0.30** | 0.11 | |
| VD-Slope | |||
| Age | 0.01 | 0.12 | |
| Self-Rated Health Δ | 0.16 | 0.12 | |
| ADLs | -0.02 | 0.14 | |
| IADLs | 0.46 | 0.37 | |
| Frailty-Int | |||
| Age | 0.23*** | 0.04 | |
| Self-Rated Health Δ | 0.02 | 0.04 | |
| ADLs | 0.32*** | 0.06 | |
| IADLs | -0.07 | 0.10 | |
| Frailty-Slope | |||
| Age | 0.10 | 0.05 | |
| Self-Rated Health Δ | -0.06 | 0.04 | |
| ADLs | -0.06 | 0.08 | |
| IADLs | 0.17 | 0.17 | |
| Mortality | |||
| Age | 0.21*** | 0.02 | |
| Self-Rated Health Δ | 0.00 | 0.02 | |
| ADLs | 0.10*** | 0.02 | |
| IADLs | 0.20*** | 0.04 | |
p<.05;
p<.01;
p<.001
VD =Vascular Depression Symptoms. ADLs=Activities of Daily Living. IADLs=Instrumental Activities of Daily Living.
As shown in Figure 2, higher vascular depression symptom scores at intercept predicted significantly slower rate of change in vascular depression symptom (β=-.35, p=.03), indicating that respondents with few vascular depression symptoms at baseline had a greater increase in these symptoms over the course of the study. Higher vascular depression symptom scores at intercept predicted higher frailty scores at intercept (β=.26, p<.001), indicating that respondents with more vascular depression symptoms at baseline (1998) had more frailty symptoms at the subsequent wave (2000). There was also a strong trend for respondents with higher vascular depression symptom slope scores to have more frailty symptoms at baseline (β=.14, p=.06). Similarly, higher vascular depression symptom slope predicted higher frailty symptom slope (β= .10, p=.02), indicating that respondents with worstening vascular depression symptoms also had increasing frailty symptoms over the course of the study. Respondents with higher baseline frailty scores had attenuated frailty symptom slopes over the course of the study (β=-.33, p<.001), indicating that those who were less frail at baseline became more so over the course of the study. The frailty symptom intercept score significantly predicted mortality (β=.02,p=.04), indicating that respondents with more frailty symptoms at baseline had higher mortality rates. The relationship between the vascular depression symptom score at baseline and mortality showed a trend toward significance (β=.01; p=.09). The indirect relationship between the vascular depression symptom intercept term and mortality through the frailty intercept term was statistically significant (β=.01, p=.04).
To test the directly competing hypothesis—that frailty symptoms lead to vascular depression symptoms and, indirectly, to mortality through vascular depression symptoms—a post-hoc model was completed in which the pathways from the vascular depression symptom intercept and slope variables to frailty intercept and slope variables were reversed. This post-hoc model produced similar fit indices as the hypothesized model (RMSEA=.05; CFI=.97; X2=234.83, p<.001). In this modified model, greater vascular depression symptom scores at baseline were significantly predicted by higher baseline frailty symptom scores (β=.69, p<.001), indicating that frailty predicts vascular depression symptoms cross-sectionally at baseline. Change in vascular depression symptoms over the course of the study was predicted by vascular depression symptom intercept (β=-.45, p=.007). Change in vascular depression symptoms, however, was not predicted by baseline frailty symptoms (β=.52, p=.28) or by change in frailty symptoms over time (β=.58, p=.73). As in the primary model above, the only primary variables with significant direct effects on mortality were the frailty intercept (β=.02, p=.04), age (β=.21, p<.001), ADL disability (β=.10, p<.001), and IADL disability (β=.20, p<.001). In sum, the results of these equations support a temporal relationship between baseline vascular depression symptomatology and later frailty, but do not support a temporal relationship between baseline frailty and later vascular depression symptomatology. In addition, the indirect pathway—from frailty through vascular depression symptomatology to mortality— is not statistically significant (β=.009, p=.06).
Finally, a mediation analysis was also completed (results not shown) testing the hypothesis that frailty symptomatology at wave 5 mediates the relationship between vascular depression symptoms at wave 4 and time to death. This analysis included 1,113 participants for whom complete data on these three variables were available and included no imputed data points. Results of this analysis showed partial mediation using the Sobel test (p<.001), and were consistent with the primary findings of this study, suggesting that these conclusions are not an artifact of the modeling strategy.
Conclusions
Our primary finding is the elucidation of a temporal ordering and pathway from vascular depression symptoms to frailty symptoms to death. In our sample of stroke-free women over the age of 80, vascular depression symptoms predicted mortality through frailty symptoms. When the ordering of the model was reversed, however, frailty symptoms did not predict longitudinal change in vascular depression symptoms. These results support the proposed theoretical framework by describing a late-life clinical trajectory characterized by high vascular depression and frailty symptoms. It is important to note past work with this same sample concluded that neither high CVB alone nor high depression symptoms alone were as good at predicting frailty and/or mortality (Paulson & Lichtenberg, in press). Support was found for the hypothesis that vascular depression symptoms are prodromal of frailty symptoms toward the end of life in older-old women. We also found higher rates of vascular depression symptoms among elders with higher levels of ADL and IADL disability. In our sample of women over age 80, frailty symptoms were more common with high ADL disability. Mortality risk was significantly predicted by age and more ADL and IADL disability. Controlling for age, ADL and IADL disability, and self-reported health change, we found a progression from vascular depression symptoms to frailty symptoms to earlier mortality.
In this analysis, respondents who were more frail at baseline were more likely to die over the following waves, while healthier respondents became increasingly frail with the passage of time. This study did not find that elders with increasing frailty slope scores had higher mortality rates, as might be expected from both the theoretical model and other work on frailty (Fried et al., 2001). Respondents with higher frailty slope scores tended to be in better health at baseline, as indicated by lower frailty intercept scores. Consequently, change in frailty over time was not predictive of mortality in this analysis. These findings are not surprising, however, in light of the advanced age of our sample and the relatively poor health at baseline and high rate of mortality across the study period.
These results support the vascular depression hypothesis (Alexopoulos et al., 1997), and clinically defined vascular depression in particular, and extend it by describing the emergence of vascular depression symptoms as an early stage in this chain of health decline. These findings also support work that relates frailty symptoms to mortality (Fried et al., 2001). The finding that frailty leads to mortality demonstrates the central importance of this syndrome among the oldest-old. In this sample, frailty predicts mortality at rates roughly similar to those found in other samples. Structural equation modeling, used in this study, is a rigorous statistical methodology with implications of causality, making this study one of the most stringent statistical tests of the frailty-mortality link in the literature.
These results also extend the frailty literature and contribute to the ongoing discussion of how we should define frailty. Emerging frailty research describes connections with various biomedical and disability-related covariates, including depression and CVB, often presenting a “chicken and egg” problem regarding the emergence of these symptoms throughout late life. Deficit accumulation models of frailty (Rockwood et al., 1999) address this problem by overlapping with or subsuming mental health or functional independence variables. By contrast, phenotypic frailty models such as that proposed by Fried et al (2001), and the conceptualization used in this paper, delineate a construct that is related to, but independent of, mental health and functional independence. Incorporating a clearly defined frailty syndrome promotes a better understanding of both this common “mechanism” of old age (Metchnikoff, 1903) and interventions that prevent (primary or secondary) or treat frailty. Specifically, past studies based on this same research found that CVB and depression independently predict mortality (Paulson, Bowen, & Lichtenberg, 2011), CVB is one cause of late-life depressive symptomatology (Paulson et al., under review), and that the combination of high CVB and depressive symptomatology is a common pathway to frailty (Paulson & Lichtenberg, in press). In combination with the current findings that vascular depression symptomatology predicts mortality indirectly through frailty, this series of papers integrates phenotypic frailty into broader theoretical framework describing a late-life clinical trajectory.
Of particular significance, identification of clinical trajectories such as the one described by this study, informs clinical conceptualizations of geriatric syndromes and guide intervention efforts. Our results suggest that interventions to reduce vascular burden throughout the lifespan may reduce depression and frailty in late-life, thereby preserving capacity for self-care and quality of life. A second course of investigation suggested by these results relates to slowing the transition to frailty and prolonging independence. Arean and colleagues' (2010) work suggests that among older adults with depression, skills-based interventions can improve capacity for self-care and reduce depression symptoms. However, whether skills-based interventions might slow the transition to frailty has not been explicitly studied.
One limitation of this study is that, while the relationship between vascular depression symptoms and mortality was small in magnitude, it may be underrepresented in this study. The most significant factor is that many respondents with high CVB were excluded from the study at baseline for having reported a stroke. Moreover, the HRS data includes community-dwelling older adults, so the most vulnerable elders may not be represented in this sample. Another significant factor is that this study includes mortality occurring between 2000 and 2008. The death of respondents who died after that time, who may have also had symptoms of vascular depression and frailty, was not reflected in this study. Nonetheless, the indirect relationship between vascular depression and mortality was significant, and deserves further study, perhaps with younger samples and over longer periods of time.
Other limitations of this study include the use of self-reported medical and depression symptom data. Future research using the HRS data could make use of biomarkers newly available in recent data releases. This might, for instance, involve blood pressure or glucose response as continuous variables that predict development of depression symptoms and frailty. At this time, the HRS data does not include diagnostic measurement of depression. Future research may build on these findings by incorporating clinical depression diagnosis, rather than depressive symptomatology as a continuous variable, as was done in this study. Additional limitations include absence of previous mental health history or information regarding current antidepressant treatment. This study also does not model the influence of current medical care, adherence to that care, or health behaviors such as diet and exercise. Additionally, this study does not include current cognitive functioning as a predictor or marker of decline.
Acknowledgments
We thank Professor Tenko Raykov for his assistance in developing the statistical methodology used in this paper.
FUNDING: This work was generously supported by the Blue Cross Blue Shield of Michigan Foundation (1680.SAP) and by the T32 grant-supported NIH Pre-Doctoral Training Program in Aging and Urban Health at the Institute of Gerontology (T-32 AG00275-06).
References
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. “Vascular depression” hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Hillsdale, NJ: Erlbaum; 1996. pp. 243–277. [Google Scholar]
- Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. American Journal of Psychiatry. 2010;167(11):1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman ATF, Deeg DJH, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression Scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychological Medicine. 1997;27(1):231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Marshall LM, Michael Y, Dam T, Ensrud KE, Barrett-Connor E, Orwoll ES. Frailty in older men: Prevalence, progression, and relationship with mortality. Journal of the American Geriatrics Society. 2007;55(8):1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- Clegg A. The frailty syndrome. Clinical Medicine. 2011;11(1):72–75. doi: 10.7861/clinmedicine.11-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: A comparison of normal and depressed elderly subjects. The American Journal of Psychiatry. 1990;147(2):187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Fainsinger RL, Carson M, Finegan BA. Cost trajectories at the end of life: The Canadian experience. Journal of Pain and Symptom Management. 2009;38(1):75–80. doi: 10.1016/j.jpainsymman.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Flicker L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clinics in Geriatric Medicine. 2010;26(1):17–27. doi: 10.1016/j.cger.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults: Evidence for a phenotype. Journal of Gerontology: Medical Sciences. 2001;56A(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Graham JW. Adding missing-data relevant variables to FIML-based structural equation models. Structural Equation Modeling. 2003;10:80–100. doi: 10.1207/S15328007SEM1001_4. [DOI] [Google Scholar]
- Heeringa SG, Conner J. Technical description of the Health and Retirement Study sample design: HRS/AHEAD documentation report DR-002. Ann Arbor: University of Michigan; 1995. [Google Scholar]
- Katz IR. Depression and frailty: The need for multidisciplinary research. American Journal of Geriatric Psychiatry. 2004;12(1):1–6. [PubMed] [Google Scholar]
- Lakey SL, LaCroix AZ, Gray SL, Borson S, Williams CD, Calhoun D, Woods NF. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. Journal of the American Geriatrics Society. 2012;60(5):854–861. doi: 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, Mack WJ. Association of depressed mood and mortality in older adults with and without cognitive impairment in a prospective naturalistic study. American Journal of Psychiatry. 2010;167:589–597. doi: 10.1176/appi.ajp.2009.09020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness JM, Caine ED, King DA, Conwell Y, Cox C, Duberstein PR. Cerebrovascular risk factors and depression in older primary care patients: Testing a vascular brain disease model of depression. American Journal of Geriatric Psychiatry. 1999;7(3):252–258. doi: 10.1097/00019442-199908000-00010. [DOI] [PubMed] [Google Scholar]
- Mast BT. Vascular depression: Cardiovascular implications for mental health. Annual Review of Gerontology and Geriatrics. 2010;30(1):135–154. [Google Scholar]
- Mast BT, Neufeld S, MacNeill SE, Lichtenberg PA. Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. American Journal of Geriatric Psychiatry. 2004;12(1):93–101. [PubMed] [Google Scholar]
- Metchnikoff E. The nature of man: Studies in optimistic philosophy. New York and London: G. P. Putnam's Sons; 1903. [Google Scholar]
- Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: A systematic review. International Journal of Geriatric Psychiatry. doi: 10.1002/gps.2807. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Lohman M, Dumenci L, Lapane K. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. American Journal of Geriatric Psychiatry. doi: 10.1016/j.jagp.2012.12.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Muir SW, Hall M, Doherty TJ, Kloseck M, Beauchet O, Speechley M. Gait variability is associated with frailty in community-dwelling older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66A(5):568–576. doi: 10.1093/gerona/glr007. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus (Version 5.0) Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Paulson D, Bowen ME, Lichtenberg PA. Cardiovascular risk factors, cognitive functioning and depression symptoms in women over 80. Journal of Aging Research, 2011. 2011:7. doi: 10.4061/2011/912680. 2011. [DOI] [Google Scholar]
- Paulson D, Bowen ME, Lichtenberg PA. Does Brain Reserve Protect Older Women from Vascular Depression? doi: 10.1093/geronb/gbt007. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA. Late-Life Depression is a Prodrome for Frailty. Paper presented at the Gerontological Society of America; Boston, MA: 2011. [Google Scholar]
- Paulson D, Lichtenberg PA. The Paulson-Lichtenberg Frailty Index: Construct validity for a self-report measure of frailty. Wayne State University; Detroit, MI: 2012. [Google Scholar]
- Paulson D, Lichtenberg PA. Vascular depression: An early warning sign of frailty. doi: 10.1080/13607863.2012.692767. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. Frailty: Help or hindrance? Journal of the Royal Society of Medicine. 1997;90(32):23–26. doi: 10.1177/014107689709032s07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiology of Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lunn M, Wong D. Death, Dropout, and Longitudinal Measurements of Cognitive Change in Old Age. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2008;63B(5):P271–P278. doi: 10.1093/geronb/63.5.P271. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rainer MK, Mucke H, Zehetmayer S, Krampla W, Kuselbauer T, Weissgram S, Fischer P. Data from the VITA study do not support the concept of vascular depression. American Journal of Geriatric Psychiatry. 2006;14(6):531–537. doi: 10.1097/01.JGP.0000218326.91287.66. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. Second. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Raykov T. Longitudinal analysis with regressions among random effects: A latent variable modeling approach. Structural Equation Modeling. 2007;14(1):146–169. [Google Scholar]
- Raykov T, Marcoulides GA. An introduction to applied multivariate analysis. New York: Routledge; 2008. [Google Scholar]
- Rockwood K, Stadnyk K, MacKnight C, McDowell I, Herbert R, Hoban DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9162):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. It's about time: Using discrete-time survival analysis to study duration and the timing of events. Journal of Educational Statistics. 1993;18(2):155–195. [Google Scholar]
- Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: Evidence of internal validity. Biological Psychiatry. 2008;64(6):491–497. doi: 10.1016/j.biopsych.2008.03.032. S0006-3223(08)00403-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffick DE. Documentation of affective functioning measures in the health and retirement study HRS Documentation Report DR-005. Ann Arbor: Survey Research Center at the Institute for Social Research; 2000. [Google Scholar]
- Yochim B, Mast BT, Lichtenberg PA. Cerebrovascular risk factors and depressed mood in inner city older adults. Clinical Psychologist. 2003;7(1):11–20. [Google Scholar]