Abstract
We completed a phase II clinical trial evaluating rapamycin-resistant allogeneic T cells (T-Rapa) and now are evaluating a T-Rapa product manufactured in 6-days (T-Rapa6) rather than 12-days (T-Rapa12). Using gene expression microarrays, we addressed our hypothesis that the two products would express a similar phenotype. The products had similar phenotypes using conventional comparison methods of cytokine secretion and surface markers. Unsupervised analysis of 34,340 genes revealed that T-Rapa6 and T-Rapa12 products clustered together, distinct from culture input CD4+ T cells (CD4). Statistical analysis of T-Rapa6 products revealed differential expression of 19.3% of genes (n=6641) compared to input CD4 cells; similarly, 17.8% of genes (n=6147) were differentially expressed between T-Rapa12 products and input CD4 cells. Compared to input CD4 cells, T-Rapa6 and T-Rapa12 products were similar in terms of major gene families up-regulated (cell cycle, stress response, glucose catabolism, DNA metabolism) and down-regulated (inflammatory response, immune response, apoptosis, transcriptional regulation). However, when directly compared, T-Rapa6 and T-Rapa12 products showed differential expression of 5.8% of genes (n=1994; T-Rapa6 vs. T-Rapa12). Second-generation T-Rapa6 cells therefore possess a similar yet distinct gene expression profile relative to first-generation T-Rapa12 cells, and thus may mediate differential effects after adoptive transfer.
Keywords: Cell Therapy, Graft vs Host Disease, Sirolimus, T-Lymphocytes
Introduction
Although gene expression microarrays represents a feasible and unbiased method for the characterization of global cellular function[1], initial studies are only now underway to use this technology for the characterization of ex vivo manipulated clinical cellular products[2]. We reasoned that, in the setting of cellular therapy, expression profiling may have value in several respects, including: (1) as a quality control measure, with results providing a ‘genetic fingerprint’ that can be utilized to help ensure cell product efficacy; (2) the identification of putative functional pathways, thereby contributing to an increased understanding of cellular mechanisms of action; and (3) facilitation of an assessment of whether modifications of the ex vivo manufacturing process alter the global cellular phenotype.
In murine models, we have found that ex vivo manufacture of allogeneic CD4+ T cells in rapamycin, which inhibits mTOR and alters cellular function through a variety of mechanisms[3], generates a robust T cell population that can be adoptively transferred to beneficially modulate the balance of immune reactions that occur after hematopoietic cell transplantation. Such rapamycin-resistant T-helper (Th) cells could be manufactured ex vivo in either a Th1 or Th2 cytokine phenotype depending on whether antigen-presenting-cell (APC) free co-stimulation was performed in the presence of IL-12 or IL-4 polarizing cytokines, respectively[4]. Importantly, rapamycin-resistant T cells of both Th1 and Th2 phenotype had increased in vivo function after adoptive transfer into allogeneic hosts[4]. These results stood in contrast to previous results that found rapamycin to tolerize T cells[5] or induce an immune suppressive regulatory T (TREG) phenotype[6] but are consistent with emerging data that indicate an immune augmentation effect of rapamycin on CD8+ T cell function[7].
Infusion of rapamycin-resistant donor Th2 cells represented a novel approach to balancing Th1/Th2 immunity after experimental murine allogeneic bone marrow transplantation for the mediation of graft-versus-tumor (GVT) effects with reduced graft-versus-host disease (GVHD)[8] and for the prevention of fully MHC-disparate graft rejection[9, 10]. The enhanced in vivo efficacy of rapamycin-resistant murine and human T cells in allogeneic[9] and xenogeneic[11] transplantation models was due in part to a multi-faceted anti-apoptotic phenotype that dictated increased in vivo T cell persistence after adoptive transfer. Finally, consistent with the known role of rapamycin-induced mTOR blockade in the promotion of autophagy[12], we have identified that the anti-apoptotic phenotype of rapamycin-resistant T cells emanated from a mitochondrial autophagy process[11, 13].
In a clinical translation of this research, we developed a method for the ex vivo manufacture of cytokine polarized allogeneic human CD4+ T cells in rapamycin and evaluated such cells on a phase II clinical trial (NCT #00074490). This method consisted of APC-free co-stimulation of purified CD4+ T cells in rapamycin, IL-4, and IL-2 over a 12-day culture interval; the resultant T-Rapa cell product was comprised of minimally differentiated effector T cells that secreted a balanced pattern of Th1 and Th2 cytokines[14]. In the setting of HLA-matched sibling allogeneic hematopoietic cell transplantation using low-intensity chemotherapy conditioning, we found that the delayed infusion of allogeneic T-Rapa cells at day 14 post-transplant induced a balanced pattern of immune reconstitution involving both Th1 and Th2 cytokines, promoted alloengraftment as indicated by conversion of mixed chimerism towards full donor elements, associated with a low rate of classical acute GVHD (4 cases out of 40 patients), and resulted in sustained complete remissions in patients with chemotherapy refractory hematologic malignancy[15]. The main cause of post-transplant mortality was malignant disease progression, and as such, we are now evaluating whether alternative methods of T-Rapa cell manufacturing might augment GVT effects.
In experimental models of syngeneic anti-tumor T cell therapy, the state of T cell differentiation is an important determinant of T cell efficacy after adoptive transfer, with less differentiated T cells mediating more potent in vivo effects[16]. In our studies performed in the allogeneic transplantation setting involving rapamycin-resistant murine Th2 cells, which were manufactured by a truncated 6-day culture method, we found that: (1) such cells were minimally differentiated at the time of T cell transfer, as indicated by very low cytokine secretion potential and expression of cell surface molecules such as CD62L and CCR7[4]; and (2) in spite of this minimally differentiated state, adoptive transfer of such cells induced approximately one-log higher in vivo cytokine levels relative to control T cells not manufactured in rapamycin[8]. In sum, these data indicate that minimally differentiated T cells represent a suitable phenotype for adoptive T cell transfer.
Given this background and our desire to generate a more robust allogeneic T-Rapa cell product for more potent mediation of GVT effects, we truncated our manufacturing process from 12-days (T-Rapa12 cells) to 6-days (T-Rapa6 cells). Our current translational efforts are evaluating this second-generation T-Rapa6 cell therapy on a phase II clinical trial using the exact same transplant conditions utilized in our evaluation of the first-generation T-Rapa12 cells. In this project, we have utilized gene expression profiling to compare and contrast the global phenotype of the T-Rapa12 and T-Rapa6 clinical products.
Material and Methods
T Cell Isolation and Culture
Experiments were performed according to a Standard Operating Procedures (SOP) established to support the phase II clinical trial evaluating T-Rapa12 cell therapy at the NIH Clinical Center (NCI Protocol 04-C-0055). Peripheral blood mononuclear cells (PBMCs) from 6 healthy donors were collected by apheresis (Amicus Separator, Fenwal; Lake Zurich, IL, USA) on an IRB-approved protocol in the Department of Transfusion Medicine, National Institutes of Health (Bethesda, Maryland, USA). CD4+ T cells were isolated by positive selection to > 99% purity using a CliniMACS system (Miltenyi Biotec; Bergish Gladbach, Germany). CD4 cells were cryopreserved using a controlled rate freezer (Kryosave, Integra, Planer plc; Sunbury-on-Thames, UK) and media containing 5% DMSO (Edwards Lifesciences; Inc, Ca), 6% pentastarch (Pharmaceutical Development Section, Pharmacy Dept, Clinical Center, NIH), and 4% human serum albumin (Baxter Health Care Corporation; Ca, USA). Thawed CD4+ T cells were cultured in PL732 bags in X-VIVO 20 media containing 5% heat inactivated human AB plasma, recombinant human IL-4 (1,000 I.U./mL; Schering; Whitehouse Station, NJ, USA), recombinant human IL-2 (20 I.U./mL; Chiron Corporation; Emeryville, CA, USA), and rapamycin (Rapamune, 1 uM; Wyeth Ayerst; West Greenwich, RI, USA). T cells were co-stimulated once at culture initiation with tosylactivated paramagnetic M-450 beads (Dynal Invitrogen; Oslo, Norway) coated with anti-CD3 (OKT3; Ortho Biotech) and anti-CD28 (clone 9.3; kindly provided by Dr. Bruce Levine of the University of Pennsylvania Cancer Center) at a bead to cell ratio of 3:1. The number of CD4+ T cells loaded into each culture bag ranged from 62 to 154 × 106 cells per culture; each culture was initiated at a T cell concentration of 1.5 × 106 cells/ml. On culture days 2 and 4, 10× cytokines (in 10% total culture volumes) were added to each bag and as cultures were monitored for cell growth. In addition, 1× cytokine-containing media (in 10–25% volume amounts) was also added to each bag to ensure that cell concentrations did not exceed 0.5 to 1 × 106 cells/ml. The cells were cultured at 37° C in incubators with 5% CO2 for either 6 or 12 days to generate the T-Rapa6 and T-Rapa12 populations, respectively.
Assessment of T Cell Cytokine Secretion Profile
T cell culture supernatants of input CD4 cells, T-Rapa6 cells, and T-Rapa12 cells were generated by adjusting the T cells to a concentration of 1 × 106 cells/ml and co-stimulating at a 3:1 bead to cell ratio for 24 hours; then, the cell-free supernatants were harvested and tested for cytokine content by Luminex multiplex assays following the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA, USA). To further assess cytokine secretion potential, T-Rapa6 and T-Rapa12 populations were co-stimulated and cultured for an additional 6 days in X-VIVO 20 media supplemented with heat inactive AB plasma and IL-2 (20 IU/ml) with or without polarizing cytokines (IL-4 or IFN-γ; then, the resultant expanded T cells were co-stimulated again and the 24 hour supernatants were tested for cytokine content by Luminex.
Total RNA Isolation
Total RNA was extracted from the input CD4+ T cells and the T-Rapa6 and T-Rapa12 populations using the mRNeasy mini kit (Qiagen; Valencia, CA, USA). Quality of the isolated RNA was evaluated by the Agilent Bioanalyzer 2100 using an RNA 6000 Nano-LabChip (Agilent Technologies; Santa Clara, CA, USA).
Microarray Expression Analysis
Microarray expression experiments were performed on 4×44 K Whole Human Genome Microarray (Agilent Technologies), according to the manufacturer's instructions. Universal Human Reference RNA (Stratagene, Santa Clara, CA, USA) was used as a common reference. Test samples (6 samples for each cell product) and reference RNA were amplified and labeled using Agilent kit according to the manufacturer’s instructions. Images of the arrays were acquired using a microarray scanner G2505B (Agilent Technologies) and image analysis was performed using feature extraction software version 9.5 (Agilent Technologies). Resulting data files were uploaded to the mAdb database (http://nciarray.nci.nih.gov/) and further analyzed using BRBArrayTools developed by the Biometric Research Branch, National Cancer Institute (http://linus.nci.nih.gov/BRB-ArrayTools.html).
Statistical and Microarray Data Analysis
The raw data set was filtered according to a standard procedure to exclude spots below a minimum intensity of 10 in both fluorescence channels and not represented in more than 80% of samples. Class comparison was conducted with BRB Array Tools using a random variance model. Significant genes were defined as p-value < 0.001 and an FDR < 0.10. Partek Genomic Suite 6.4 (Partek Inc., St. Louis, MO, USA) was used for data visualization, hierarchical cluster analysis, and Principal Component Analysis (PCA)[17]. Enrichment of biological pathways for differentially expressed genes and Gene Ontology designations were determined using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics resource[18]; specific gene annotations were retrieved on Gene Cards (http://www.genecards.org/index.shtml). The microarray data used in this study were deposited on the NCBI GEO database (GSE34911).
Results
T-Rapa6 vs. T-Rapa12 Cells: Differential Yield But Similar Cytokine Phenotype
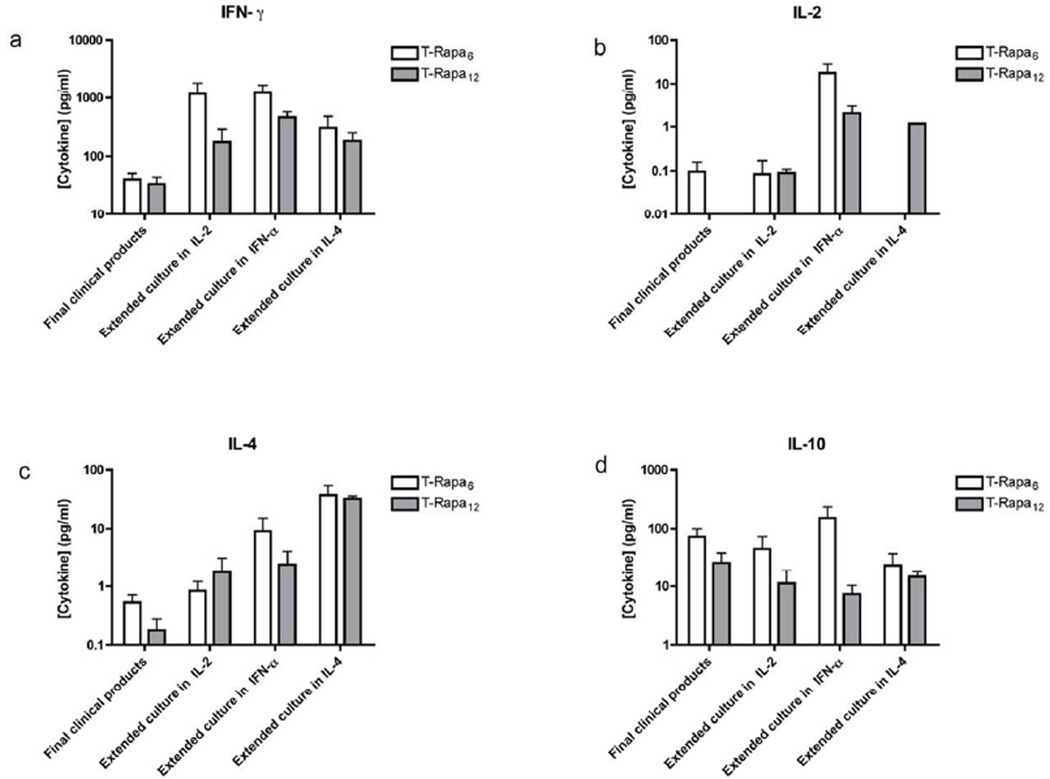
Relative to the absolute number of input CD4+ T cells at culture initiation, there was reduced T cell expansion in the T-Rapa6 culture condition relative to the T-Rapa12 culture condition (mean fold-expansion ± SEM, n=6: 2.2 ± 0.3 vs. 17.8 ± 3.0, p < 0.05). However, there were no consistent differences in T-Rapa6 and T-Rapa12 capacity to secrete IFN-γ, IL-2, IL-4, and IL-10; of the 16 comparisons in totality, we only found statistically significant differences in two comparisons (Figures 1A through 1D). At the end of T-Rapa6 and T-Rapa12 cell manufacturing, T cell capacity for cytokine secretion was minimal, thereby consistent with a minimally differentiated state; specifically, median cytokine secretion values were: IFN-γ < 100 pg/ml, IL-2 < 1 pg/ml, IL-4 < 1 pg/ml, and IL-10 < 100 pg/ml (all comparisons of T-Rapa6 vs. T-Rapa12: p = NS). Extended culture of T-Rapa6 and T-Rapa12 cell products resulted in T cells with an enhanced capacity for cytokine secretion, in particular IFN-γ and IL-4. Extended culture in the presence of a type I polarizing cytokine (IFN-γ) similarly increased both T-Rapa6 and T-Rapa12 cell capacity for IL-2 secretion, whereas extended culture in the presence of the type II polarizing cytokine IL-4 similarly increased both T-Rapa6 and T-Rapa12 cell capacity for IL-4 secretion. Because both T-Rapa6 and T-Rapa12 cell products maintained a balanced pattern of secretion of IFN-γ, IL-2, IL-4, and IL-10, each population was comprised of a mix of Th1 and Th2 effectors that manifested minimal T-helper subset differentiation plasticity.
Figure 1. Cytokine expression analysis of T-Rapa6 and T-Rapa12 cells generated from CD4+ cells (n=6).
Cell supernatants were analyzed for cytokine content by Luminex assay. Cytokine levels were measured in cell supernatants generated over a 24 hr restimulation period. In contrast, cytokine levels in supernatants of cultures generated over a 24 hr restimulation period after extending the cultures for an additional 6 days in absence of Rapamycin were setup under costimulation conditions with IL-2 alone, IL-2 and IFN-α, or IL-2 and IL-4. The production of IFN-γ (a), IL-2 (b), IL-4 (c) and IL-10 (d) are shown.
Gene Expression Unsupervised Analysis Differentiates T-Rapa6 and T-Rapa12 Cell Products
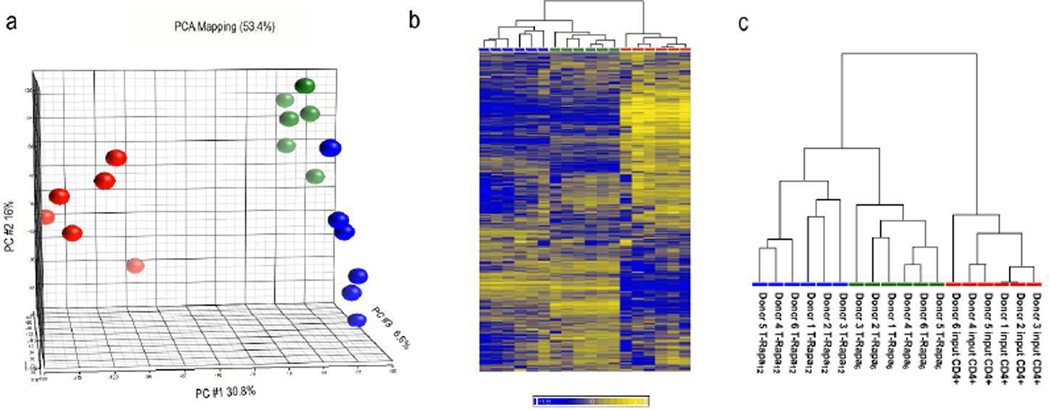
Principal component analysis (PCA) of the entire gene expression dataset (34,340 genes) separated the three T cell populations into three distinct groups: (1) input CD4+ T cells; (2) T-Rapa12 cells; and (3) T-Rapa6 cells (Fig. 2A). The first principal component (PC #1) clearly separated input CD4+ T cells from each of the T-Rapa cell populations, whereas the third principal component (PC #3) separated the T-Rapa6 and T-Rapa12 cell populations. Given the high signal to noise ratio in microarray data[17, 19], we performed an unsupervised hierarchical clustering on the most informative genes by removing genes that had low variation across the dataset (as defined by a coefficient of variation < 0.05). The unsupervised hierarchical clustering of the resulting 14,780 genes revealed two distinct main clusters: (1) one with all of the input CD4+ T cell samples (Fig. 2B); and (2) one with all of the T-Rapa6 cell samples separated from all of the T-Rapa12 cell samples (Fig. 2C).
Figure 2. Gene expression analysis of T-Rapa6 and T-Rapa12 cells.
a) Principal component analysis of the whole dataset (34340 genes); b) Unsupervised hierarchical clustering analysis of 14780 genes that remained after filtering out genes with a coefficient of variation < 0.05. The heat map is average corrected. c) Magnification of the dendrogram in b showing sample identifiers. The T-Rapa6 cells, T-Rapa12 cells and input CD4+ cells are indicated in green, blue, and red, respectively.
The Majority of Differentially Expressed Genes are of Limited Magnitude
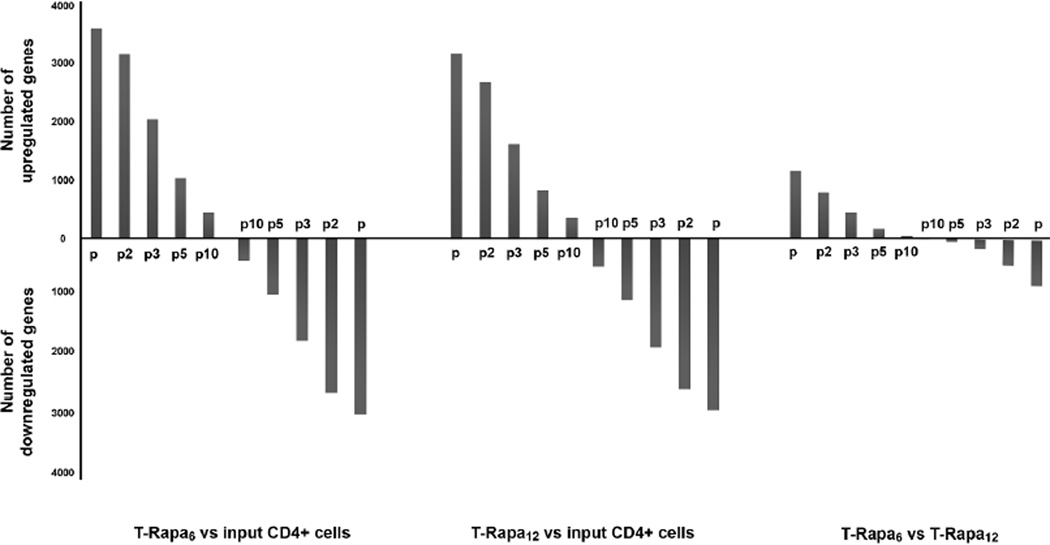
To better delineate differential gene expression across the three T cell populations, we compared the number of differentially expressed genes using an additional filter that incorporated relative fold-change of gene expression (Fig. 3). The total number of differentially expressed genes between the input CD4+ T cells and the T-Rapa6 cells was 6641 (p-value < 0.001 and FDR < 0.10; 19.3% of all evaluable genes), with a relatively equal number of genes up-regulated (n=3614) or down-regulated (n=3027) by the T-Rapa6 culture condition. Similarly, the total number of differentially expressed genes between the input CD4+ T cells and the T-Rapa12 cells was 6147 (p-value < 0.001 and FDR < 0.10; 17.8% of all evaluable genes), again with a relatively equal number of genes up-regulated (n=3185) or down-regulated (n=2962) by the T-Rapa12 culture condition. Instead, the total number of differentially expressed genes between the T-Rapa6 and T-Rapa12 cells was 1994 (p-value < 0.001 and FDR < 0.10; 5.8% of all evaluable genes; 1144 genes up-regulated in T-Rapa6 cells, 800 genes down-regulated in T-Rapa6 cells); furthermore, whereas both T-Rapa6 and T-Rapa12 cells had more than 2000 genes that differed from the input CD4+ T cells by relatively large fold-changes of ≥ 5-fold, less than 200 genes were of that magnitude difference when comparing the T-Rapa6 and T-Rapa12 cell populations. As such, the T-Rapa6 and T-Rapa12 cell products showed fewer differences from each other than from the input CD4+ T cells.
Figure 3. The number of T-Rapa6 and T-Rapa12 cells that differ from input CD4+ cells.
The number of genes up- and down-regulated among the three cell populations for which differences in expression exceeded specified levels of fold change (2-, 3-, 5- and 10-fold change as indicated by p, p2, p3, p5, p10 respectively) and whose difference in expression was highly significant (p-value < 0.001). The y-axis indicates the number of genes selected by filtering only using the p-value < 0.001 or the p-value plus 2-, 3-, 5- and 10-fold difference in expression.
T-Rapa6 and T-Rapa12 Cell Products Each Up-regulate Cell Cycle and Metabolic Genes and Each Down-regulate Apoptosis and Immune Response Genes
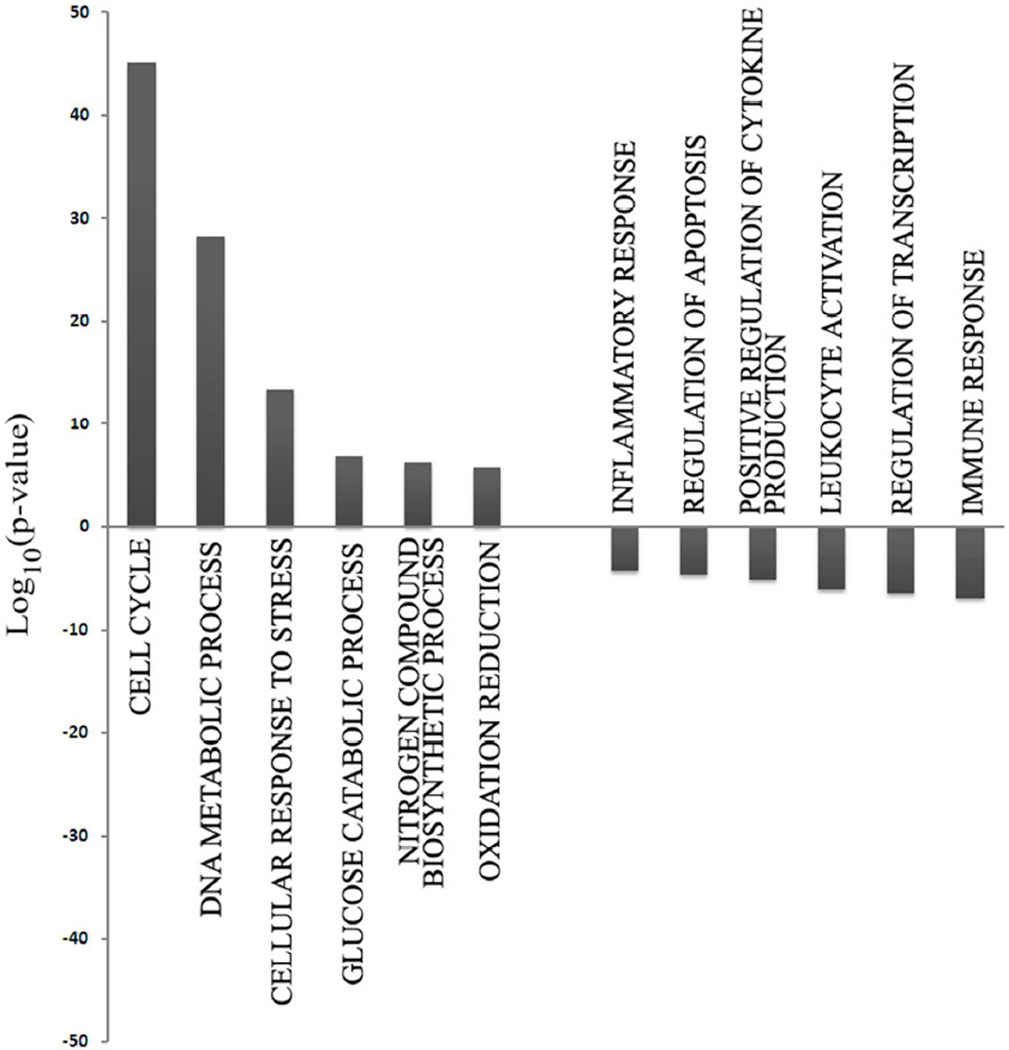
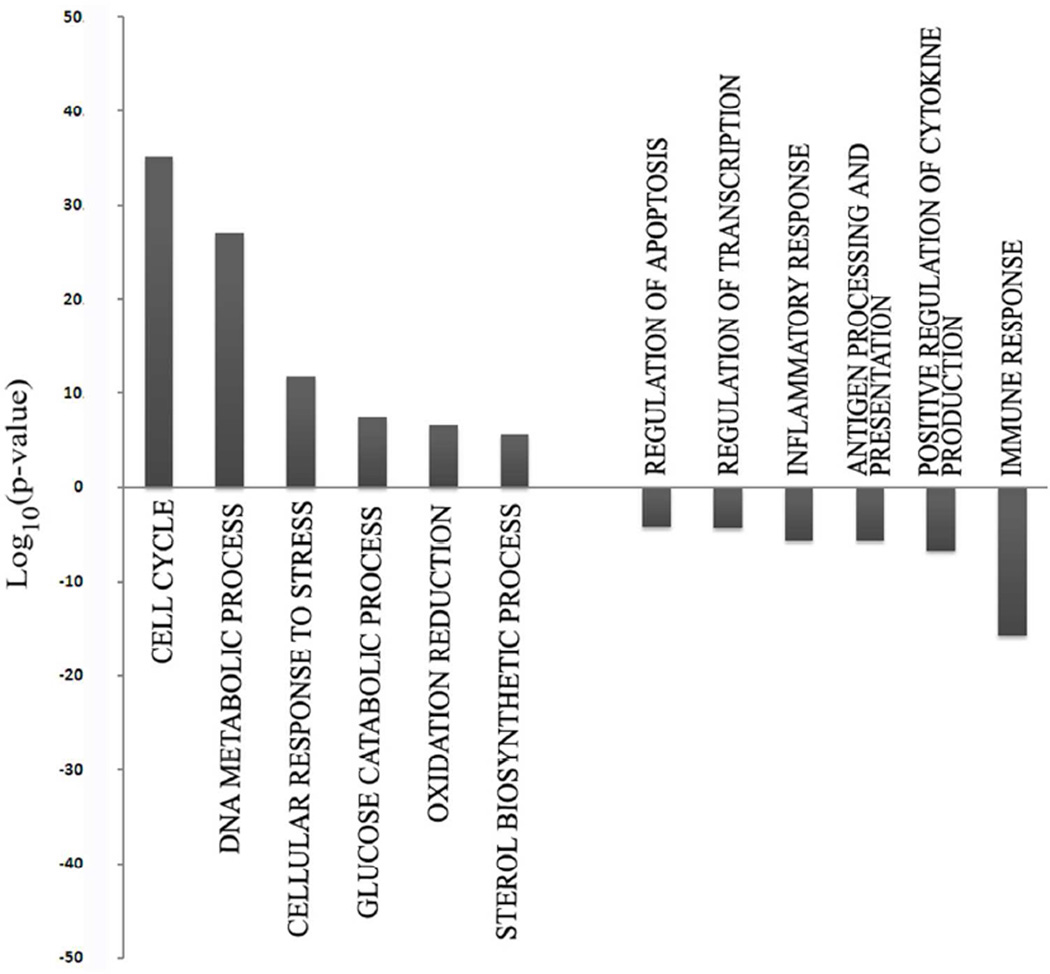
Next, we performed Gene Ontology (GO) analysis using DAVID software to determine which gene families were most significantly altered in their expression when comparing either T-Rapa6 or T-Rapa12 cell populations to the input CD4+ T cells. Relative to input CD4+ T cells, gene families that were the most significantly over-represented in the genes up-regulated in T-Rapa12 cell clinical products included cell cycle, modulation of DNA metabolism, cellular response to stress, glucose catabolic process, nitrogen compound biosynthetic process, and oxidative reduction (Fig. 4). On the other hand, gene families that were the most significantly over-represented in the genes down-regulated in the T-Rapa12 cell clinical products included the inflammatory response, regulation of apoptosis, positive regulation of cytokine production, leukocyte activation, regulation of transcription, and the immune response. Remarkably, 10 out of 12 of these gene families were also the most significant families that had differential gene expression when comparing T-Rapa6 cell clinical products to input CD4+ T cells (Fig. 5). Taken together, these gene family ontology results indicate that the majority of the gene expression differences induced by the culture system were apparent by day 6 of culture (T-Rapa6 cell product) and maintained throughout day 12 of culture (T-Rapa12 cell product). Table I details specific genes from these families that were commonly differentially expressed in the T-Rapa12 and T-Rapa6 cells relative to the day 0 input CD4+ T cells.
Figure 4. Gene ontology analysis of genes differentially expressed between T-Rapa12 cells and input CD4+ cells.
The most overrepresented GO families among T-Rapa12 cells are represented as −Log10(p-value) for up-regulated genes and Log10(p-value) for down-regulated ones.
Figure 5. Gene ontology analysis of genes differentially expressed between T-Rapa6 cells and input CD4+ cells.
Most overrepresented GO families among T-Rapa6 are represented as −Log10(p-value) for up-regulated genes and Log10(p-value) for down-regulated genes.
Table I.
Genes differentially regulated in both T-Rapa12 and T-Rapa6 cells (vs. day 0 input cells).
| Gene Family | Gene Symbol (Reference) |
T-Rapa12 | T-Rapa6 | |
|---|---|---|---|---|
| Up-Regulated Families | ||||
| Cell Cycle | DTL ([20]) | 63-fold up | 57-fold up | |
| DNA Metabolism | ESCO2 ([21]) | 49-fold up | 38-fold up | |
| Stress Response | TOP2A ([22]) | 23-fold up | 31-fold up | |
| Glucose Catabolism | OGDHL ([23]) | 92-fold up | 23-fold up | |
| Oxidative Reduction | MAOA ([24]) | 35-fold up | 41-fold up | |
| Down-Regulated Families | ||||
| Apoptosis | IL-1B ([25]) |
134-fold down | 90-fold down | |
| Transcription | EREG ([26]) | 45-fold down | 100-fold down | |
| Inflammation | S100A8 ([27]) | 341-fold down | 451-fold down | |
| Cytokine Production | NLRP3 ([28]) | 173-fold down | 11-fold down | |
| Immune Response | CXCL2 ([29]) | 35-fold down | 98-fold down | |
T-Rapa6 and T-Rapa12 Cell Products Differentially Express Genes Related to Hypoxia, Cell Death, and Immune Function
We next utilized GO analysis to evaluate families of genes that were up- or down-regulated in the second-generation T-Rapa6 cell product relative to the first-generation T-Rapa12 cells. Moreover, it is important to note that the T-Rapa6 cell products were similar to the T-Rapa12 products in terms of expression of genes in the Th2 family, the Th1 family, and the TREG family (Table II); consistent with the cytokine secretion results (Figure 1), these results are consistent with the conclusion that both the T-Rapa12 and T-Rapa6 cell products can be characterized as having a mixed Th1/Th2 phenotype.
Table II.
T-Rapa12 and T-Rapa6 cell products: similar expression of Th2, Th1, and T-Reg genes.
| Gene Family |
Gene Symbol (Ref.) |
T-Rapa12 (Ref.) |
T-Rapa6 (vs. Day 0) |
T-Rapa6 (vs. T-Rapa12) |
|---|---|---|---|---|
| Th2 Genes | ||||
| IL4 ([30]) | 4-fold up | 6-fold up | NS | |
| IL13 ([31]) | 17-fold up | 27-fold up | NS | |
| NFIL3 ([32]) | 4-fold up | 4-fold up | NS | |
| Th1 Genes | ||||
| IFNG ([33]) | NS | NS | NS | |
| IL12Rβ2 ([34]) | 21-fold up | 24-fold up | NS | |
| HAVCR2 ([35]) | 6-fold up | 6-fold up | NS | |
| T-Reg Genes | ||||
| FOXP3 ([36]) | NS | NS | NS | |
| NRP1 ([37]) | NS | NS | NS | |
| IKZF2 ([38]) | NS | NS | NS |
NS: not statistically significant
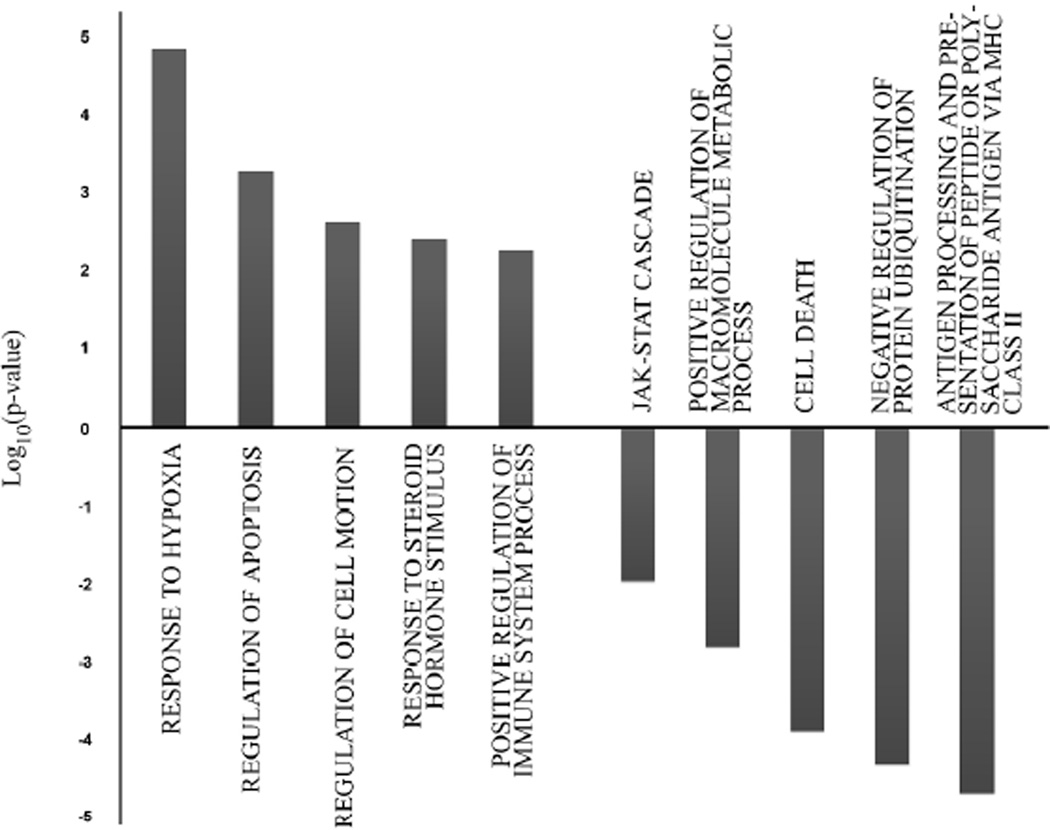
The gene families that were differentially expressed between the T-Rapa6 and T-Rapa12 cell products are shown in Figure 6. Relative to the T-Rapa12 cell product, the T-Rapa6 cell product had up-regulation of hypoxia response genes, anti-apoptosis genes, cell motility genes, sterol hormone synthesis genes, and regulation of the immune system genes; and conversely, relative to the T-Rapa12 cell product, the T-Rapa6 cell product had down-regulation of families of genes relating to antigen processing and presentation, negative regulation of protein ubiquitination, cell death, positive regulation of macromolecule metabolic process, and the JAK-STAT cascade. It should be noted that the levels of significance of the changes in these gene families were markedly lower than the levels of significance observed when comparing either T-Rapa cell product to the input CD4+ T cells (that is, the most significant family difference p value was in the 10−5 range rather than in the 10−50 range). Table III details specific genes from these families that were differentially expressed when comparing the T-Rapa12 and T-Rapa6 cell products. In general, the magnitude of these fold-changes were less than the fold-changes observed when comparing the T-Rapa cell products to the day 0 CD4+ T cells.
Figure 6. Gene ontology analysis of genes differentially expressed between T-Rapa6 and T-Rapa12 cells.
Most overrepresented GO families are represented as −Log10(p-value) for genes up-regulated in T-Rapa6 and Log10(p-value) for those down-regulated.
Table III.
Genes differentially regulated T-Rapa6 cells relative to T-Rapa12 cells.
| Gene Ontology Family Up | Gene Symbol (Reference) |
T-Rapa6 vs. T- Rapa12 |
|
|---|---|---|---|
| Up-Regulated Families | |||
| Response to Hypoxia | FLT1 ([39]) | 14-fold up | |
| Regulation of Apoptosis | IER3 ([40]) | 7-fold up | |
| Regulation of Cell Motion | PDGFA ([41]) | 4-fold up | |
| Sterol Hormone Synthesis | ADM ([42]) | 5-fold up | |
| Immune System Regulation | CD86 ([43]) | 18-fold up | |
| Down-Regulated Families | |||
| Antigen Processing and Presentation | HLA-DRA([44]) | 4-fold down | |
| Protein Ubiquitination | CDKN2A ([45]) | 3-fold down | |
| Cell Death | XAF1 ([46]) | 9-fold down | |
| Macromolecule Metabolic Process | KLF2 ([47]) | 78-fold down | |
| JAK-STAT Cascade | JAK2 ([48]) | 4-fold down | |
Discussion
Gene expression profiling represents an important tool to more fully characterize ex vivo manufactured T cell products in an unbiased manner, can be utilized to provide a T cell ‘fingerprint’ as a component of a quality control program, and may offer new insights into T cell biology and the modulation of transplantation responses. These attributes of microarray analysis are particularly relevant to our clinical translation efforts using ex vivo manufactured allogeneic donor T-Rapa cells because our murine models indicated that such T cells had diverse functional characteristics above and beyond classical definitions of T cell function such as the Th1/Th2 cytokine profile, including a post-autophagy state, an anti-apoptotic phenotype, and altered mitochondrial biology. A more detailed investigation of T cell product characteristics is particularly important as we have now transitioned from an initial 12-day interval of T-Rapa cell manufacturing to the current culture interval of 6 days. In this report, we have found that the T-Rapa12 and T-Rapa6 populations, which are relatively indistinguishable from one another in terms of conventional definitions of Th1/Th2 cytokine secretion, were also indistinguishable from one another in terms of the majority of genes that were up- or down-regulated during T cell culture. However, we did identify that the T-Rapa12 and T-Rapa6 populations differentially expressed a significant number of genes, thereby indicating that these T cell populations would best be viewed as distinct cellular products.
In comparing the T-Rapa12 and T-Rapa6 cell products, conventional methods of T cell phenotyping did not identify differences that might indicate that the T cell products were functionally distinct. That is, although the T-Rapa12 cell product expanded to a greater degree in culture relative to the T-Rapa6 cell product, the two products were nearly identical in terms of their ability to secrete the Th1 cytokines IL-2 and IFN-γ or the Th2 cytokines IL-4 and IL-10 in response to anti-CD3, anti-CD28 co-stimulation. In addition, we assessed the important functional characteristics of T cell differentiation plasticity by performing an extended ex vivo culture without rapamycin and in the presence of variable cytokine polarizing conditions, and again found the T-Rapa12 and T-Rapa6 cell products to be nearly identical. It is possible that protein-based methods of cell product phenotyping may have limited applicability in the setting of rapamycin-based manufacturing due to the ability of mTOR blockade to inhibit protein translation. The above-mentioned functional similarities that we observed between the two T-Rapa clinical products necessitated that we utilize additional methods such as gene expression microarray to identify differentially expressed genes and identify differences in less expected functions.
Given these functional similarities using conventional assays, it was somewhat surprising to observe that the T-Rapa12 and T-Rapa6 populations could be easily segregated from one another using global microarray analytical tools such as the principal component analysis and unsupervised hierarchical clustering. Remarkably, the T-Rapa12 and T-Rapa6 populations had similar numbers of differentially expressed genes relative to the input CD4+ T cells (17.8% and 19.3 % of all genes, respectively). Although these results indicate that T cell culture using co-stimulation, polarizing cytokines, and rapamycin altered expression of a multitude of genes, the great majority of these genes had small magnitudes of differential expression relative to the day 0 input CD4+ T cells (< 3-fold). Nonetheless, several hundred genes were massively up- or down-regulated in these culture conditions (>10-fold difference relative to input CD4+ T cells), thereby indicating the dramatic effect that culture in rapamycin and polarizing media had on T cell biology; it should be emphasized that further studies will be required to delineate which of the gene expression changes were due to individual culture components (that is, co-stimulation, cytokines, or rapamycin). Using this fold-change approach to the microarray analysis, one can clearly conclude that the T-Rapa12 and T-Rapa6 cell products were more similar than different: specifically, only a few genes were differentially expressed at a level of greater than 10-fold when comparing these two clinical T cell products.
Using GO to better understand patterns of differential gene expression, we found that the first-generation T-Rapa12 and second-generation T-Rapa6 cell populations were virtually identical in terms of the major families that were altered. With both T cell products, there was a great up-regulation of genes that promote the cell cycle; potentially, this attribute of the T-Rapa cell products may help ensure cell product expansion in vivo after adoptive transfer and thus may be a critical factor in any observed clinical effects. Several families related to cellular metabolism and stress were commonly up-regulated in both T-Rapa cell products, including the family of genes related to DNA metabolic process, glucose catabolic process, and oxidative reduction. The T-Rapa12 cells had a further signature of up-regulated nitrogen compound biosynthetic process whereas the T-Rapa6 cells had a further signature of up-regulated sterol biosynthetic process. In sum, these data indicate that both the T-Rapa12 and T-Rapa6 populations had up-regulation of multiple pathways related to cellular energetics.
In a similar way, the T-Rapa12 and T-Rapa6 populations were virtually identical in terms of the gene ontology families that were consistently down-regulated. That is, apoptosis, transcription, inflammation, cytokine production, and overall immune response genes were commonly down-regulated, with T-Rapa12 cells also having down-regulation of the leukocyte activation family of genes and T-Rapa6 cells also having downregulation of antigen processing and presentation genes. Reduction in apoptosis genes is consistent with our previous results, which found that murine and human rapamycin-resistant T cells expressed an anti-apoptotic phenotype that conferred increased T cell survival in vivo[9, 11]. Furthermore, the observed reduction of inflammatory and immune response genes is consistent with our previous findings that rapamycin-resistant murine[8] and human[14] T cells existed in a minimally-differentiated effector status. Although such a relatively primitive T cell effector state may seem counter-productive, we[8] and others[16] have shown that T cells possessing nominal effector function at the time of T cell transfer associate with an increased in vivo efficacy.
Although these results in sum indicate that the T-Rapa12 and T-Rapa6 cell products are remarkably similar in terms of the number and types of genes differentially regulated relative to the day 0 input CD4+ T cells, it is also recognized that these populations are distinct, and to the extent, it is important to consider in greater detail the differences that exist. There were several gene ontology families that were not identified in the initial comparisons to the day 0 input CD4+ T cells; specifically, relative to the T-Rapa12 cells, the T-Rapa6 cells had increased expression of hypoxia response genes and cell motility genes and reduced expression of genes in the JAK-STAT cascade and protein ubiquitination families. Further studies are required to better characterize the differential gene expression that exists between these two T-Rapa cell products and to evaluate whether such differences are associated with differences in clinical outcomes.
In conclusion, using gene expression microarray, we have characterized two ex vivo manufactured T cell populations currently being evaluated in the setting of allogeneic hematopoietic cell transplantation, the first-generation T-Rapa12 and the second-generation T-Rapa6 cell products. Using this unbiased approach to T cell analysis, we have determined that these T cell products were remarkably similar in their differential expression of genes relative to the CD4+ T cells used for culture initiation. The families of genes that were commonly differentially expressed would predictably associate with an increased T cell function after adoptive cell transfer: that is, in general, both the T-Rapa12 and T-Rapa6 cell populations had a favorable genetic profile for increased cell cycle machinery, an anti-apoptotic phenotype, and a minimally-differentiated effector state. Nonetheless, the T-Rapa12 and T-Rapa6 populations were easily segregated on a gene expression basis, and as such, future research will more closely evaluate the functional differences that appear to exist between these two T cell products. Specifically, it will be important to validate these research findings in an expanded dataset during clinical trial implementation, to confirm differences at the protein level and through functional assays, and to correlate T cell product phenotype and transcription profile with clinical outcomes after allogeneic transplantation, including engraftment, graft-versus-host disease, and graft-versus-tumor effects.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, Clinical Center and National Cancer Institute. We would like to thank our collaborator, Dr. Bruce Levine of the University of Pennsylvania, for his assistance with the manufacturing of the anti-CD3, anti-CD28 co-stimulation beads.
Abbreviations
- APC
antigen-presenting-cell
- GO
Gene Ontology
- GVT
graft-versus-tumor
- GVHD
graft-versus-host disease
- PCA
Principal component analysis
- Th
T-helper cells
- TREG
regulatory T cells
Footnotes
Disclosure of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Gorreta F, Carbone W, Barzaghi D. Genomic profiling: cDNA arrays and oligoarrays. Methods Mol Biol. 2012;823:89–105. doi: 10.1007/978-1-60327-216-2_7. [DOI] [PubMed] [Google Scholar]
- 2.Jin P, Han TH, Ren J, Saunders S, Wang E, Marincola FM, et al. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010;8:4. doi: 10.1186/1479-5876-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of Immune Responses by mTOR. Annu Rev Immunol. 2011 Mar 24; doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung U, Foley JE, Erdmann AA, Toda Y, Borenstein T, Mariotti J, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006 Sep;12(9):905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999 Mar 1;162(5):2775–2784. [PubMed] [Google Scholar]
- 6.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005 Jun 15;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 7.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 Jul 2;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley JE, Jung U, Miera A, Borenstein T, Mariotti J, Eckhaus M, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005 Nov 1;175(9):5732–5743. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 9.Mariotti J, Foley J, Jung U, Borenstein T, Kantardzic N, Han S, et al. Ex vivo rapamycin generates apoptosis-resistant donor Th2 cells that persist in vivo and prevent hemopoietic stem cell graft rejection. J Immunol. 2008 Jan 1;180(1):89–105. doi: 10.4049/jimmunol.180.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, et al. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway. Blood. 2008 Dec 1;112(12):4765–4775. doi: 10.1182/blood-2008-05-154278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amarnath S, Flomerfelt FA, Costanzo CM, Foley JE, Mariotti J, Konecki DM, et al. Rapamycin generates anti-apoptotic human Th1/Tc1 cells via autophagy for induction of xenogeneic GVHD. Autophagy. 2010 May 16;6(4) doi: 10.4161/auto.6.4.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995 Feb 3;270(5):2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 13.Amarnath S, Fowler DH. Harnessing autophagy for adoptive T-cell therapy. Immunotherapy. 2012 Jan;4(1):1–4. doi: 10.2217/imt.11.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossoba ME, Mariotti J, Yan XY, Hakim FT, Sabatini DM, Stroncek D, et al. T rapa cell clinical products contain a balance of minimally differentiated Th2/Th1 effector cells depleted of Treg cells. Blood. 2010;116(21):158–159. [Google Scholar]
- 15.Fowler DH, Mossoba ME, Hakim FT, Kurlander R, Schuver BB, Sabatino M, et al. T Rapa Cell DLI Safely Balances Th1/Th2 Cytokine Activation After Low-Intensity Allogeneic Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2011;17(2) Supplement S157 [Google Scholar]
- 16.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005 Jun;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, Dai Y. Principal component analysis based methods in bioinformatics studies. Brief Bioinform. 2011 Nov;12(6):714–722. doi: 10.1093/bib/bbq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang da W, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007 Jul;35:W169–W175. doi: 10.1093/nar/gkm415. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing EP, Karp RM. CLIFF: clustering of high-dimensional microarray data via iterative feature filtering using normalized cuts. Bioinformatics. 2001;17(Suppl 1):S306–S315. doi: 10.1093/bioinformatics/17.suppl_1.s306. [DOI] [PubMed] [Google Scholar]
- 20.Liu CL, Yu IS, Pan HW, Lin SW, Hsu HC. L2dtl is essential for cell survival and nuclear division in early mouse embryonic development. J Biol Chem. 2007 Jan 12;282(2):1109–1118. doi: 10.1074/jbc.M606535200. [DOI] [PubMed] [Google Scholar]
- 21.Whelan G, Kreidl E, Wutz G, Egner A, Peters JM, Eichele G. Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. Embo J. 2011 Jan 4;31(1):71–82. doi: 10.1038/emboj.2011.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008 Apr 4;133(1):103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunik VI, Degtyarev D. Structure-function relationships in the 2-oxo acid dehydrogenase family: substrate-specific signatures and functional predictions for the 2-oxoglutarate dehydrogenase-like proteins. Proteins. 2008 May 1;71(2):874–890. doi: 10.1002/prot.21766. [DOI] [PubMed] [Google Scholar]
- 24.Dabrowska M, Skoneczny M, Rode W. Functional gene expression profile underlying methotrexate-induced senescence in human colon cancer cells. Tumour Biol. 2011 Oct;32(5):965–976. doi: 10.1007/s13277-011-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999 Jan;19(1):1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 26.Yun J, Song SH, Park J, Kim HP, Yoon YK, Lee KH, et al. Gene silencing of EREG mediated by DNA methylation and histone modification in human gastric cancers. Lab Invest. 2012 Apr 16; doi: 10.1038/labinvest.2012.61. [DOI] [PubMed] [Google Scholar]
- 27.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007 Sep;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 28.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010 Sep;30(5):628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 29.Owen JL, Criscitiello MF, Libreros S, Garcia-Areas R, Guthrie K, Torroella-Kouri M, et al. Expression of the inflammatory chemokines CCL2, CCL5 and CXCL2 and the receptors CCR1-3 and CXCR2 in T lymphocytes from mammary tumor-bearing mice. Cell Immunol. 2011;270(2):172–182. doi: 10.1016/j.cellimm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010 Apr;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr Opin Investig Drugs. 2010 May;11(5):527–534. [PubMed] [Google Scholar]
- 32.Kashiwada M, Cassel SL, Colgan JD, Rothman PB. NFIL3/E4BP4 controls type 2 T helper cell cytokine expression. Embo J. 2011 May 18;30(10):2071–2082. doi: 10.1038/emboj.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cimmino L, Martins GA, Liao J, Magnusdottir E, Grunig G, Perez RK, et al. Blimp-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J Immunol. 2008 Aug 15;181(4):2338–2347. doi: 10.4049/jimmunol.181.4.2338. [DOI] [PubMed] [Google Scholar]
- 34.Boisson-Dupuis S, El Baghdadi J, Parvaneh N, Bousfiha A, Bustamante J, Feinberg J, et al. IL-12Rbeta1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey. PLoS One. 2011;6(4):e18524. doi: 10.1371/journal.pone.0018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun KJ, Lee MJ, Shin DC, Woo MY, Kim K, Park S. Identification of CCL1 as a Gene Differentially Expressed in CD4 T Cells Expressing TIM-3. Immune Netw. 2011 Aug;11(4):203–209. doi: 10.4110/in.2011.11.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger TL, Tauro S. Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Hum Immunol. 2012 Mar;73(3):232–239. doi: 10.1016/j.humimm.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004 Mar;34(3):623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 38.Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, et al. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7(3):e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, et al. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol. 2007 Jul;212(1):236–243. doi: 10.1002/jcp.21022. [DOI] [PubMed] [Google Scholar]
- 40.Shen L, Guo J, Santos-Berrios C, Wu MX. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J Biol Chem. 2006 Jun 2;281(22):15304–15311. doi: 10.1074/jbc.M600054200. [DOI] [PubMed] [Google Scholar]
- 41.Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004 Jun;131(11):2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- 42.Harmancey R, Senard JM, Pathak A, Desmoulin F, Claparols C, Rouet P, et al. The vasoactive peptide adrenomedullin is secreted by adipocytes and inhibits lipolysis through NO-mediated beta-adrenergic agonist oxidation. Faseb J. 2005 Jun;19(8):1045–1047. doi: 10.1096/fj.04-2868fje. [DOI] [PubMed] [Google Scholar]
- 43.Shimazu T, Iida R, Zhang Q, Welner RS, Medina KL, Alberola-Lla J, et al. CD86 is expressed on murine hematopoietic stem cells and denotes lymphopoietic potential. Blood. 2012 May 24;119(21):4889–4897. doi: 10.1182/blood-2011-10-388736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011 Sep;37(2):104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell Cycle. 2011 Aug 1;10(15):2497–2503. doi: 10.4161/cc.10.15.16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun PH, Zhu LM, Qiao MM, Zhang YP, Jiang SH, Wu YL, et al. The XAF1 tumor suppressor induces autophagic cell death via upregulation of Beclin-1 and inhibition of Akt pathway. Cancer Lett. 2011 Nov 28;310(2):170–180. doi: 10.1016/j.canlet.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 47.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009 Jul 17;31(1):122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008 Jun;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]