Abstract
Currently, no reliable biomarkers are available to predict transformation from smoldering myeloma (SMM) to multiple myeloma (MM). Using an ultrasensitive enzyme-linked immunosorbent assay (ELISA) we assessed the levels of a broad range of cytokines and chemokines in the peripheral blood (PB) and bone marrow (BM) supernatant collected from 14 SMM and 38 MM patients and compared to healthy donors. We found significantly increased levels of key cytokines, in particular CXCL8 (IL-8), associated with progressive disease state (controls→SMM→MM). Cytokine profiles were found similar in PB and BM. Five of fourteen SMM patients (36%) progressed to MM. Our findings, although based on a limited number of patients, suggest that serum-based cytokines may have a future role as biomarkers for disease progression and could potentially be assessed as novel targets for treatment.
INTRODUCTION
Currently, two clinical risk models (Mayo Clinic and Spanish PETHEMA model) are available to predict progression from smoldering myeloma (SMM) to multiple myeloma (MM) (1, 2). Both these models are based on data derived from retrospective singlecenter studies. Recently, a prospective head-to-head comparison of the two models showed a high degree of discordance when defining individual patients risk using the two models in parallel (3).
In order to expand our knowledge on biological markers of progression, we assessed a broad range of cytokines and chemokines proposed to play a role in myelomagenesis (4), including IL-1β, IL-2, IL-4, IL-5, IL-6, CXCL8, IL-10, IL-12 p70, IL-13, IFNγ, and TNFα. We assayed these markers in peripheral blood and bone marrow supernatant from SMM and MM patients and compared our results to samples obtained from healthy donor controls.
PATIENTS AND METHODS
Bone marrow and peripheral blood samples (serum) were obtained with informed patient consent. Peripheral blood samples were obtained from 7 healthy donors, 14 SMM patients and 38 MM patients. BM supernatant samples were obtained from 17 MM patients and from 9 healthy donors. The samples were assayed in two ELISA multi-array experiments using an ultra-sensitive Human TH1/TH2 10-plex multi-spot plate and an ultra-sensitive multi-array plate for IL-6 detection (Meso Scale Discovery®) A set of known concentration calibrators was added to the 96-well plate to generate a standard curve for each cytokine. The derived standard curves were used to calculate cytokine concentrations in each of the clinical samples. All samples were added to the plates in duplicate. The assays were repeated twice (Figure 1). A two-tailed Mann-Whitney test was performed for statistical analysis using Prism software. Gene expression profile (GEP) data were collected from the Gene Expression Omnibus accession number GSE6477. All microarray data derived from Affymetrix U133A chip.
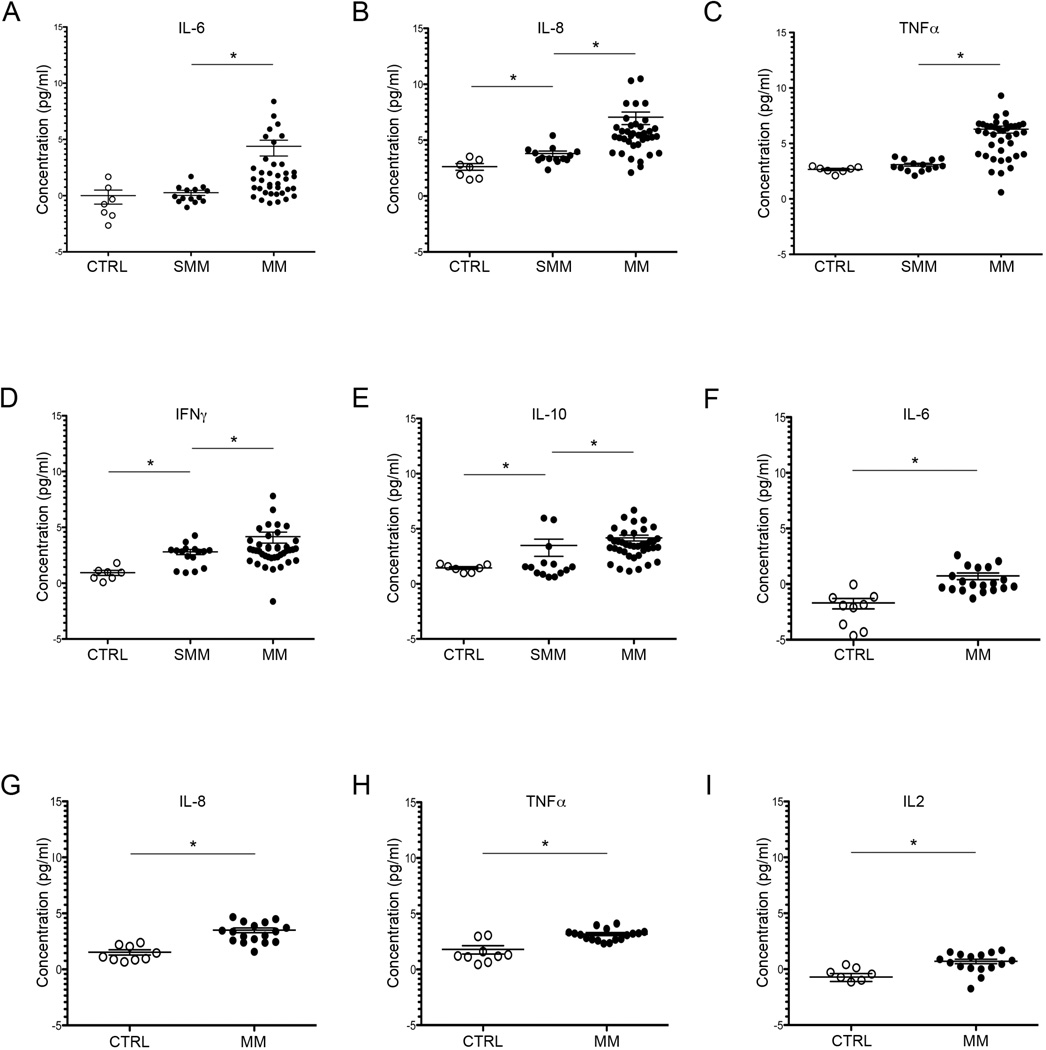
Figure 1. Comparison of cytokine and chemokine levels in peripheral blood (PB) and bone marrow (BM) supernatant of SMM and MM patients. PB (serum).
(A) Values are expressed as log2. Level of IL-6 significantly elevated in MM patients (21 ± 9.5 pg/mL) compared to SMM (1.2 ± 0.7 pg/mL) compared to controls (1.0 ±0.4 pg/mL). *p= 0.006 and *p= 0.001 respectively. (B) CXCL8 (or IL-8) concentration elevated in SMM patients (13.9±2.4 pg/mL) and MM patients (133.2 ± 48 pg/mL) versus controls (6.2 ± 1.2 pg/mL. *p=0.008 and *p< 0.0001 respectively. (C) TNF-alpha level increased in SMM patients (8.5±2.9pg/mL) and MM (78±16.9 pg/mL) compared to controls (6.3±1.1 pg/mL). MM vs SMM:*p=0.0005). (D) Levels of IFN-gamma in SMM (7.0±1.1 pg/mL) and in MM (17.9±5.9 pg/mL) compared to controls (1.9±0.3pg/ml) *p=0.002 and 0.001 respectively. (E) Level of IL-10 significantly increased in MM patients (18.08 pg/mL±3.2) versus SMM (11.16±5.4) and controls (2.7±0.6 pg/mL). *p= 0.001 and 0.0003 respectively. BM supernatant. (F) Level of IL-6 increased in MM patients (1.66 ±0.3 pg/mL) compared to controls (0.3±0.09pg/ml) * p= 0.0003. (G) Level of CXCL8 increased in MM patients (11.3±1.6 pg/mL) compared to controls (2.8±0.4 pg/mL)*p=0.0001. (H) TNF-alpha elevated in MM patients (9.03±0.8 pg/mL) compared to controls (3.49±0.8 pg/mL) *p=0.0008. (I) Level of IL-2 elevated in MM patients (1.6±0.2 pg/mL) versus controls (0.6±0.1 pg/mL). *p=0.007.
All samples were assayed in two ELISA multi-array experiments using an ultra-sensitive Human TH1/TH2 10-plex multi-spot plate for quantifying the levels of IL-1β, IL-2, IL-4, IL-5, CXCL-8, IL-10, IL-12 p70, IL-13, IFN-gamma, TNF-alpha; and an ultra-sensitive multi-array plate for IL-6 detection (Meso Scale Discovery®).
RESULTS AND DISCUSSION
Using a multi-array assay, we quantified the level of a broad range of cytokines and chemokines both in PB blood and bone marrow supernatants of SMM, MM patients and healthy donors. In PB obtained from SMM patients, we found significantly increased levels of CXCL8 (IL-8) (p=0.008) and IFNγ (p=0.002) compared to healthy controls (Figure 1). The same cytokines were found to be further increased in PB from MM patients compared to SMM and controls: CXCL8 (p=0.0009 and p=<0.0001); IFNγ (p=0.001 MM versus controls.) (Figure1). Furthermore, additional cytokines were elevated in PB of MM patients compared to SMM and controls including: IL-6 (p=0.0009, p=0.003) and IL-10 (p=0.001, p=0.0003); TNF-alpha was elevated in MM versus controls (p=00005). In addition, in the PB of three of SMM patients we also found elevated concentrations of IL-10 compared to controls (Figure 1).
As a second step, we assessed patterns of cytokines assayed in BM supernatants derived from 38 MM tumors and found a profile similar to that defined in PB. Specifically, levels of IL-6, CXCL8 and TNFα were significantly greatly increased in MM patients compared to controls (p= 0.0003, p=0.0001, p=0.0008,). Levels of IL-2 were also increased in BM of MM patients (p=0.007) (Figure 1). Our findings are consistent with a previous study showing high levels of chemokine IL-8 in SMM and MM patients in an in vitro model of human stromal cells cultured in the presence of BM supernatant derived from MGUS, SMM and MM patients (5).
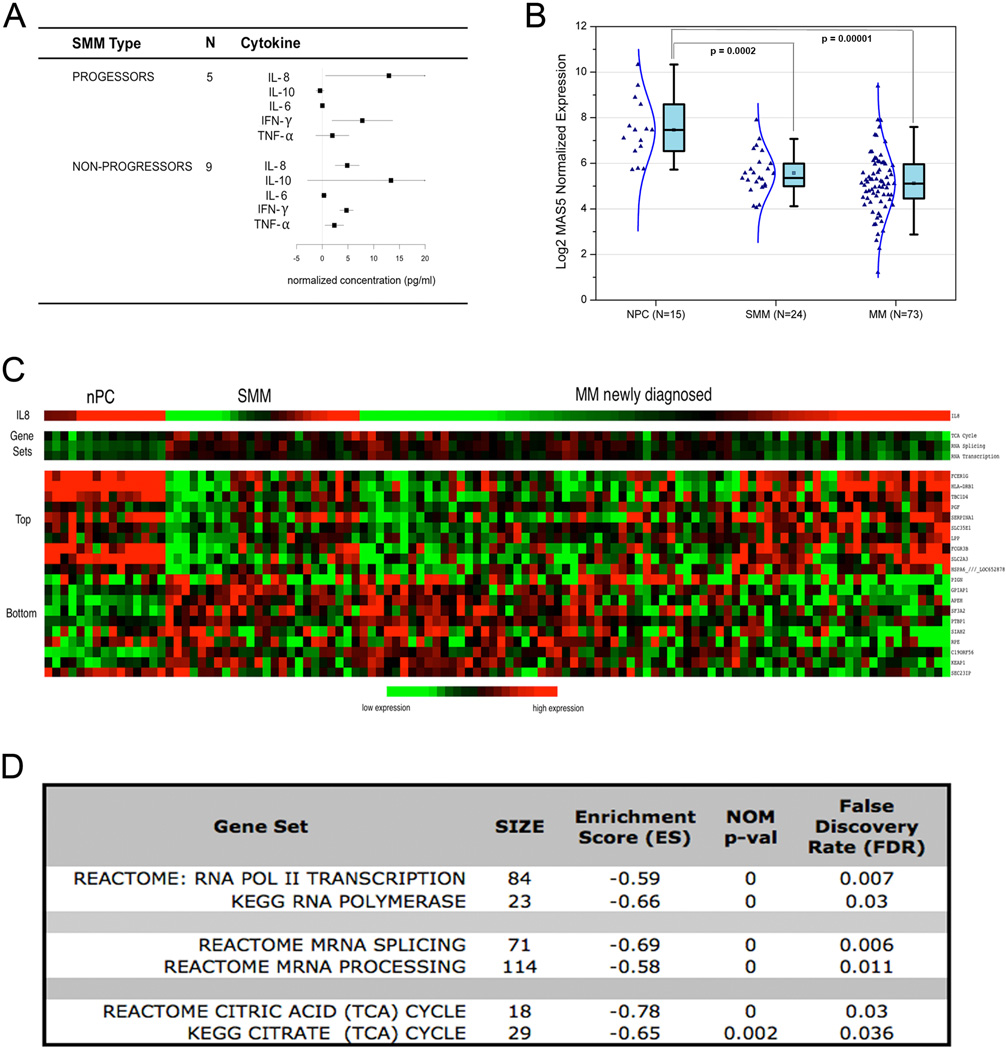
Of 14 SMM patients analyzed, five (36%) progressed to MM in a two-year follow-up (Figure 2A). Although the statistical difference in the level of CXCL8 between the group of SMM patients with progressive disease (PD) and the rest of SMM patients with stable disease (SD) was limited due to sample size, the mean concentration of CXCL8 in PD patients was elevated three-fold (12.9±6.1 pg/mL) compared to SD patients (4.8±1.3 pg/mL) (Figure 2 A). CXCL8 (or IL-8) is a pro-inflammatory chemokine acting as an autocrine growth factor within the BM microenvironment (6). Chemokines sustain cancer proliferation and survival through an interaction with receptors expressed by the cancer cells. CXCL8 binding receptors CXCR1 and CXCR2 have been characterized in cancer cells, endothelial cells, tumor-associated macrophages and infiltrating neutrophils (7). IL-8 signaling has been reported to activate multiple pathways including Phosphotidylinositol-3-kinase (PI-3K), Phospholipase C, Protein Kinase C (PKC) MEK-ERK, p38MAPK, Akt, NF-kB and Rho-GTPase proteins, key regulators of the actin cytoskeleton (8, 9). CXCL8 signaling also induces angiogenesis through phosphorylation of the receptor for vascular endothelial growth factor (VEGFR-2) (10).
Figure 2.
(A) Change of cytokine and chemokines levels in SMM progressed to MM versus SMM with stable disease (SD). CXCL8 is the most elevated chemokine within the five SMM patients (36%) that progressed to MM. (B) Transformation from normal PC to MM is associated with a decreased CXCL8 transcript level. Gene expression profile data including 15 normal PC, 25 SMM and 73 MM patients show that CXCL8 expression is significantly decreased in SMM and MM patients compared to PC controls (p=0.0002 and 0.00001 respectively). (C) Heatmap displaying a CXCL8 signature. A CXCL-8 signature was defined including the top ten genes most positively and negatively correlated with CXCL8 expression in using the above subset of samples. SMM patients show a profile more similar to MM patients than the normal PC. (D) List of gene sets that correlate with CXCL8 expression. By GSEA analysis we defined three main sets of genes negatively correlated with CXCL8 expression, including genes regulating cellular metabolism (Tricyclic Acid Cycle), RNA splicing and RNA trancsription.
It has been shown that while chemotherapeutic agents kill cancer cells, they also trigger a parallel microenvironment reaction leading to the production of chemokines and growth factors that provides proliferative and anti-apoptotic signals to tumor cells (11). A recent in vitro study demonstrated that high levels of CXCL8 directly correlate with resistance to bortezomib, high levels of both CXCL8 and VEGF correlate with resistance to melphalan, whereas IL-6 concentration correlate to resistance to both agents (12). In this system, neutralizing antibodies against these cytokines restored sensitivity to drugs (12).
Given the significant difference of IL-8 chemokine detected at the protein level during disease progression, we decided to further investigate the role of CXCL8 expression in normal PC versus SMM and MM. Using GEP data we queried 168 patients including 73 newly diagnosed untreated MM, 24 SMM and 15 normal PC from the Gene Expression Omnibus (GSE6477) and we found that CXCL8 expression is significantly decreased in SMM and MM patients compared to PC controls (p=0.0002 and 0.00001 respectively) (Figure 2 B). We then defined a CXCL8 signature comprising the top ten genes most positively and negatively correlated with CXCL8 expression within the MM patient set, and found SMM and MM patients sharing a similar CXCL8 signature while normal PC demonstrated a different profile (Figure 2C). We then applied gene set enrichment analysis (GSEA) and found Tricyclic Acid (TCA) Cycle, regulation of RNA transcription and RNA splicing gene sets to be significantly enriched in genes negatively correlated with CXCL8 expression (Figure 2 D). Our data suggests that CXCL8 expression in CD138+ cells decreases with progression from normal PC→SMM→MM (Figure 2 B) and thus the increased CXCL8 protein level detected in the PB and BM supernatant is due mostly to stromal cell production. As CXCL8 expression decreased with disease progression there is an apparent increase of the TCA cycle, RNA transcription and RNA splicing levels (Figure 2 C, D). This may be expected in increasingly malignant cells with greater metabolic demand and production of paraprotein.
In addition, 20% of SMM patients studied showed also increased levels of interleukin IL-10 similar to that detected in the PB of MM patients (Figure 1). The mean concentration of IL-10 in the SMM patients versus controls was 11.16±5.4 pg/ml and 2.74±5.5 pg/ml, respectively (data not shown). One SMM patient with the highest level of CXCL8 was also found with the highest level of IL-10 (data not shown).
IL-10 affects myeloma cell growth and survival by stimulating oncostatin (OSM) and correlated with increased expression of c-MAF.(13) Over-expression of c-MAF has also been involved in the development of drug-resistance (14). Recent studies demonstrate that targeting NF-kB signaling can overcome the growth and survival advantage conferred both by tumor cell binding to BMSCs and cytokine secretion in the BM microenvironment (15). Given the implication of these pathways in myelomagenesis (13, 15), the identification of factors produced by the BM microenvironment might provide candidate targets for new therapeutic approaches.
Our study supports a role for chemokine signaling as a marker for tumor progression and a potential candidate for intervention.
Highlights.
SMM and MM patients exhibit altered cytokine and chemokine profiles
Progressive disease is associated with increased levels of CXCL8.
CXCL8 is a potential predictor of tumor progression
Footnotes
AUTHORSHIP
A.Z. and O.L. wrote the paper. A.Z., W.W., M.C., and R.P. performed research and analyzed data. S.P. analyzed gene expression data. A.Z., K.R.C. and O.L. designed research and analyzed data. K.R.C., Y.Z., A.M.R. and I.M.G., contributed vital reagents. N.K., M.K., E.M., N.T., M.M. D.Z., M.Y., and M.R. analyzed data. All the authors reviewed the paper and made editorial suggestions.
DISCLOSURES
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010 Jun;24(6):1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zingone A, Kuehl WM. Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. Semin Hematol. 2011 Jan;48(1):4–12. doi: 10.1053/j.seminhematol.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry BM, Korde N, Kwok M, Manasanch E, Bhutani M, Mulquin M, et al. Modeling risk of progressuion to multiple myeloma: results from a prospective clinical study. Leuk Lymphoma. 2012;2013 doi: 10.3109/10428194.2013.764419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline M, Donovan K, Wellik L, Lust C, Jin W, Moon-Tasson L, et al. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leuk Res. 2007 May;31(5):591–598. doi: 10.1016/j.leukres.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008 Nov 1;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 7.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 8.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol. 2003 Dec 15;171(12):6714–6722. doi: 10.4049/jimmunol.171.12.6714. [DOI] [PubMed] [Google Scholar]
- 10.Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007 Dec;18(12):5014–5023. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3(8):e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maharaj L, Popat R, Gribben JG, Joel S. IL-6, IL-8 and VEGF Neutralisation Restores Drug Sensitivity to Conventional and Novel Treatment Combinations in a Multiple Myeloma Bone Marrow Micro-Environment Model. Blood. 2012;120(21) Abstract 2949. [Google Scholar]
- 13.Otsuki T, Yata K, Sakaguchi H, Uno M, Fujii T, Wada H, et al. IL-10 in myeloma cells. Leuk Lymphoma. 2002 May;43(5):969–974. doi: 10.1080/10428190290021579. [DOI] [PubMed] [Google Scholar]
- 14.Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004 Feb;5(2):191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002 May 10;277(19):16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]