Abstract
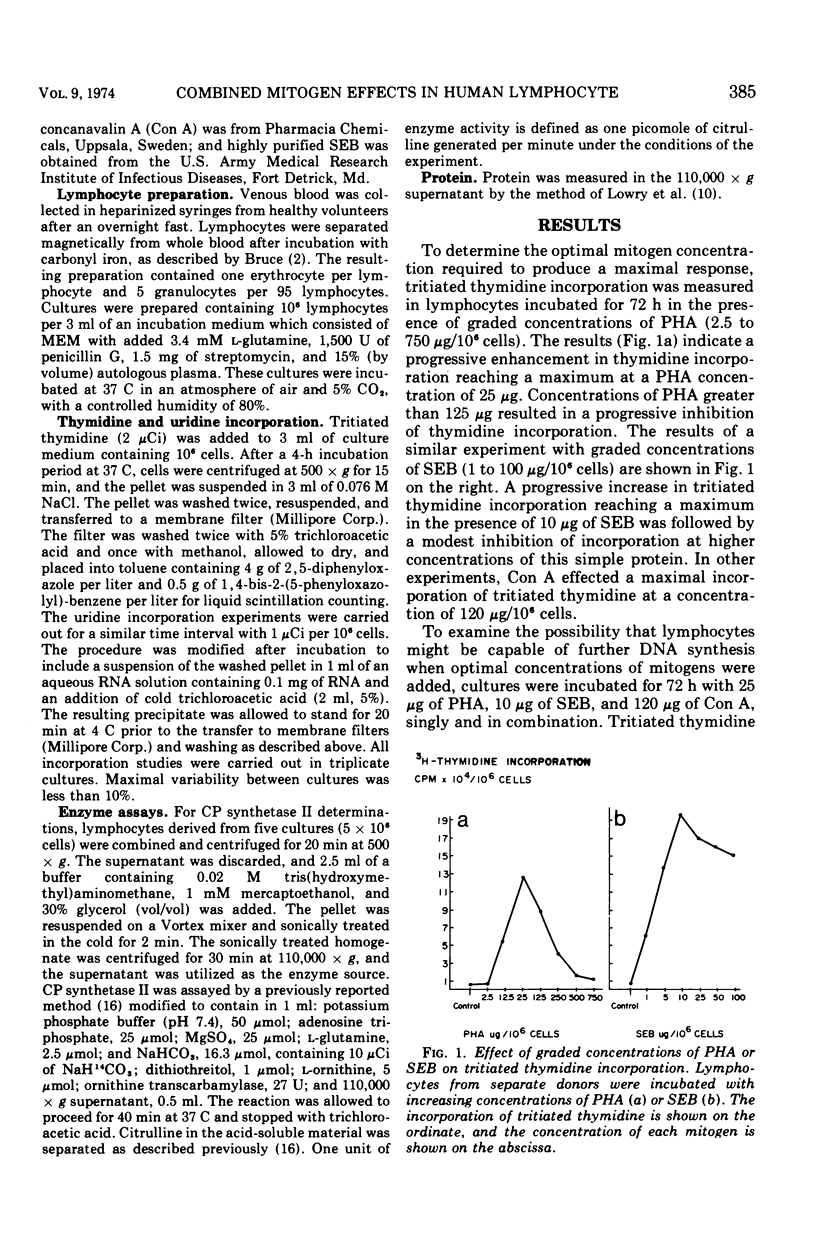
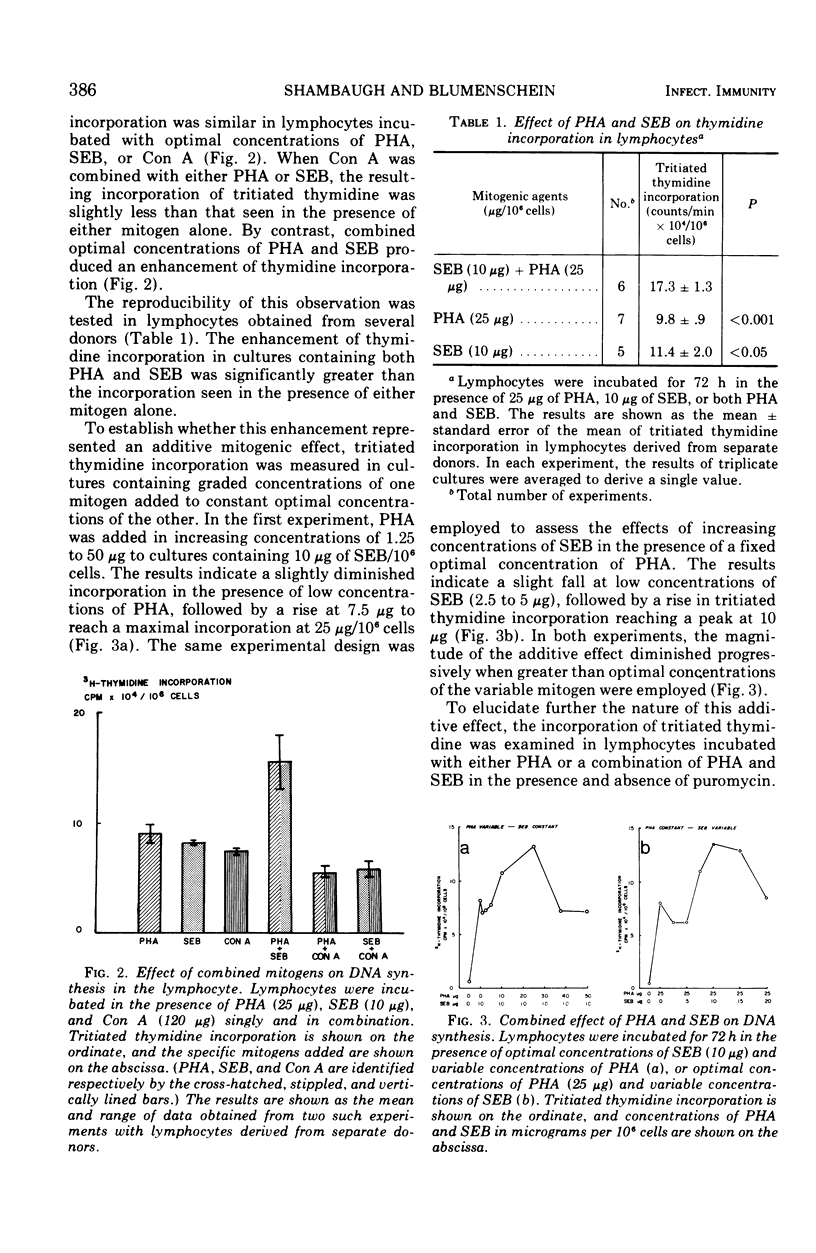
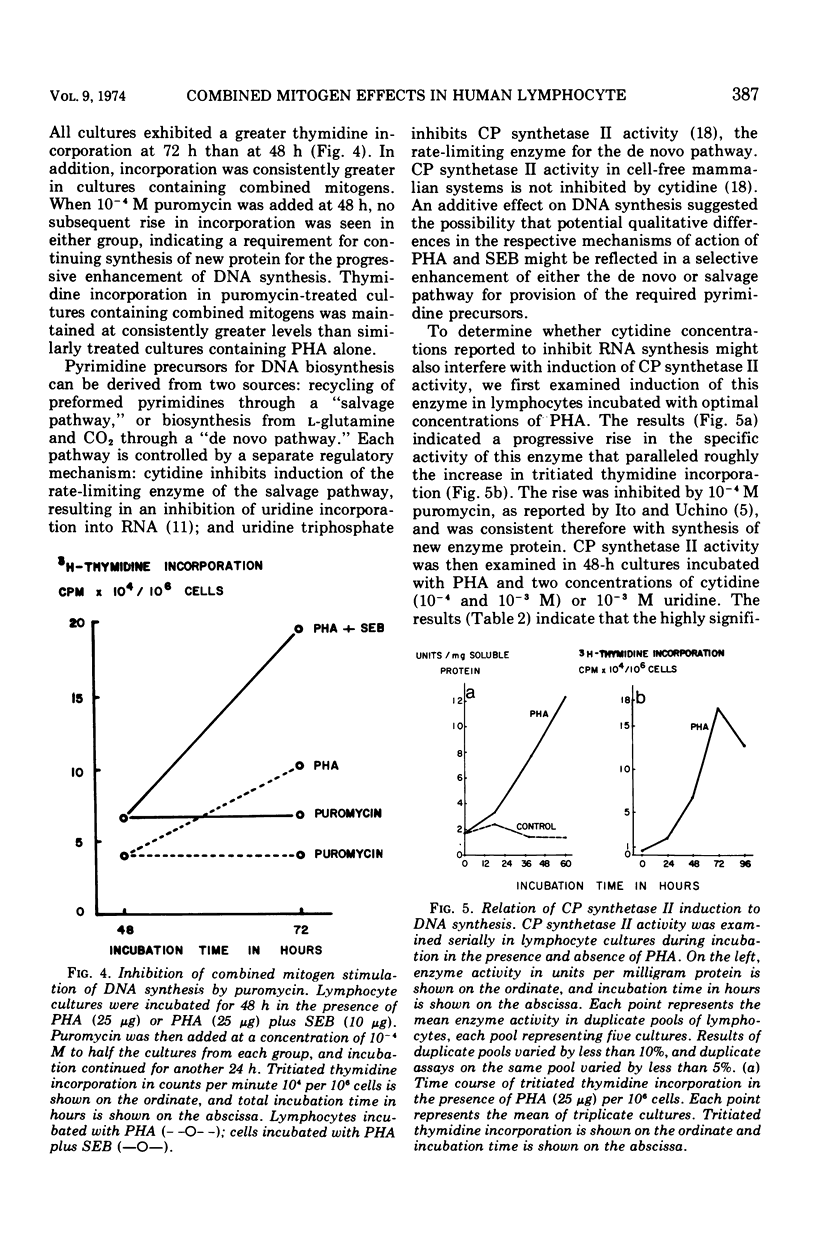
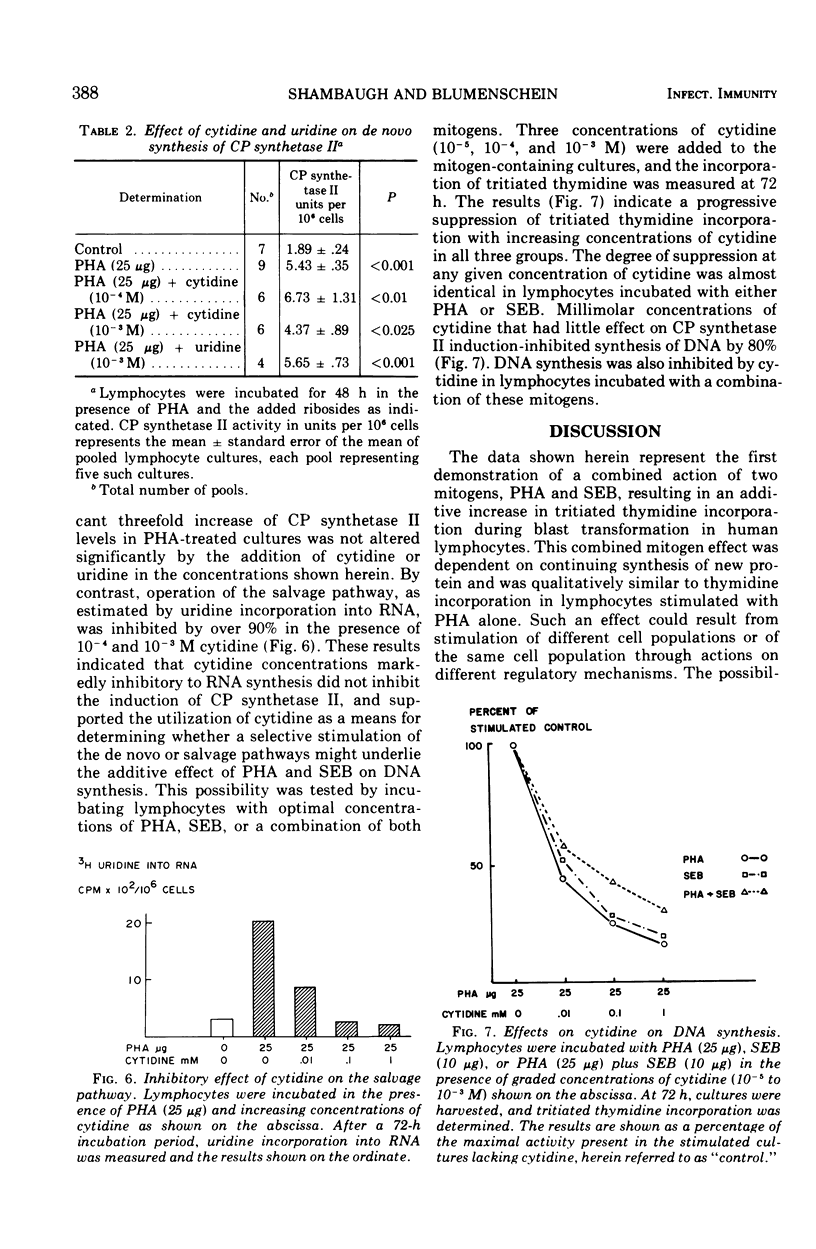
Three mitogenic agents, phytohemagglutinin (PHA), staphylococcal enterotoxin B (SEB), and concanavalin A (Con A) were tested for their effects on deoxyribonucleic acid (DNA) synthesis in the normal human lymphocyte. When optimal concentrations of PHA and SEB were combined, tritiated thymidine incorporation in lymphocytes derived from several donors was enhanced significantly. In the presence of graded concentrations of one of these mitogens added to fixed optimal concentrations of the other, this enhancement was shown to be additive. By contrast, when PHA or SEB were combined with Con A, the resulting thymidine incorporation was slightly lower than for either mitogen alone. An inhibition of further thymidine incorporation when puromycin was added to lymphocytes incubated with PHA and SEB suggested that the additive effect of these mitogens was due to increased enzyme synthesis. To define potential differences in mechanisms of action underlying the additive effect of SEB and PHA, the relative contribution of the de novo and salvage pathways for pyrimidine biosynthesis was tested with cytidine, a specific salvage pathway inhibitor. Cytidine (10−3 M) inhibited synthesis through the salvage pathway, but did not significantly alter induction of carbamyl phosphate synthetase II, the rate-limiting enzyme for the de novo pathway. An inhibition of DNA synthesis by millimolar cytidine concentrations in lymphocytes incubated with PHA or SEB, singly or in combination, suggested that pyrimidines for the observed enhancement of DNA synthesis were derived largely via the salvage pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce D. L. Immunologically competent anesthesiologists. Anesthesiology. 1972 Jul;37(1):76–78. doi: 10.1097/00000542-197207000-00015. [DOI] [PubMed] [Google Scholar]
- Heilman D. H. Mitogenic activity of bacterial fractions in lymphocyte cultures. Endotoxins and other derivatives of gram-negative bacteria. Int Arch Allergy Appl Immunol. 1970;39(4):415–425. doi: 10.1159/000230369. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Brittinger G., Hirschhorn K., Weissmann G. Studies on lysosomes. XII. Redistribution of acid hydrolases in human lymphocytes stimulated by phytohemagglutinin. J Cell Biol. 1968 May;37(2):412–423. doi: 10.1083/jcb.37.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Uchino H. Control of pyrimidine biosynthesis in human lymphocytes. Induction of glutamine-utilizing carbamyl phosphate synthetase and operation of orotic acid pathway during blastogenesis. J Biol Chem. 1971 Jun 25;246(12):4060–4065. [PubMed] [Google Scholar]
- Killander D., Rigler R., Jr Initial changes of deoxyribonucleoprotein and synthesis of nucleic acid in phytohemagglutinine-stimulated human leucocytes in vitro. Exp Cell Res. 1965 Sep;39(2):701–704. doi: 10.1016/0014-4827(65)90075-3. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy R., Levy S., Rosenberg S. A., Simpson R. T. Selective stimulation of nonhistone chromatin protein synthesis in lymphoid cells by phytohemagglutinin. Biochemistry. 1973 Jan 16;12(2):224–228. doi: 10.1021/bi00726a008. [DOI] [PubMed] [Google Scholar]
- Lucas Z. J. Pyrimidine nucleotide synthesis: regulatory control during transformation of lymphocytes in vitro. Science. 1967 Jun 2;156(3779):1237–1240. doi: 10.1126/science.156.3779.1237. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Rosenberg S. A., Levy R. Synthesis of nuclear-associated proteins by lymphocytes within minutes after contact with phytohemagglutinin. J Immunol. 1972 Apr;108(4):1105–1109. [PubMed] [Google Scholar]
- Shambaugh G. E., 3rd, Metzger B. E., Freinkel N. Glutamine-dependent carbamyl phosphate synthetase in placenta and fetal structures of the rat. Biochem Biophys Res Commun. 1971 Jan 22;42(2):155–158. doi: 10.1016/0006-291x(71)90081-7. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Carbamyl phosphate synthetase of the hematopoietic mouse spleen and the control of pyrimidine biosynthesis. Biochem Biophys Res Commun. 1967 Jan 23;26(2):221–227. doi: 10.1016/0006-291x(67)90238-0. [DOI] [PubMed] [Google Scholar]



